Secondary Metabolic Profiles of Two Cultivars of Piper nigrum (Black Pepper) Resulting from Infection by Fusarium solani f. sp. piperis
Abstract
:1. Introduction
2. Results
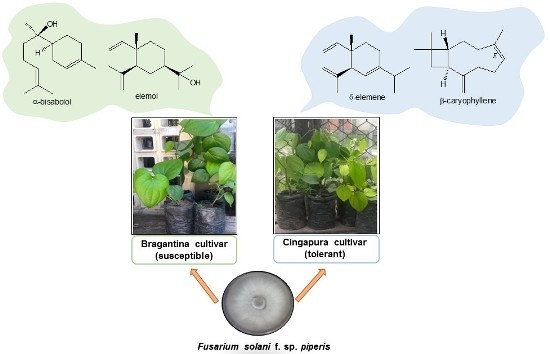
2.1. Evaluation of Susceptibility and Tolerance Of Black Pepper Cultivars after Inoculation with Fusarium solani f. sp. piperis
2.2. Profiles of Volatile Compounds of the Black Pepper Cultivars
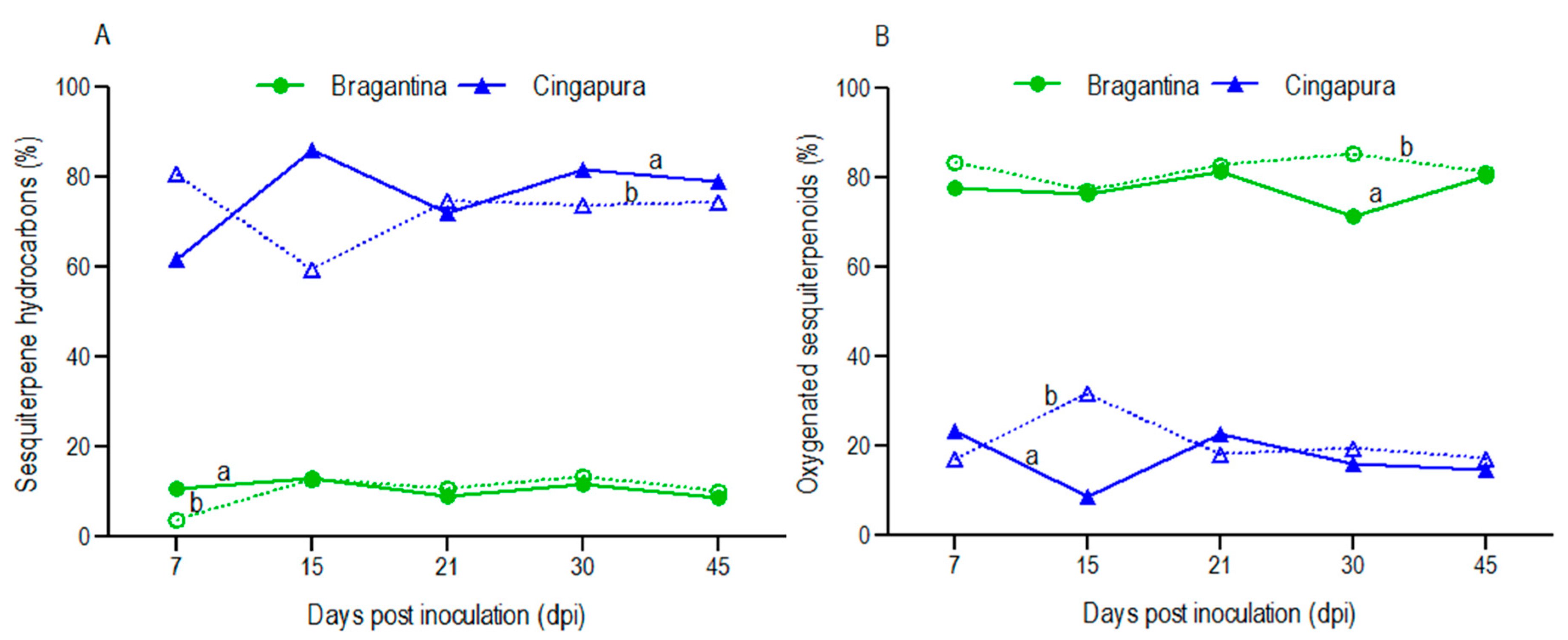
2.3. Variation in Volatile Profiles of the Black Pepper Cultivars, after Fusarium Infection
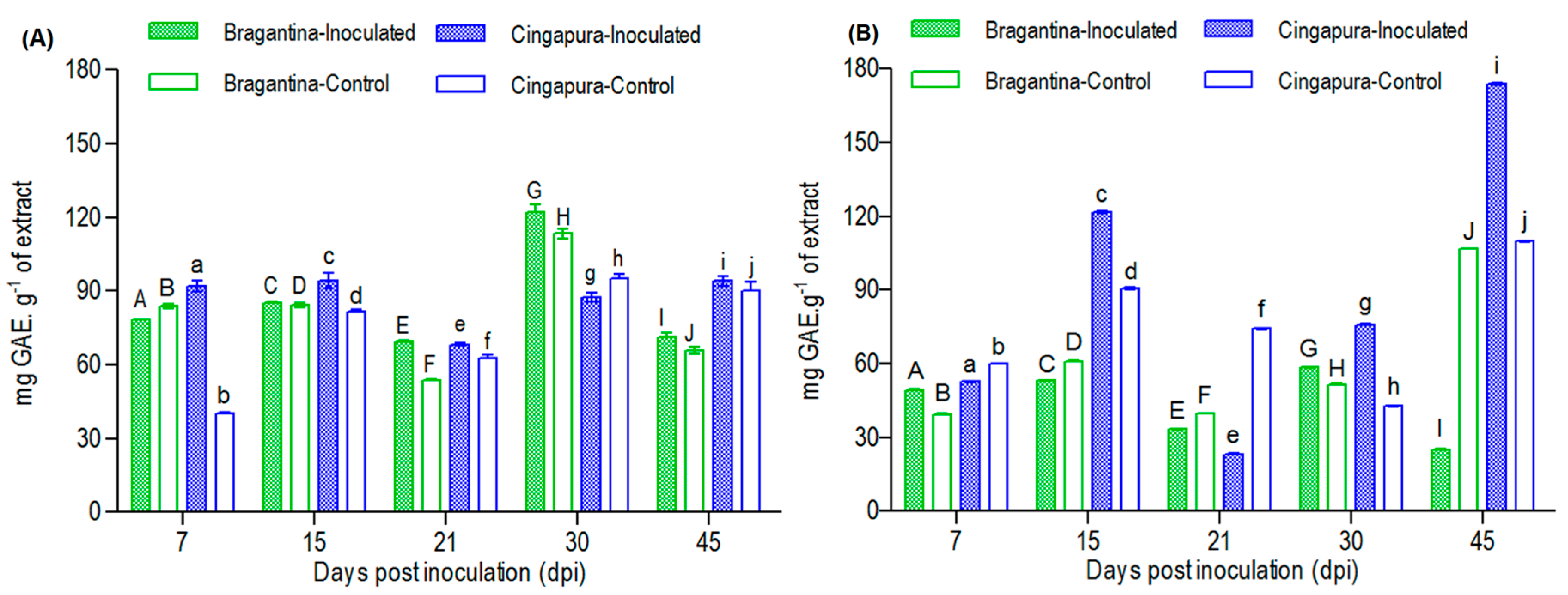
2.4. Variation in Total Phenolic Compounds Profiles of the Black Pepper Cultivars, after the Fusarium Infection
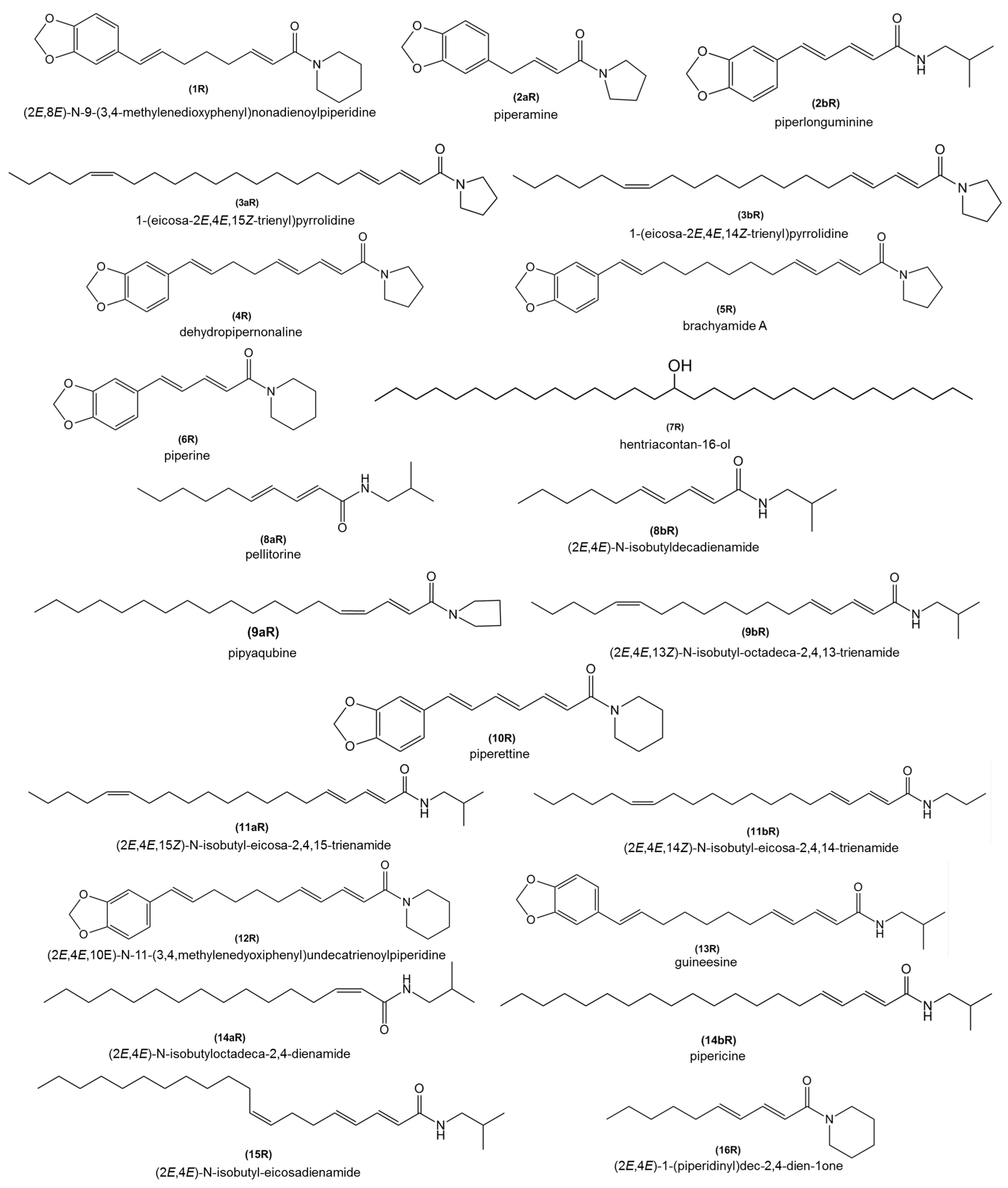
2.5. Chemical Profiles of the Root Extracts from Bragantina and Cingapura Cultivars, after Fusarium Infection
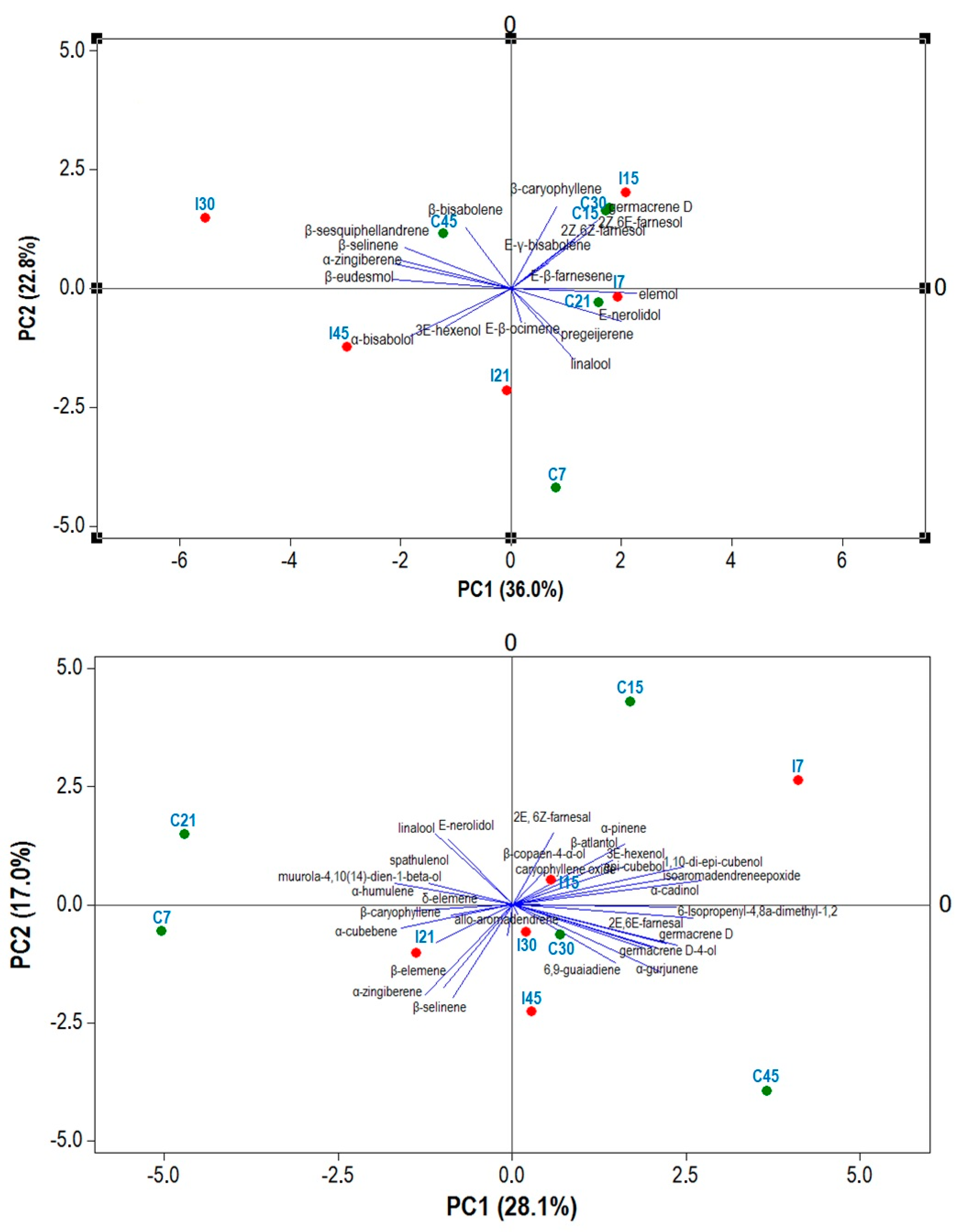
2.6. PCA Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Fungal Strains and Inoculation on Black Pepper
4.3. Volatile Concentrates and Extracts Preparation
4.4. Volatile Concentrates Analysis
4.5. Total Phenolics Content
4.6. Extract Samples Analysis
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joy, N.; Abraham, Z.V.; Soniya, E. A preliminary assessment of genetic relationships among agronomically important cultivars of black pepper. BMC Genet. 2007, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Damanhouri, Z.A.; Ahmad, A. A Review on Therapeutic Potential of Piper nigrum L. (Black Pepper): The King of Spices. Med. Aromat. Plants 2014, 3, 161. [Google Scholar] [CrossRef]
- Nascimento, S.B.; Cascardo, J.C.M.; Menezes, I.C.; Duarte, M.L.R.; Darnet, S.; Harada, M.L.; Souza, C.R.B. Identifying Sequences Potentially Related to Resistance Response of Piper tuberculatum to Fusarium solani f. sp. piperis by Suppression Subtractive Hybridization. Protein Pept. Lett. 2009, 16, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Poltronieri, M.C.; Lemos, O.F.; Albuquerque, F.C. Pimenta-do-Reino (Piper nigrum L.), Empresa Brasileira de Pesquisa Agropecuária. In Programa de Melhoramento Genético e de Adaptação de Espécies Vegetais Para a Amazônia Oriental; Embrapa (Coleção Documentos, 16): Belém, Brazil, 1999; pp. 127–137. [Google Scholar]
- Lemos, O.F.; Poltronieri, M.C.; Rodrigues, S.M.; Menezes, I.C.; Mondin, M. Conservação e Melhoramento Genético da Pimenteira do Reino (Piper nigrum L.) em Associação com as Técnicas de Biotecnologia; Embrapa Amazônia Oriental: Belém, Brazil, 2011; ISSN 1983-0513. [Google Scholar]
- Gordo, S.M.C.; Pinheiro, D.G.; Moreira, E.C.O.; Rodrigues, S.M.; Poltronieri, M.C.; Lemos, O.F.; da Silva, I.T.; Ramos, R.T.J.; Silva, A.; Schneider, H.; et al. High-throughput sequencing of black pepper root transcriptome. BMC Plant Biol. 2012, 12, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meireles, E.N.; Xavier, L.P.; Ramos, A.R.; Maia, J.G.S.; Setzer, W.N.; da Silva, J.K.R. Phenylpropanoids Produced by Piper divaricatum, A Resistant Species to Infection by Fusarium solani f. sp. piperis, the Pathogenic Agent of Fusariosis in Black Pepper. J. Plant Pathol. Microbiol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Wishart, D.S. Quantitative metabolomics using NMR. Trac-Trends Anal. Chem 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Davies, H.V.; Shepherd, L.V.T.; Stewart, D.; Frank, T.; Röhlig, R.M.; Engel, K.H. Metabolome variability in crop plant species—When, where, how much and so what? Regul. Toxicol. Pharmacol. 2010, 58, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nadella, K.D.; Marla, S.S.; Kumar, P.A. Metabolomics in Agriculture. J. Int. Biol. 2012, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Misra, A.K.; Modi, D.R. Current status of mango malformation in India. Asian J. Plant Sci. 2011, 10, 1–23. [Google Scholar] [CrossRef]
- Hamzehzarghani, H.; Paranidharan, V.; Abu-Nada, Y.; Kushalappa, A.C.; Dion, Y.; Rioux, S.; Comeau, A.; Yaylayan, V.; Marshall, W.D. Metabolite profiling coupled with statistical analyses for potential high-throughput screening of quantitative resistance to fusarium head blight in wheat. Can. J. Plant Pathol. 2008, 30, 24–36. [Google Scholar] [CrossRef]
- Balmer, D.; Papajewski, D.V.; Planchamp, C.; Glauser, G.; Mauch-Mani, B. Induced resistance in maize is based on organ-specific defence responses. Plant J. 2013, 74, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Hantao, L.W.; Aleme, H.G.; Passador, M.M.; Furtado, E.L.; Ribeiro, F.A.L.; Poppi, R.J.; Augusto, F. Determination of disease biomarkers in Eucalyptus by comprehensive two-dimensional gas chromatography and multivariate data analysis. J. Chromatogr. A 2013, 1279, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J. Plant Defense Compounds: Systems Approaches to Metabolic Analysis. Annu. Rev. Phytopathol. 2012, 50, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Ralchenko, Y. NIST Atomic Spectra Database; Mem. Sait. Suppl: Gaithersburg, MD, USA, 2005; pp. 96–102. Available online: http://physics.nist.gov/PhysRefData/ASD/index.html (accessed on 17 May 2016).
- Adams, R.P. Identification of Essential Oil Components by gas Chromatography—Mass Spectrometry; Allured Publishing Corporation: DuPage County, IL, USA, 2007. [Google Scholar]
- Laine, A.; Burdon, J.J.; Dodds, P.N.; Thrall, P.H. Spatial variation in disease resistance: From molecules to metapopulations. J. Ecol. 2011, 99, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Tack, A.J.M.; Thrall, P.H.; Barrett, L.G.; Burdon, J.J.; Laine, A. Variation in infectivity and aggressiveness in space and time in wild host-pathogen systems—Causes and consequences. J. Evol. Biol. 2012, 25, 1918–1936. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.L.S.; Lemos, O.F.; Tremacoldi, C.R.; Moraes, F.K.C.; Santos, L.R.R.; Pinheiro, H.A. Susceptibility of in vitro black pepper plant to the filtrate from a Fusarium solani f. sp. piperis culture. Plant Cell Tissue Organ Cult. 2016, 127, 263–268. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Padmakumari, K.P.; Jayalekshmy, A.; Narayanan, C.S. Gas chromatographic analysis and odour profiles of few Indian genotypes of Piper nigrum L. J. Essent. Oil Res. 1993, 5, 247–253. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B.; Geissler, M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase micro extraction-gas chromatography, solid-phase micro extraction gas chromatography–mass spectrometry and olfactometry. J. Chromatogr. A 2002, 976, 265–275. [Google Scholar] [CrossRef]
- Menon, A.N.; Padmakumari, K.P.; Jayalekshmy, A.; Gopalakrishnan, M.; Narayanan, C.S. Essential oil composition of four popular Indian cultivars of black pepper (Piper nigrum L.). J. Essent. Oil Res. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Menon, A.N.; Padmakumari, K.P.; Jayalekshmy, A.J. Essential oil composition of four major cultivars of black pepper (Piper nigrum L.). J. Essent. Oil Res. 2002, 14, 84–86. [Google Scholar] [CrossRef]
- Menon, A.N.; Padmakumari, K.P.; Jayalekshmy, A. Essential oil composition of four major cultivars of black pepper (Piper nigrum L.) III. J. Essent. Oil Res. 2003, 15, 155–157. [Google Scholar] [CrossRef]
- Zachariah, T.J.; Safeer, A.L.; Jayarajan, K.; Leela, N.K.; Vipin, T.M.; Saji, K.V.; Shiva, K.N.; Parthasarathy, V.A.; Mammootty, K.P. Correlation of metabolites in the leaf and berries of selected black pepper varieties. Sci. Hortic. 2010, 123, 418–422. [Google Scholar] [CrossRef]
- Quaglia, M.; Fabrizi, M.; Zazzerini, A.; Zadra, C. Role of pathogen-induced volatiles in the Nicotiana tabacume Golovinomyces cichoracearum interaction. Plant Physiol. Biochem. 2012, 52, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Godard, K.; White, R.; Bohlmann, J. Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry 2008, 69, 1838–1849. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Kurishita, S.; Hisamitsu, A.; Kajiwara, T. A lipid-hydrolysing activity involved in hexenal formation. Biochem. Soc. Trans. 2000, 28, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Shiojiri, K.; Ozawa, R.; Matsui, K.; Kishimoto, K.; Kugimiya, S.; Takabayashi, J. Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J. Chem. Ecol. 2006, 32, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Gouinguené, S.P.; Turlings, T.C.J. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002, 129, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Kessler, A.; Kahl, J.; Lorenz, A.; Baldwin, I.T. Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuate. Oecologia 2000, 124, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Nascetti, P.; Graverini, A.; Mannozzi, M. Emission and content of monoterpenes in intact and wounded needles of the mediterranean pine, Pinus pinea. Funct. Ecol. 2000, 14, 589–595. [Google Scholar] [CrossRef]
- Singsaas, E. Terpenes and the thermotolerance of photosynthesis. New Phytol. 2000, 146, 1–4. [Google Scholar] [CrossRef]
- Huang, J.; Cardoza, Y.J.; Schmelz, E.A.; Raina, R.; Engelberth, J.; Tumlinson, J.H. Differential volatile emissions and salicylic acid levels from tobacco plants in response to different strains of Pseudomonas syringae. Planta 2003, 217, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, Y.J.; Alborn, H.T.; Tumlinson, J.H. In-vivo volatile emissions from peanut plants induced by simultaneous fungal infection and insect damage. J. Chem. Ecol. 2002, 28, 161–174. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal. Behav. 2012, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.X.; Xiang, C.Y.; Li, J.X.; Yang, C.Q.; Hu, W.L.; Wang, L.J. The rice (E)-β-caryophyllene synthase (Os TPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 2007, 68, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.M.; Herrfurth, C.; Irmisch, S.; Köllner, T.G.; Feussner, I.; Karlovsky, P.; Splivallo, R. Infection of corn ears by Fusarium spp. induces the emission of volatile sesquiterpenes. J. Agric. Food Chem. 2014, 62, 5226–5236. [Google Scholar] [CrossRef] [PubMed]
- Vandana, V.V.; Bhai, R.S.; Azeez, S. Biochemical defense responses of black pepper (Piper nigrum L.) lines to Phytophthora capsici. Physiol. Mol. Plant 2014, 88, 18–27. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rusjan, D.; Halbwirth, H.; Stich, K.; Mikulic-Petkovsek, M.; Veberic, R. Biochemical response of grapevine variety ‘Chardonnay’ (Vitis vinifera L.) to infection with grapevine yellows (Bois noir). Eur. J. Plant Pathol. 2012, 134, 231–237. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Tampar, F.S.; Veberic, R. Accumulation of phenolic compounds in apple in response to infection by the scab pathogen, Venturia inaequalis. Physiol. Mol. Plant Pathol. 2009, 74, 60–67. [Google Scholar] [CrossRef]
- Treutter, D.; Feucht, W. Accumulation of flavan-3-ols in fungus-inoculated leaves of Rosaceae. Pflanzen 1990, 97, 634–641. [Google Scholar]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Weber, N.; Veberic, R.; Stampar, F.; Munda, A.; Koron, D. Alteration of the Content of Primary and Secondary Metabolites in Strawberry Fruit by Colletotrichum nymphaeae Infection. J. Agric. Food Chem. 2013, 61, 5987–5995. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, W.A.; Regnier, T.; Combrinck, S.; Botha, B.M. Metabolic profiling of mango cultivars to identify biomarkers for resistance against Fusarium infection. Phytochem. Lett. 2014, 10, 104–110. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, M.; Schafer, T.; Dollard, C.; Sasner, J. Utilization of Folin-Ciocalteu reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Boonen, J.; Bronselaer, A.; Nielandt, J.; Veryser, L.; Tre, G.D.; Spiegeleer, B.D. Alkamid database: Chemistry, occurrence and functionality of plant N-alkylamides. J. Ethnopharmacol. 2012, 142, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y. Natural Alkamides: Pharmacology, Chemistry and Distribution, Drug Discovery Research in Pharmacognosy; Vallisuta, O., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0213-7. [Google Scholar]
- Rahalison, L.; Hamburger, M.; Monod, M.; Frenk, E.; Hostettmann, K. Antifungal tests in phytochemical investigations: Comparison of bioautographic methods using phytopathogenic and human pathogenic fungi. Planta Med. 1994, 60, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.V.; Navickiene, H.M.D.; Kato, M.J.; Bolzani, V.S.; Méda, C.I.; Young, M.C.M.; Furlan, M. Antifungal amides from Piper arboreum and Piper tuberculatum. Phytochemistry 2002, 59, 521–527. [Google Scholar] [CrossRef]
- Navickiene, H.M.D.; Alécio, A.C.; Kato, M.J.; Bolzani, V.S.; Young, M.C.M.; Cavalheiro, A.J.; Furlan, M. Antifungal amides from Piper hispidum and Piper tuberculatum. Phytochemistry 2000, 55, 621–626. [Google Scholar] [CrossRef]
- Coulon, D.; Faure, L.; Salmon, M.; Wattelet, V.; Bessoule, J.J. N-Acylethanolamines and related compounds: Aspects of metabolism and functions. Plant Sci. 2012, 184, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Chapman, K.D.; Blanca Flor, E.B. Fatty acid amide lipid mediators in plants. Plant Sci. 2010, 178, 411–419. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. Generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Da Silva, J.K.R.; Sousa, P.J.C.; Andrade, E.H.A.; Maia, J.G.S. Antioxidant Capacity and Cytotoxicity of Essential Oil and Methanol Extract of Aniba canelilla (H.B.K.) Mez. J. Agric. Food Chem. 2007, 55, 9422–9426. [Google Scholar] [CrossRef] [PubMed]

| Bragantina Cultivar | 7 dpi | 15 dpi | 21 dpi | 30 dpi | 45 dpi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constituents | RIa | RIb | IP | CP | IP | CP | IP | CP | IP | CP | IP | CP |
| 3E-Hexenol | 845 | 844 | 0.3 | 0.5 | 0.3 + | 0.1 | 0.6 * | 0.7 * | 0.2 * | |||
| Limonene | 1022 | 1024 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||
| E-β-Ocimene | 1029 | 1044 | 1.2 + | 0.1 | 0.5 | 1.1 | 0.8 | 1.0 + | 0.5 | |||
| Linalool | 1091 | 1095 | 0.5 | 0.7 | 0.3 | 0.4 | 0.4 | 0.3 | 0.3 + | 0.1 | ||
| Pregeijerene | 1269 | 1285 | 0.3 | 0.5 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | |||
| 2-Undecanone | 1285 | 1293 | 0.1 * | 0.1 | 0.1 | 0.1 | 0.1 | |||||
| δ-Elemene | 1325 | 1335 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||||
| α-Ylangene | 1359 | 1373 | 0.1 * | 0.1 | 0.1 | 0.1 | ||||||
| β-Bourbonene | 1374 | 1387 | 0.1 * | |||||||||
| β-Elemene | 1380 | 1389 | 0.2 + | 0.1 | 0.2 | 0.2 | 0.1 | |||||
| β-Caryophyllene | 1407 | 1417 | 1.0 + | 0.4 | 1.1 | 1.2 | 0.7 | 0.9 | 0.9 | 0.9 | 0.6 | 0.8 |
| γ-Elemene | 1418 | 1434 | 0.1 | 0.1 | 0.2 + | 0.1 | 0.1 | |||||
| Geranyl acetone | 1440 | 1453 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 * | |||||
| E-β-Farnesene | 1443 | 1454 | 0.7 + | 0.2 | 0.8 | 0.8 | 0.7 | 0.7 | 0.7 | 0.5 | ||
| Germacrene D | 1470 | 1484 | 4.2 + | 1.4 | 5.7 | 5.1 | 3.4 | 4.3 | 2.2 − | 6.9 | 2.5 | 4.1 |
| β-Selinene | 1476 | 1489 | 0.1 | 0.1 | 0.1 − | 0.2 | 0.5 * | 0.2 | 0.2 | |||
| α-Zingiberene | 1483 | 1493 | 0.2 + | 0.1 | 0.2 − | 0.4 | 0.2 | 0.9 * | 0.9 | 0.7 | ||
| α-Muurolene | 1490 | 1500 | 0.1 * | 0.1 | 0.1 | 0.4 * | 0.2 + | 0.1 | ||||
| β-Bisabolene | 1497 | 1505 | 3.6 * | 1.1 | 4.3 | 4.1 | 4.2 | 4.2 | 6.0 + | 3.6 | 3.0 | 2.8 |
| β-Sesquiphellandrene | 1514 | 1521 | 0.1 * | 0.3 | 0.4 | 0.7 * | 0.6 | 0.5 | ||||
| E-γ-Bisabolene | 1517 | 1529 | 0.1 | 0.1 | 2.0 | |||||||
| Elemol | 1535 | 1548 | 19.0 | 27.3 | 24.4 | 22.3 | 15.3 | 28.4 | 0.7 − | 32.9 | 3.4 − | 9.2 |
| Germacrene B | 1545 | 1559 | 0.3 * | 0.3 | 0.1 | 0.1 | ||||||
| E-Nerolidol | 1551 | 1561 | 1.8 | 1.7 | 1.5 | 2.0 | 1.7 | 1.2 | 1.1 | 1.2 | 1.0 | |
| Caryophyllene oxide | 1569 | 1582 | 0.2 | 0.2 | 0.1 * | 0.1 | ||||||
| Longiborneol | 1591 | 1599 | 0.1 | 0.1 | 0.1 * | 0.1 | 0.1 | |||||
| β-Eudesmol | 1640 | 1649 | 0.3 | 0.3 | 0.3 | 0.3 | 10.7 * | 14.9 | 12.2 | |||
| α-Bisabolol | 1679 | 1685 | 51.4 | 53.9 | 44.8 | 47.9 | 64.3 | 51.4 | 60.1 | 49.8 | 60.7 | 54.6 |
| (2Z,6Z)-Farnesol | 1696 | 1698 | 1.6 * | 1.9 | 1.5 | 0.5 | 1.5 | |||||
| (2Z,6E)-Farnesol | 1704 | 1722 | 3.2 * | 3.5 | 3.2 | 1.1 | 1.5 | 2.5 | ||||
| Monoterpene hydrocarbons | 1.4 | 0.3 | 0.1 | 0.7 | 1.1 | 0.8 | 1.2 | 0.6 | ||||
| Oxygenated monoterpenes | 0.9 | 1.2 | 0.6 | 0.7 | 0.4 | 0.6 | 0.6 | 0.4 | ||||
| Sesquiterpene hydrocarbons | 10.9 | 3.7 | 13.2 | 12.8 | 9.0 | 10.7 | 11.6 | 13.4 | 8.8 | 10.2 | ||
| Oxygenated sesquiterpenes | 77.6 | 83.5 | 76.3 | 77.2 | 81.3 | 82.9 | 71.5 | 85.3 | 80.3 | 81.2 | ||
| Others | 0.3 | 0.5 | 0.3 | 0.1 | 0.6 | 0.7 | 0.2 | |||||
| Total | 91.1 | 89.2 | 90.5 | 91.5 | 92.4 | 95.0 | 83.8 | 98.7 | 91.1 | 92.4 | ||
| Cingapura Cultivar | 7 dpi | 15 dpi | 21 dpi | 30 dpi | 45 dpi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Constituents | RIa | RIb | IP | CP | IP | CP | IP | CP | IP | CP | IP | CP |
| 3E-Hexenol | 846 | 844 | 1.6 * | 0.3 * | 0.2 + | 0.1 | ||||||
| α-Pinene | 928 | 932 | 0.5 * | 0.1 | 0.1 | |||||||
| β-Pinene | 970 | 974 | 0.1 | |||||||||
| Myrcene | 983 | 988 | 0.1 | |||||||||
| δ-2-Carene | 1004 | 1001 | 0.2 * | 0.1 * | ||||||||
| Limonene | 1023 | 1024 | 0.2 * | |||||||||
| E-β-Ocimene | 1040 | 1044 | 0.4 * | 0.2 | 0.2 | |||||||
| Linalool | 1092 | 1095 | 2.6 | 2.0 | 1.0 | 1.4 | 1.2 − | 2.5 | 1.5 + | 0.6 | 0.5 | 0.8 |
| Methyl citronellate | 1250 | 1257 | 0.1 | |||||||||
| Carvenone | 1252 | 1255 | 0.1 * | 0.1 | 0.1 | 0.1 | 0.1 | |||||
| 2-Undecanone | 1285 | 1293 | 0.1 | |||||||||
| δ-Elemene | 1322 | 1335 | 45.9 | 57.6 | 73.6 + | 48.7 | 48.8 | 55.1 | 61.2 | 57.1 | 55.8 | 48.1 |
| δ-Cubebene | 1337 | 0.6 * | ||||||||||
| α-Cubebene | 1365 | 1345 | 0.4 − | 2.1 | 0.4 | 0.3 | 0.6 * | 0.5 | 0.9 | 0.7 | ||
| α-Copaene | 1365 | 1374 | 1.0 * | |||||||||
| α-Ylangene | 1367 | 1373 | 1.2 * | 0.8 + | 0.4 | 1.1 * | 1.2 | 1.2 | 1.4 | |||
| β-Elemene | 1379 | 1389 | 2.7 | 4.1 | 2.1 | 1.6 | 3.1 | 3.0 | 3.2 | 2.7 | 3.1 | 3.9 |
| α-Gurjunene | 1396 | 1409 | 0.7 * | 0.4 | 0.3 | 0.8 | 0.8 | 0.7 | 1.0 | 1.1 | ||
| β-Caryophyllene | 1407 | 1417 | 3.0 | 3.8 | 2.3 | 3.1 | 6.6 | 8.7 | 3.9 | 3.0 | 5.4 | 4.9 |
| 6,9-Guaiadiene | 1430 | 1442 | 0.1 * | 0.5 * | 0.7 | 1.2 | ||||||
| α-Humulene | 1443 | 1452 | 0.6 − | 2.2 | 0.2 | 0.5 | 0.9 * | 0.8 * | 0.9 * | |||
| Thujopsadiene | 1449 | 1465 | 0.3 * | 0.3 | 0.5 * | 0.4 * | 0.3 * | |||||
| allo-Aromadendrene | 1461 | 1458 | 0.1 * | 0.1 * | 0.5 * | 0.4 * | ||||||
| 9-epi-E-Caryophyllene | 1465 | 1464 | 0.2 * | 0.1 − | 0.2 | 0.4 | ||||||
| Germacrene D | 1470 | 1484 | 0.6 * | 0.6 + | 0.3 | 0.6 * | 0.7 | 0.6 | 0.5 | 0.7 | ||
| β-Selinene | 1476 | 1489 | 2.2 | 4.2 | 1.8 | 1.3 | 3.3 | 2.9 | 3.4 | 2.5 | 3.6 | 4.4 |
| α-Zingiberene | 1482 | 1493 | 2.9 − | 6.9 | 2.9 | 2.3 | 5.2 | 5.0 | 5.2 | 4.3 | 5.5 | 6.8 |
| Z- Cycloisolongifol-5-ol | 1498 | 1513 | 0.3 | |||||||||
| epi-Cubebol | 1503 | 1493 | 0.3 * | 0.1 − | 0.7 | 0.9 * | 0.3 | 0.4 | ||||
| δ-Amorphene | 1507 | 1511 | 0.7 * | 0.3 * | 0.7 + | 0.4 | 0.4 | 0.7 | ||||
| γ-Vetivenene | 1534 | 1531 | 0.2 * | 0.1 | 0.1 | |||||||
| γ-Elemene | 1545 | 1465 | 0.1 | |||||||||
| E-Nerolidol | 1549 | 1561 | 2.9 | 4.8 | 1.3 − | 8.5 | 3.9 | 6.6 | 2.7 | 4.0 | 2.7 | 2.2 |
| Germacrene D-4-ol | 1564 | 1574 | 0.2 * | 0.1 | 0.3 | 0.3 * | 0.4 | 0.4 | 0.5 | |||
| Caryophyllene oxide | 1576 | 1582 | 6.6 | 6.3 | 2.9 | 3.9 | 3.6 | 3.8 | ||||
| Spathulenol | 1577 | 1577 | 3.1 | 6.1 | 6.1 | 3.6 | 4.5 | 0.3 * | ||||
| β-Copaen-4α-ol | 1595 | 1590 | 2.8 | 1.5 * | 1.7 | 1.7 | 0.8 * | |||||
| β-Atlantol | 1596 | 1608 | 3.4 * | |||||||||
| Ledol | 1602 | 1602 | 0.4 | |||||||||
| 1,10-di-epi-Cubenol | 1607 | 1618 | 1.6 * | 0.6 − | 1.2 | 1.0 * | 0.8 | 1.3 | 1.1 | |||
| Muurola-4,10(14)-dien-1β-ol | 1613 | 1630 | 4.3 | 5.9 | 2.0 − | 4.9 | 6.5 | 5.5 | 3.7 | 5.3 | 3.8 | 3.1 |
| α-Cadinol | 1642 | 1652 | 0.5 * | 0.2 − | 0.4 | 0.4 * | 0.3 * | 0.3 | 0.5 | |||
| allo-Aromadendrene epoxide | 1649 | 1639 | 0.2 * | 0.1 * | 0.1 − | 0.2 | ||||||
| Khusinol | 1659 | 1679 | 0.7 * | 0.3 − | 0.6 | 0.4 * | 0.2 | 0.5 | ||||
| Germacra-4(15),5,10(14)-trien-1α-ol | 1672 | 1685 | 0.2 * | 0.1 | 0.3 | 0.1 | ||||||
| 2Z,6Z-Farnesal | 1684 | 1684 | 0.2 * | 0.1 * | 0.1 | 0.1 | ||||||
| 2E, 6Z-Farnesal | 1696 | 1713 | 0.6 | |||||||||
| 2Z,6E-Farnesal | 1696 | 1715 | 0.2 * | 0.4 * | ||||||||
| Eudesma-4,11-dien-2α-ol | 1704 | 1704 | 1.2 * | 0.6 − | 1.9 | 0.9 * | 1.2 | 1.1 | 1.1 − | 2.2 | ||
| E, E, E-7,10,13-Hexadecatrienal | 1718 | 1824 | 0.8 * | |||||||||
| iso-Aromadendrene epoxide | 1719 | 1719 | 0.6 * | 0.3 − | 0.6 | 0.4 | 0.5 | 0.3 | 0.5 | |||
| 2E,6E-Farnesal | 1725 | 1725 | 0.4 * | 1.0 | 0.8 | 0.7 | 0.8 − | 2.0 | ||||
| Monoterpene hydrocarbons | 1.3 | 0.4 | 0.7 | |||||||||
| Oxygenated monoterpenes | 2.7 | 2.0 | 1.1 | 1.7 | 1.2 | 2.5 | 1.5 | 0.6 | 0.6 | 0.9 | ||
| Sesquiterpene hydrocarbons | 61.8 | 80.6 | 86.1 | 59.5 | 72.0 | 74.7 | 81.9 | 73.7 | 79.0 | 74.4 | ||
| Oxygenated sesquiterpenes | 23.3 | 17.0 | 8.7 | 31.7 | 22.8 | 18.2 | 16.0 | 19.5 | 14.8 | 17.2 | ||
| Others | 1.6 | 0.3 | 0.2 | 0.1 | ||||||||
| Total | 90.7 | 99.9 | 96.4 | 93.4 | 95.7 | 95.4 | 99.4 | 93.8 | 94.6 | 92.6 | ||
| RT | Alkamides | MW | MF | 7 dpi | 15 dpi | 21 dpi | 30 dpi | 45 dpi | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (min) | (Figure 4) | (Molecular Weight) | (Molecular Formula) | IP | CP | IP | CP | IP | CP | IP | CP | IP | CP |
| 17.1 | 1R | 341.4402 | C21H27NO3 | X | X | − | X | − | X | ||||
| 18.8 | 2aR/2bR | 273.3243 | C16H19NO3 | + | X | X | |||||||
| 19.7 | 3aR/3bR | 359.5827 | C24H41NO | − | X | X | X | ||||||
| 20.5 | 4R | 339.4246 | C21H25NO3 | X | X | − | X | ||||||
| 21.4 | 5R | 381.5035 | C24H31NO3 | X | X | X | X | X | X | X | X | ||
| 22.2 | 6R | 285.3350 | C17H19NO3 | X | X | X | X | X | X | X | X | X | X |
| 22.6 | 7R | 452.8303 | C31H64O | + | |||||||||
| 23.3 | 8aR/8bR | 224.3587 | C14H26NO | X | X | X | X | X | X | X | X | X | X |
| 23.8 | 9aR/9bR | 333.5457 | C22H39NO | X | X | + | |||||||
| 24.1 | 10R | 311.3720 | C19H21NO3 | + | |||||||||
| 25.6 | 11aR/11bR | 361.5983 | C24H43NO | − | X | X | X | ||||||
| 26.2 | 12R | 367.4772 | C23H29NO3 | X | X | X | X | X | X | X | X | X | X |
| 26.8 | 13R | 383.5191 | C24H33NO3 | X | X | X | X | X | X | X | X | X | X |
| 27.7 | 9aR/9bR | 333.5457 | C22H39NO | X | X | + | − | X | X | X | X | X | |
| 28.3 | 14aR/14bR | 335.5613 | C22H41NO | X | X | X | X | X | X | X | X | X | X |
| 29.0 | 15aR/15bR | 363.6139 | C24H45NO | − | X | ||||||||
| RT | Alkamides | MW | MF | 7 dpi | 15 dpi | 21 dpi | 30 dpi | 45 dpi | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (min) | (Figure 4) | (Molecular Weight) | (Molecular Formula) | IP | CP | IP | CP | IP | CP | IP | CP | IP | CP |
| 22.2 | 6R | 285.3350 | C17H19NO3 | X | X | X | X | X | X | − | X | X | X |
| 23.3 | 8aR/8bR | 224.3587 | C14H26NO | X | X | X | X | X | X | X | X | X | X |
| 23.9 | 9aR/9bR | 333.5457 | C22H39NO | X | X | − | X | X | X | − | X | X | X |
| 24.0 | 14aR/14bR | 335.5613 | C22H41NO | + | X | X | − | X | X | X | |||
| 24.1 | 9aR/9bR | 333.5457 | C22H39NO | X | X | X | X | − | X | X | X | ||
| 24.4 | 16R | 235.3616 | C15H25NO | X | X | X | X | X | X | − | X | X | X |
| 26.3 | 12R | 367.4772 | C23H29NO3 | X | X | X | X | X | X | X | X | X | X |
| 26.8 | 13R | 383.5191 | C24H33NO3 | X | X | X | X | X | X | X | X | X | X |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Luz, S.F.M.; Yamaguchi, L.F.; Kato, M.J.; De Lemos, O.F.; Xavier, L.P.; Maia, J.G.S.; Ramos, A.D.R.; Setzer, W.N.; Da Silva, J.K.d.R. Secondary Metabolic Profiles of Two Cultivars of Piper nigrum (Black Pepper) Resulting from Infection by Fusarium solani f. sp. piperis. Int. J. Mol. Sci. 2017, 18, 2434. https://doi.org/10.3390/ijms18122434
Da Luz SFM, Yamaguchi LF, Kato MJ, De Lemos OF, Xavier LP, Maia JGS, Ramos ADR, Setzer WN, Da Silva JKdR. Secondary Metabolic Profiles of Two Cultivars of Piper nigrum (Black Pepper) Resulting from Infection by Fusarium solani f. sp. piperis. International Journal of Molecular Sciences. 2017; 18(12):2434. https://doi.org/10.3390/ijms18122434
Chicago/Turabian StyleDa Luz, Shirlley F. M., Lydia F. Yamaguchi, Massuo J. Kato, Oriel F. De Lemos, Luciana P. Xavier, José Guilherme S. Maia, Alessandra De R. Ramos, William N. Setzer, and Joyce Kelly do R. Da Silva. 2017. "Secondary Metabolic Profiles of Two Cultivars of Piper nigrum (Black Pepper) Resulting from Infection by Fusarium solani f. sp. piperis" International Journal of Molecular Sciences 18, no. 12: 2434. https://doi.org/10.3390/ijms18122434