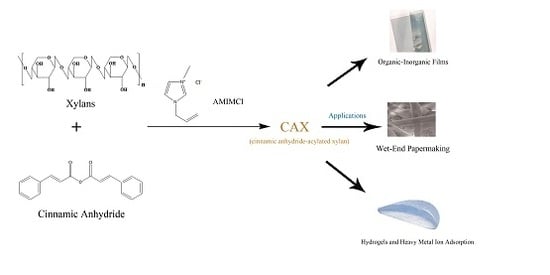
Ionic Liquid-Mediated Homogeneous Esterification of Cinnamic Anhydride to Xylans
Abstract
:1. Introduction
2. Results and Discussion
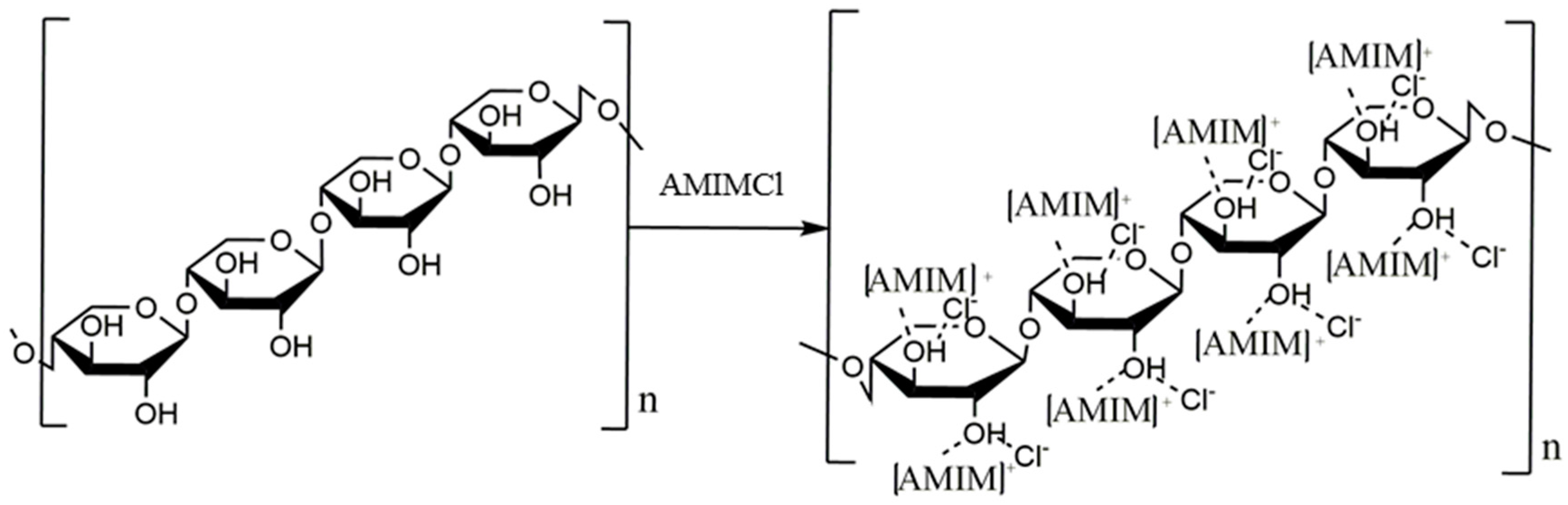
2.1. Dissolution Mechanism of Xylan in Ionic Liquid
2.2. Effects of Reaction Conditions on the Degrees of Substitution (DS) of Cinnamic Anhydride Xylan (CAX)
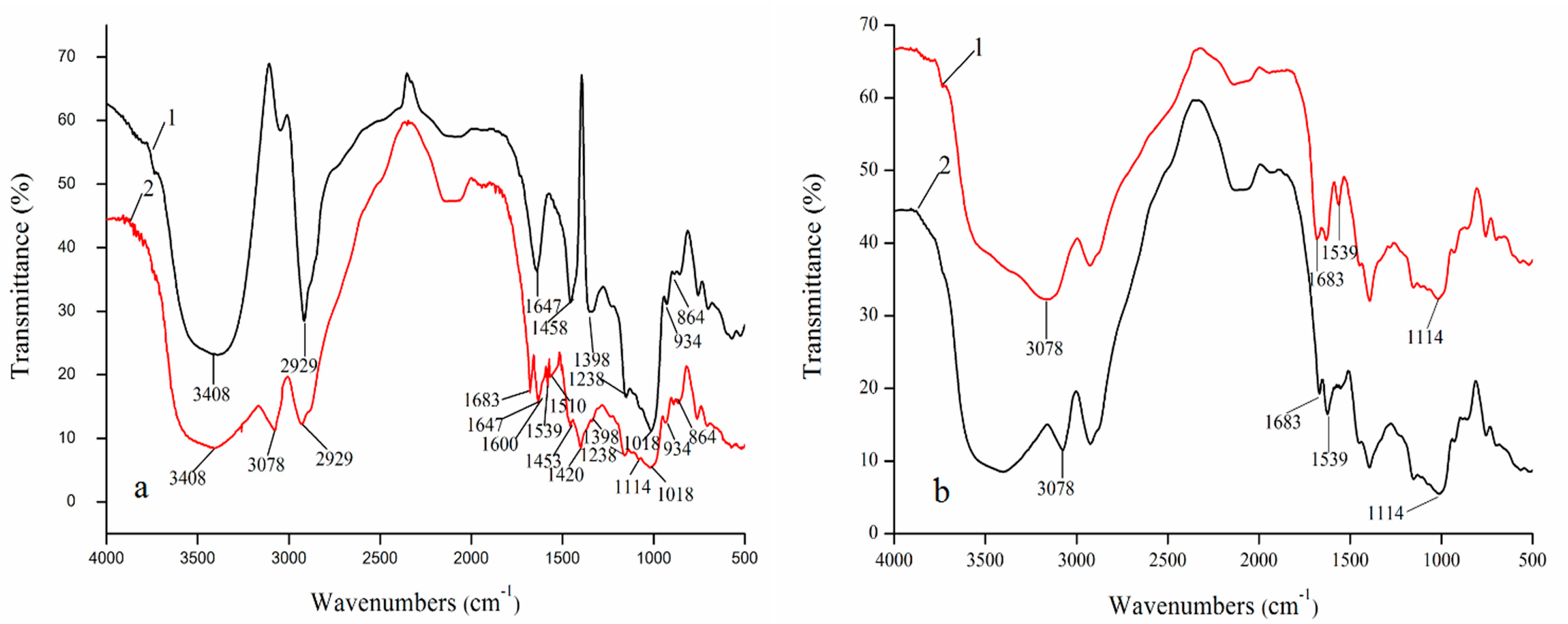
2.3. FT-IR Spectra Analyses
2.4. 13C-NMR Spectra
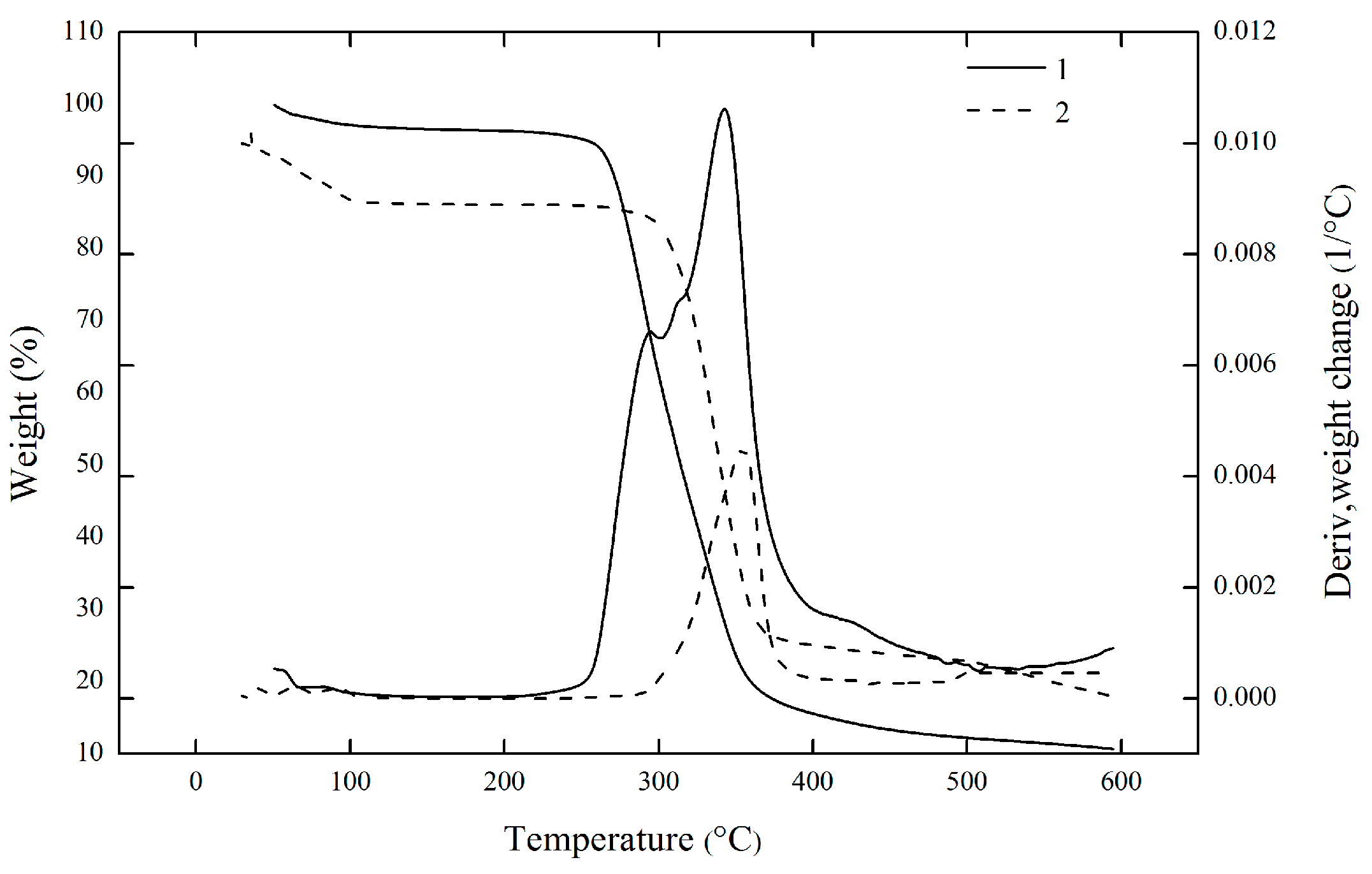
2.5. Thermal Analysis
2.6. Applications of CAX
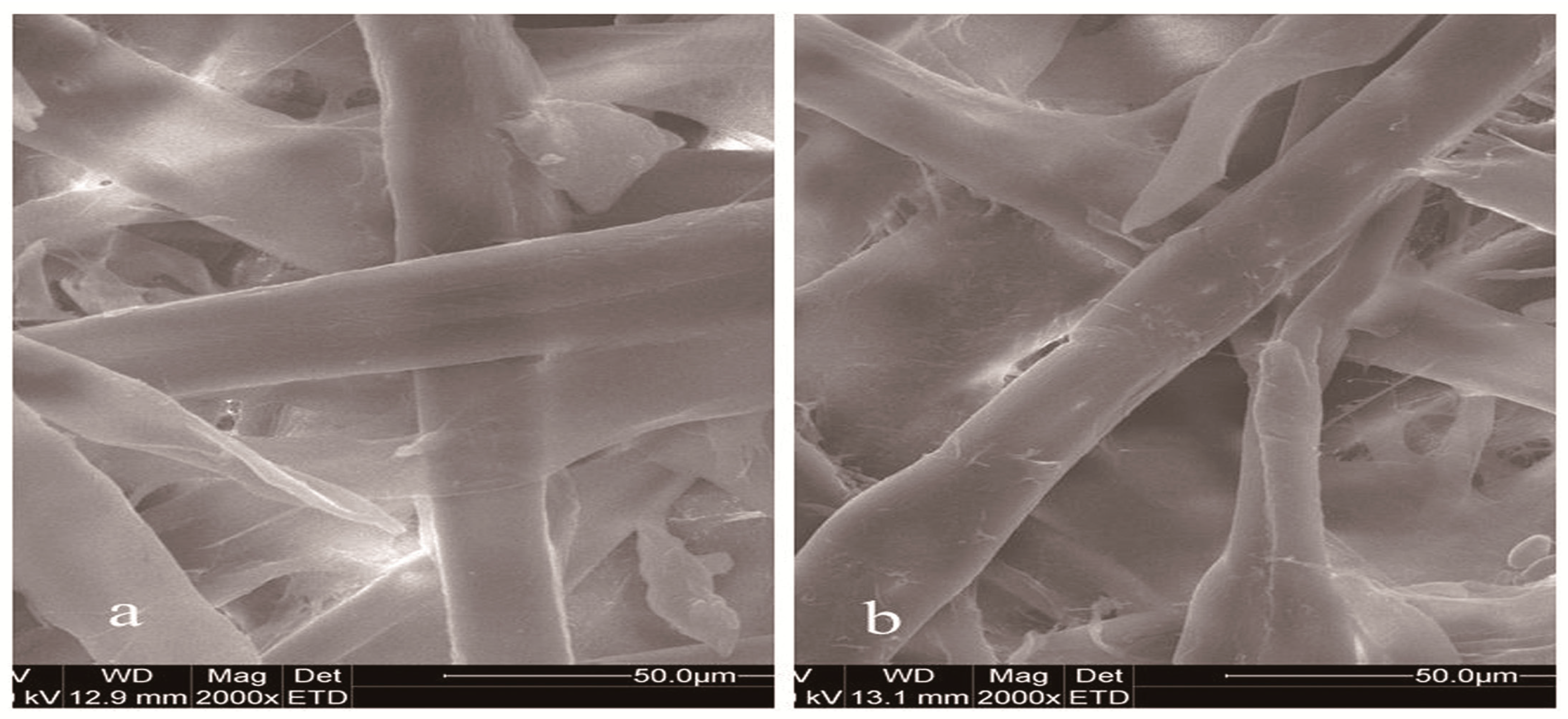
2.6.1. Physical Properties of Paper
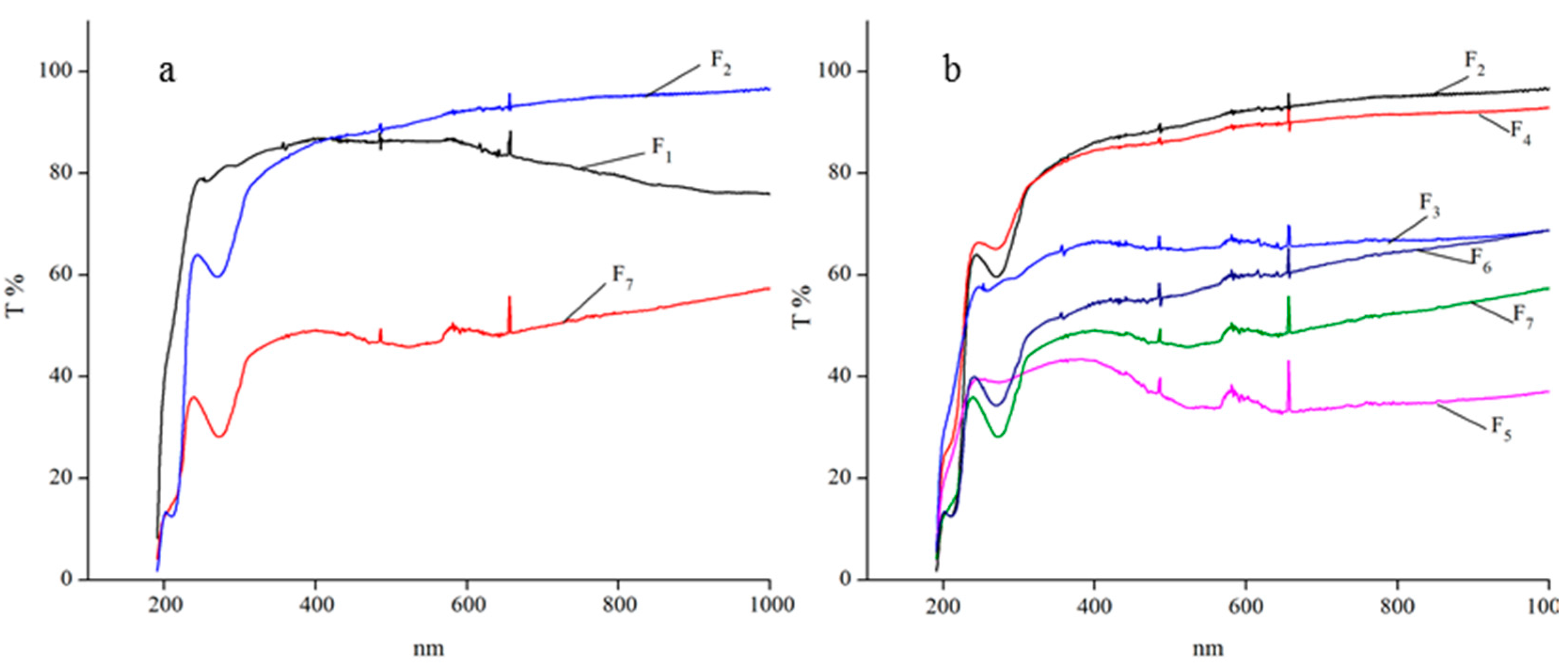
2.6.2. Organic–Inorganic Films
2.6.3. Hydrogels and Heavy Metal Ion Adsorption
3. Materials and Methods
3.1. Materials
3.2. Homogeneous Esterification of Xylan in AMIMCl
3.3. Determination of Degree of Substitution
3.4. Characterization of CAX
3.5. Application of CAX
3.5.1. Wet-End of Papermaking
3.5.2. Preparation of Organic–Inorganic Composite Films
3.5.3. Preparation of Hydrogel and Adsorption of Heavy Metal Ions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Satgé, C.; Verneuil, B.; Branland, P.; Granet, R.; Krausz, P.; Rozier, J. Rapid homogeneous esterification of cellulose induced by microwave irradiation. Carbohydr. Polym. 2002, 49, 373–376. [Google Scholar] [CrossRef]
- Hansen, N.M.L.; David, P. Sustainable films and coatings from hemicelluloses: A review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Parajó, J.C.; Domínguez, H.; Domínguez, J. Biotechnological production of xylitol. Part 1: Interest of xylitol and fundamentals of its biosynthesis. Bioresour. Technol. 1998, 65, 191–201. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Xu, F. Biomass converting enzymes as industrial biocatalysts for fuels and chemicals: Recent developments. Catalysts 2012, 2, 244–263. [Google Scholar] [CrossRef]
- Hartman, J.; Albertsson, A.C.; Lindblad, M.S.; Sjöberg, J. Oxygen barrier materials from renewable sources: Material properties of softwood hemicellulose-based films. J. Appl. Polym. Sci. 2006, 100, 2985–2991. [Google Scholar] [CrossRef]
- Lindblad, M.S.; Ranucci, E.; Albertsson, A.C. Biodegradable polymers from renewable sources. New hemicellulose-based hydrogels. Macromol. Rapid Commun. 2001, 22, 962–967. [Google Scholar] [CrossRef]
- Lima, D.U.; Oliveira, R.C.; Buckeridge, M.S. Seed storage hemicelluloses as wet-end additives in papermaking. Carbohydr. Polym. 2003, 52, 367–373. [Google Scholar] [CrossRef]
- Roel, H. Ethanol’s energy return on investment: A survey of the literature 1990-present. Environ. Sci. Technol. 2006, 40, 1744–1750. [Google Scholar]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Lucia, L.A.; Hubbe, M.A. Book review: Materials, chemicals & energy from forest biomass. Bioresources 2008, 3, 668–669. [Google Scholar]
- Grondahl, M.; Eriksson, L.; Gatenholm, P. Material properties of plasticized hardwood xylans for potential application as oxygen barriern films. Biomacromolecules 2004, 5, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Suzuki, S.; Kawasaki, N.; Endo, K.; Takahashi, A.; Attwood, D. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int. J. Pharm. 2001, 229, 29–36. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.L.; Peng, B.; Xu, F.; Sun, R.C.; Sun, J.X. Rapid homogeneous lauroylation of wheat straw hemicelluloses under mild conditions. Carbohydr. Res. 2008, 343, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Peng, F.; Sun, R.C. Preparation of hemicellulosic derivatives with bifunctional groups in different media. J. Agric. Food Chem. 2008, 56, 11209–11216. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Xu, F.; Sun, R.C.; Peng, B.; Sun, J.X. Studies of the lauroylation of wheat straw hemicelluloses under heating. J. Agric. Food Chem. 2008, 56, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Sun, R.C.; Peng, F. Carboxymethylation of hemicelluloses isolated from sugarcane bagasse. Polym. Degrad. Stab. 2008, 93, 786–793. [Google Scholar] [CrossRef]
- Ren, J.L.; Sun, R.C.; Liu, C.F.; Lin, L.; He, B.H. Synthesis and characterization of novel cationic SCB hemicelluloses with a low degree of substitution. Carbohydr. Polym. 2007, 67, 347–357. [Google Scholar] [CrossRef]
- Ren, J.L.; Sun, R.C.; Liu, C.F.; Cao, Z.N.; Luo, W. Acetylation of wheat straw hemicelluloses in ionic liquid using iodine as a catalyst. Carbohydr. Polym. 2007, 70, 406–414. [Google Scholar] [CrossRef]
- André, P.; Marsh, K.N.; Shusheng, P.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar]
- Peng, X.W.; Ren, J.L.; Peng, F.; Sun, R.C. Rapid carboxymethylation of xylan-rich hemicelluloses by microwave irradiation. Adv. Mater. Res. 2011, 236–238, 292–296. [Google Scholar] [CrossRef]
- Fang, J.M.; Sun, R.C.; Tomkinson, J.; Fowler, P. Acetylation of wheat straw hemicellulose B in a new non-aqueous swelling system. Carbohydr. Polym. 2000, 41, 379–387. [Google Scholar] [CrossRef]
- Xu, F.; Sun, R.; Sun, X.; Geng, Z.; Xiao, B.; Sun, J. Analysis and characterization of acetylated sugarcane bagasse hemicelluloses. Int. J. Polym. Anal. Charact. 2004, 9, 229–244. [Google Scholar] [CrossRef]
- Fang, J.M.; Sun, R.; Fowler, P.; Tomkinson, J.; Hill, C.A.S. Esterification of wheat straw hemicelluloses in the N,N -dimethylformamide/lithium chloride homogeneous system. J. Appl. Polym. Sci. 1999, 74, 2301–2311. [Google Scholar] [CrossRef]
- Thiebaud, S.; Borredon, M.E. Analysis of the liquid fraction after esterification of sawdust with octanoyl chloride—Production of esterified hemicelluloses. Bioresour. Technol. 1998, 63, 139–145. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yuan, T.Q.; Xu, F.; Sun, R.C. Enhanced hydrophobicity and thermal stability of hemicelluloses by butyrylation in [BMIM]Cl ionic liquid. Ind. Crop. Prod. 2013, 107, 68–72. [Google Scholar] [CrossRef]
- Thomas, H.; Katrin, S.; Susann, B. Ionic liquids as reaction medium in cellulose functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar]
- Barthel, S.; Heinze, T. Acylation and carbanilation of cellulose in ionic liquids. Green Chem. 2006, 8, 301–306. [Google Scholar] [CrossRef]
- Anderson, J.L.; Jie, D.; Thomas, W.; Armstrong, D.W. Characterizing ionic liquids on the basis of multiple solvation interactions. J. Am. Chem. Soc. 2002, 124, 14247–14254. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilib. 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Olivier-Bourbigou, H.; Magna, L. Ionic liquids: Perspectives for organic and catalytic reactions. J. Mol. Catal. A Chem. 2003, 34, 419–437. [Google Scholar] [CrossRef]
- Wei, Z.; Li, G.; Fang, Y.; Wang, X. Maleic anhydride surface-modification of crosslinked chitosan membrane and its pervaporation performance. J. Membr. Sci. 2007, 295, 130–138. [Google Scholar]
- El-Rehim, H.A.A.; Hegazy, E.A.; Ali, E.H. Selective removal of some heavy metal ions from aqueous solution using treated polyethylene-g-styrene/maleic anhydride membranes. React. Funct. Polym. 2000, 43, 105–116. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M.J. Modified chitosan 4. Superabsorbent hydrogels from poly (acrylic acid-co-acrylamide) grafted chitosan with salt- and pH-responsiveness properties. Eur. Polym. J. 2004, 40, 1399–1407. [Google Scholar] [CrossRef]
- Rokhade, A.P.; Agnihotri, S.A.; Patil, S.A.; Mallikarjuna, N.N.; Kulkarni, P.V.; Aminabhavi, T.M. Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. Carbohydr. Polym. 2006, 65, 243–252. [Google Scholar] [CrossRef]
- Grombe, R.; Gouzy, M.F.; Nitschke, M.; Komber, H.; Werner, C. Preparation and characterization of glycosylated maleic anhydride copolymer thin films. Colloids Surf. A 2006, 284, 295–300. [Google Scholar] [CrossRef]
- Pompe, T.; Zschoche, S.; Herold, N.; Salchert, K.; Gouzy, M.F.; Claudia Sperling, A. Maleic anhydride copolymersa versatile platform for molecular biosurface engineering. Biomacromolecules 2003, 4, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.P.K.; Korhonen, H.; Tuominen, J.; Seppälä, J.V. Synthesis, characterization and crosslinking of functional star-shaped poly (ε-caprolactone). Transplant. Proc. 2002, 33, 795–805. [Google Scholar]
- Wrigstedt, P.; Kylli, P.; Pitkänen, L.; Nousiainen, P.; Tenkanen, M.; Sipilä, J. Synthesis and antioxidant activity of hydroxycinnamic acid xylan esters. J. Agric. Food Chem. 2010, 58, 6937–6943. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Guo, S. Novel amphiphilic ternary polysaccharide derivates chitosan-g-PCL-b-MPEG: Synthesis, characterization, and aggregation in aqueous solution. Biopolymers 2007, 86, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Yukinobu, F.; Akiko, S.; Hiroyuki, O. Superior solubility of polysaccharides in low viscosity, polar, and halogen-free 1,3-dialkylimidazolium formats. Biomacromolecules 2006, 7, 3295–3297. [Google Scholar]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Peng, X.W.; Ren, J.L.; Sun, R.C. Homogeneous esterification of xylan-rich hemicelluloses with maleic anhydride in ionic liquid. Biomacromolecules 2010, 11, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Madan, R.N.; Bansal, M.C. Chemical composition of pinus caribaea hemicellulose. Tappi J. 1987, 38, 88–90. [Google Scholar]
- Singh, S.; Koehler, B.; Fett, W.F. Effect of osmolarity and dehydration on alginate production by fluorescent pseudomonads. Curr. Microbiol. 1992, 25, 335–339. [Google Scholar] [CrossRef]
- Sun, R.; Mott, L.; Bolton, J. Isolation and fractional characterization of ball-milled and enzyme lignins from oil palm trunk. J. Agric. Food Chem. 1998, 46, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Z.L. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Hadano, S.; Onimura, K.; Tsutsumi, H.; Yamasaki, H.; Oishi, T. Syntheses of chemical-modified cellulose obtained from waste pulp. J. Appl. Polym. Sci. 2003, 90, 2059–2065. [Google Scholar] [CrossRef]


| Samples a | Conditions | Esterification of Hemicellulose | |||
|---|---|---|---|---|---|
| Esterification Molar Ratio of CA/Xylan b | Reaction Time (min) | Temperature (°C) | Catalyst (0.025 mol/L) | DS c | |
| 1 | 2:1 | 80 | 80 | NaOH | 0.15 |
| 2 | 2:1 | 80 | 80 | KOH | 0.11 |
| 3 | 2:1 | 80 | 80 | LiOH | 0.06 |
| 4 | 2:1 | 20 | 80 | NaOH | 0.15 |
| 5 | 2:1 | 40 | 80 | NaOH | 0.18 |
| 6 | 2:1 | 60 | 80 | NaOH | 0.21 |
| 7 | 2:1 | 80 | 80 | NaOH | 0.46 |
| 8 | 2:1 | 100 | 80 | NaOH | 0.25 |
| 9 | 2:1 | 80 | 70 | NaOH | 0.15 |
| 10 | 2:1 | 80 | 80 | NaOH | 0.45 |
| 11 | 2:1 | 80 | 90 | NaOH | 0.36 |
| 12 | 2:1 | 80 | 100 | NaOH | 0.29 |
| 13 | 2:1 | 80 | 110 | NaOH | 0.22 |
| 14 | 1:1 | 80 | 80 | NaOH | 0.15 |
| 15 | 2:1 | 80 | 80 | NaOH | 0.13 |
| 16 | 4:1 | 80 | 80 | NaOH | 0.33 |
| 17 | 6:1 | 80 | 80 | NaOH | 0.57 |
| 18 | 8:1 | 80 | 80 | NaOH | 0.37 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Zhou, H.; Chen, J.; Lyu, G.; Xia, Y.; Lucia, L.A. Ionic Liquid-Mediated Homogeneous Esterification of Cinnamic Anhydride to Xylans. Int. J. Mol. Sci. 2017, 18, 2502. https://doi.org/10.3390/ijms18122502
Yang G, Zhou H, Chen J, Lyu G, Xia Y, Lucia LA. Ionic Liquid-Mediated Homogeneous Esterification of Cinnamic Anhydride to Xylans. International Journal of Molecular Sciences. 2017; 18(12):2502. https://doi.org/10.3390/ijms18122502
Chicago/Turabian StyleYang, Guihua, Huifang Zhou, Jiachuan Chen, Gaojin Lyu, Yuanyuan Xia, and Lucian A. Lucia. 2017. "Ionic Liquid-Mediated Homogeneous Esterification of Cinnamic Anhydride to Xylans" International Journal of Molecular Sciences 18, no. 12: 2502. https://doi.org/10.3390/ijms18122502