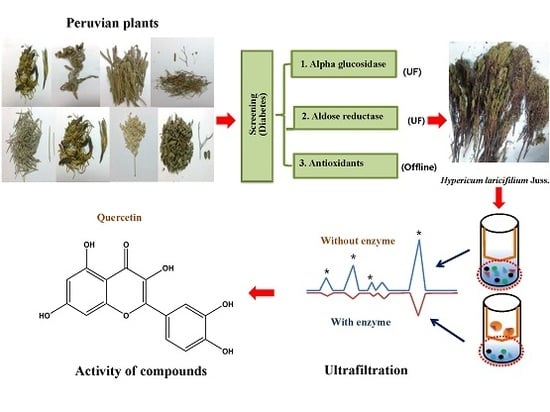
Screening In Vitro Targets Related to Diabetes in Herbal Extracts from Peru: Identification of Active Compounds in Hypericum laricifolium Juss. by Offline High-Performance Liquid Chromatography
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of α-Glucosidase and Aldose Reductase (AR) Inhibition and Antioxidant Activity of Peruvian Plants
2.2. Effect of Hypericum laricifolium Juss. (HL) on α-Glucosidase and Aldose Reductase (AR) Inhibition and Antioxidant Activity
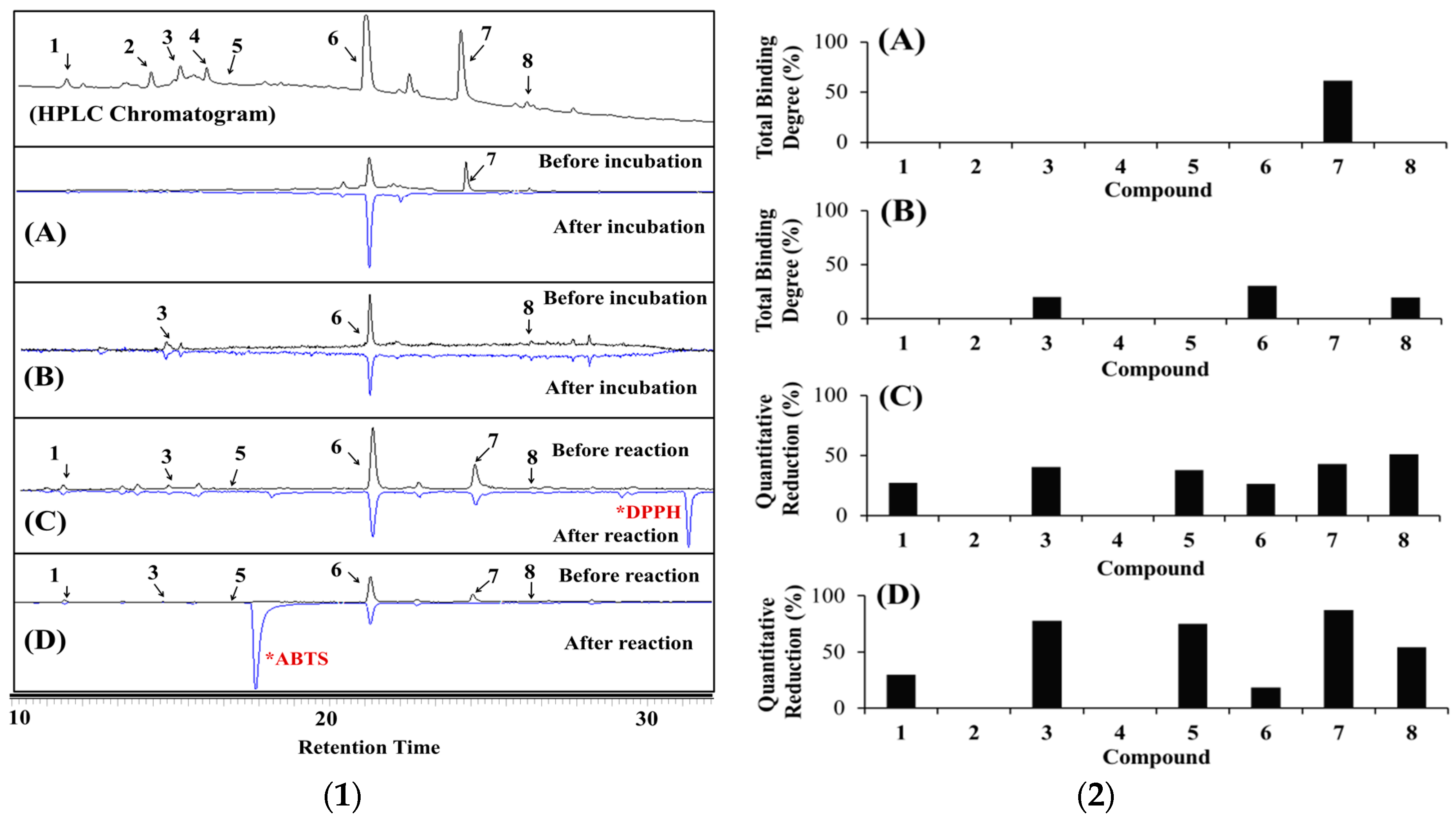
2.3. Identification of the Major Bioactive Components in Hypericum laricifolium Juss. (HL) by Offline High-Performance Liquid Chromatography (HPLC)
2.3.1. Identification of the Bioactive Compounds in HL by α-Glucosidase Ultrafiltration Combined with HPLC
2.3.2. Identification of the Bioactive Compounds in HL by Aldose Reductase (AR) and Human Recombinant AR (HRAR) Ultrafiltration Combined with HPLC
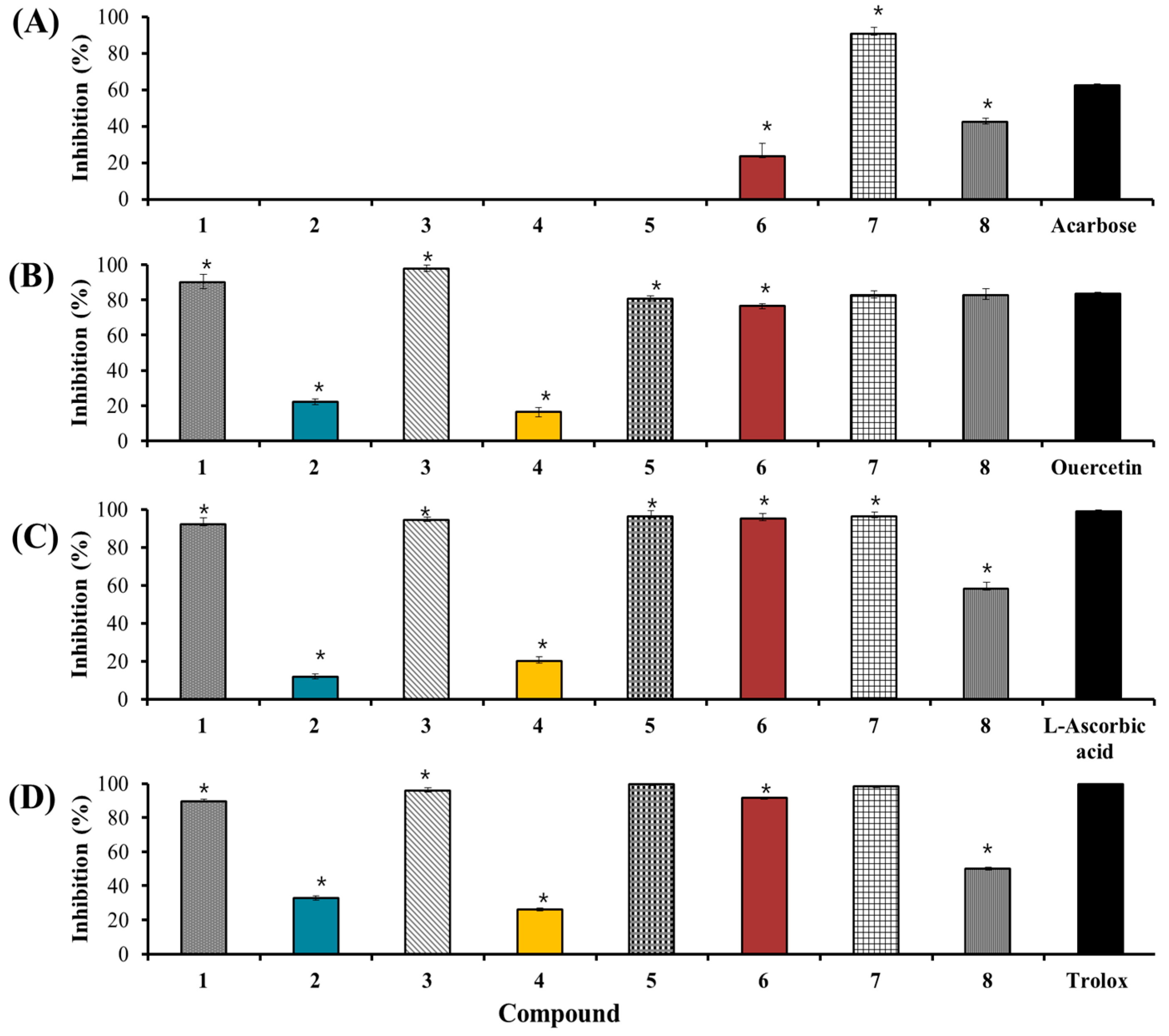
2.3.3. Identification of Antioxidants Using Offline 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)-(HPLC) and 2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS)-(HPLC)
3. Materials and Methods
3.1. Materials
3.2. Collection of Plant Material
3.3. Preparation of Extracts and Isolation of Plant Samples
3.4. Evaluation of the α-Glucosidase Inhibitory Assay
3.5. Animal Care
3.6. Evaluation of the Rat Lens Aldose Reductase (RLAR) Assay
3.7. Evaluation of the DPPH Assay
3.8. Evaluation of the ABTS Assay
3.9. Determination of the α-Glucosidase Ultrafiltration-HPLC Assay
3.10. Determination of the HRAR Ultrafiltration-HPLC Assay
3.11. Evaluation of Offline DPPH-HPLC Analysis
3.12. Evaluation of Offline ABTS-HPLC Analysis
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AR | Aldose reductase |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DMSO | Dimethyl sulfoxide |
| HL | Hypericum laricifolium Juss. |
| MeOH | Methanol |
| RLAR | Rat lens aldose reductase |
| HRAR | Human recombinant aldose reductase |
| HPLC | High-performance liquid chromatography |
| TBD | Total binding degree |
| QR | Quantitative reduction |
References
- World Health Organization. Diabetes Fact Sheet No. 312; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, 62–69. [Google Scholar]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J. Erythritol attenuates the diabetic oxidative stress through modulating glucose metabolism and lipid peroxidation in streptozotocin-induced diabetic rats. J. Agric. Food Chem. 2002, 50, 5485–5489. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Liang, J.; Zhang, Y.; Zhao, H.; Guo, Y.; Shi, S. Separation and purification of α-glucosidase inhibitors from Polygonatum odoratum by stepwise high-speed counter-current chromatography combined with Sephadex LH-20 chromatography target-guided by ultrafiltration-HPLC screening. J. Chromatogr. B 2015, 985, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Doi, U.; Osawa, T. Inhibitory Activity of Corn-Derived Bisamide Compounds Against α-Glucosidase. J. Agric. Food Chem. 2003, 51, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Minoshima, Y.; Yamamato, J.; Adachi, I.; Watson, A.A.; Nash, R.J. Protective effects of dietary chamomile tea on diabetic complications. J. Agric. Food Chem. 2008, 56, 8206–8211. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, Y.S.; Kim, S.H.; Bae, Y.S.; Lim, S.S. Inhibition of aldose reductase by phenylethanoid glycoside isolated from the seeds of Paulownia coreana. Biol. Pharm. Bull. 2011, 34, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Pari, L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2006, 79, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.X.; Ge, A.; Sineeporn, D.; Li, J.; Bai, Y.; Liu, J.; He, J.; Yang, X.; Song, L.J.; Zhang, B.L.; et al. The multi-targets integrated fingerprinting for screening anti-diabetic compounds from Chinese medicine Jinqi Jiangtang Tablet. J. Ethnopharmacol. 2015, 164, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, Z. An Ethnopharmacological Survey Conducted in the Bolivian Amazon, and Identification of N-Alkylamides and Lignans from Lepidium meyenii and Heliopsis helianthoides var. Scabra with Effects on the Central Nervous System. Ph.D. Thesis, University of Szeged, Szeged, Hungary, November 2014. [Google Scholar]
- Acosta Solis, M.A. Plantas Medicinales del Ecuador; Abya Yala: Quito, Ecuador, 1992; p. 127. [Google Scholar]
- Ramírez-Gonzáilez, I.; Amaro-Luis, J.M.; Bahsas, A. Xanthones from aerial parts of Hypericum laricifolium Juss. Nat. Prod. Commun. 2013, 8, 1731–1732. [Google Scholar] [PubMed]
- El-Seedi, H.R.; Ringbom, T.; Torssell, K.; Bohlin, L. Constituents of Hypericum laricifolium and their cyclooxygenase (COX) enzyme activities. Chem. Pharm. Bull. 2003, 51, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Ccana-Ccapatinta, G.V.; Von Poser, G.L. Acylphoroglucinol derivates from Hypericum laricifolium Juss. Phytochem. Rev. 2015, 12, 63–66. [Google Scholar]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Karato, M.; Yamaguchi, K.; Takei, S.; Kino, T.; Yazawa, K. Inhibitory effects of pasuchaca (Geranium dielsianum) extract on α-glucosidase in mouse. Biosci. Biotechnol. Biochem. 2006, 70, 1482–1484. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Paniagua-Zambrana, N.; Rivas Chamorro, M.; Molina Moreira, N.; Cuadros Negri, M.R.; Olivera, J. Perfil in the market-classification and dosage of species used as anti-diabetics in Lima, Peru. J. Ethnobiol. Ethnomed. 2013, 9, 1–37. [Google Scholar] [CrossRef] [PubMed]
- El-Moein, N.M.; Mahmoud, E.A.; Shalaby, E.A. Antioxidant Mechanism of Active Ingredients Separated from Eucalyptus globulus. Org. Chem. Curr. Res. 2012, 1, 2. [Google Scholar] [CrossRef]
- Ricco, R.A.; Agudelo, I.; Garces, M.; Evelson, P.; Wagner, M.L.; Gurni, A.A. Polifenoles y actividad antioxidante en Equisetum giganteum L. (Equisetaceae). Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 325–332. [Google Scholar]
- Bussmann, R.W.; Sharon, D. Traditional medicinal plant use in Northern Peru: Tracking two thousand years of healing culture. J. Ethnobiol. Ethnomed. 2006, 2, 1–47. [Google Scholar]
- Carraz, M.; Lavergne, C.; Jullian, V.; Wright, M.; Gairin, J.E.; Gonzales de la Cruz, M.; Bourdy, G. Antiproliferative activity and phenotypic modification induced by selected Peruvian medicinal plants on human hepatocellular carcinoma Hep3B cells. J. Ethnopharmacol. 2015, 166, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind. Crop. Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- Neto, C.C.; Owens, C.W.; Langfield, R.D.; Comeau, A.B.; St. Onge, J.; Vaisberg, A.J.; Hammond, G.B. Antibacterial activity of some Peruvian medicinal plants from the Callejon de Huaylas. J. Ethnopharmacol. 2002, 79, 133–138. [Google Scholar] [CrossRef]
- Berlowski, A.; Zawada, K.; Wawer, I.; Paradowska, K. Antioxidant Properties of Medicinal Plants from Peru. Food Nutr. Sci. 2013, 4, 71–74. [Google Scholar] [CrossRef]
- De la Cruz, H.; Vilcapoma, G.; Zevallos, P.A. Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Peru. J. Ethnopharmacol. 2007, 111, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Guillen Quispe, Y.N.; Hwang, S.H.; Wang, Z.; Lim, S.S. Screening of Peruvian plants for Tyrosinase Inhibitory Properties: Identification of Tyrosinase Inhibitors in Hypericum laricifolium Juss. Molecules 2017, 22, 402. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Jenkins, A.L.; Brissette, C.; Choleva, L.; Jovanovski, E.; Gibbs, A.L.; Bazinet, R.P.; Au-Yeung, F.; Zurbau, A.; Ho, H.V.; et al. Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: A double-blind randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Llanga Guaman, B.G. Determinacion de la Actividad Antioxidante de los Extractos de Quishuar (Buddleja incana), Aliso (Alnus acuminate) y Romerillo (Hypericum laricifolium) Localizadas en 3 Zonas Geograficas Diferentes. Ph.D. Thesis, Tecnologias y Ciencias de la Ingenieria, Riobamba, Ecuador, 8 July 2014; pp. 20–21. (In Spanish). [Google Scholar]
- Crockett, S.; Eberhardt, M.; Kunert, O.; Schuhly, W. Hypericum species in the Paramos of Central and South America: A special focus upon H. irazuense Kuntze ex N. Robson. Phytochem. Rev. 2010, 9, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Levent Altun, M.; Seever Yilmaz, B.; Erdogan, L.; Saltan Citoglu, G. Assessment of cholinesterase and tyrosinase inhibitory and antioxidant effects of Hypericum perforatum L. (St John’s wort). Ind. Crops Prod. 2013, 43, 87–92. [Google Scholar] [CrossRef]
- Galeotti, N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Roja, J.; Buitrago, A.; Rojas, L.B.; Morales, A. Chemical composition of Hypericum laricifolium Juss. Essential oil Collected from Merida—Venezuela. Med. Aromat. Plants 2013, 2, 1–3. [Google Scholar]
- Cies´la, L.; Moaddel, R. Comparison of analytical techniques for the identification of bioactive compounds from natural products. Nat. Prod. Rep. 2016, 33, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hwang, S.H.; Guillen Quispe, Y.N.; Gonzales Arce, P.H.; Lim, S.S. Investigation of the antioxidant and aldose reductase inhibitory activities of extracts from Peruvian tea plant infusions. Food Chem. 2017, 231, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC assay. Based Complement. Altern. Med. 2015, 165457. [Google Scholar] [CrossRef] [PubMed]
- Orcic, D.Z.; Mimica-Dukić, N.M.; Francišković, M.M.; Petrović, S.S.; Jovin, E.D. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem. Cent. J. 2011, 5, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gioti, E.M.; Fiamegos, Y.C.; Skalcos, D.C.; Stalikas, C.D. Antioxidant activity and bioactive components of the arterial parts of Hypericum perforatum L. from Epirus, Greece. Food Chem. 2009, 117, 398–404. [Google Scholar] [CrossRef]
- Béjaoui, A.; Salem, I.B.; Rokbeni, N.; M’rabet, Y.; Boussaid, M.; Boulila, A. Bioactive compounds from Hypericum humifusum and Hypericum perfoliatum: Inhibition potential of polyphenols with acetylcholinesterase and key enzymes linked to type-2 diabetes. Pharm. Biol. 2017, 55, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, J.K.; Jang, J.M.; Shin, K.H.; Lim, S.S. Rapid identification of the α-glucosidase inhibitory compounds from Thunberg’s Geranium (Geranium thunbergii Sieb. et Zucc.). Food Sci. Biotechnol. 2012, 2, 987–996. [Google Scholar] [CrossRef]
- Dai, X.; Huang, Q.; Zhou, B.; Gong, Z.; Liu, Z.; Shi, S. Preparative isolation and purification of seven main antioxidants from Eucommia ulmodies Oliv. (Duzhong) leaves using HSCCC guided by DPPH-HPLC experiment. Food Chem. 2013, 139, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hwang, S.H.; Wang, Z.; Yu, J.M.; Lim, S.S. Rapid Identification and Isolation of Rat Lens Aldose Reducatse and Antioxidant in Maackia amurensis. BioMed Res. Int. 2017, 4941825. [Google Scholar] [CrossRef]
| No. | Voucher | Scientific Name | Common Name | Family | Used Part 1 | References
[13,17,19,20,21,22,23,24,25,26,27,28] 2 | Yield (%) 3 | Inhibition (%) 4 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Glucosidase (500 μg/mL) 5 | AR (10 μg/mL) 6 | Antioxidants | |||||||||
| DPPH (143 μg/mL) 7 | ABTS (33 μg/mL) 8 | ||||||||||
| 1 | A5 | Annona muricata L. | Hoja de huanabana, Graviola | ANNONACEAE | L | Hypertension [ 24], Inflammation [20,24] | 19.5 | 26.3 ± 1.7 c | 0.8 ± 0.8 a | 64.2 ± 0.9 e,f,g,h,i | 31.5 ± 2.9 f,g |
| 2 | A32 | Acanthoxanthium spinosu (L.) Fourr. | Juan Alonso | ASTERACEAE | A | Diabetes [ 25], Inflammation [20] | 8.4 | 3.2 ± 2.7 a | 22.4 ± 1.2 e,f | 11.0 ± 3.5 a,b | 2.1 ± 1.8 a |
| 3 | A16 | Ambrosia arborescens Mill. | Marco | ASTERACEAE | L | Inflammation [ 25] | 12.7 | 18.5 ± 1.9 c | 31.8 ± 6.1 f,g | 3.5 ± 0.7 a,b | 6.1 ± 1.8 a,b |
| 4 | P78 | Baccharis genistelloides (Lam.) Pers. | Karqueja | ASTERACEAE | A | Diabetes [ 20,21], Inflammation [20], Burn fat [20], Cholesterol [20,21] | 19.7 | 6.2 ± 3.4 a,b | NA | 47.0 ± 9.2 c,d | 27.9 ± 1.1 e,f,g |
| 5 | P77 | Chuquiraga spinosa Less. | Huamanpinta | ASTERACEAE | A | Inflammation [ 25], Kidneys [25] | 28.7 | NA 9 | 12.2 ± 0.7 c,d | 70.3 ± 11.0 g,h,i,j | 50.3 ± 3.1 h |
| 6 | A31 | Flaveria Bidentis (L.) Kuntze | Mata gusano | ASTERACEAE | L | Inflammation [ 26] | 7.9 | 17.3 ± 8.4 b,c | 86.4 ± 1.9 k | 5.3 ± 10.8 a,b | 8.0 ± 2.1 a,b |
| 7 | P14 | Schkuhria pinnata (Lam.) Kuntze ex Thell. | Canchalagua | ASTERACEAE | A | Diabetes [ 20,21], Inflammation [20,21] | 2.4 | 0.1 ± 2.7 a | NA | 67.1 ± 3.3 f,g,h,i | 71.4 ± 8.4 j |
| 8 | A6 | Smallanthus sonchifolius (Poepp.) H. Rob. | Hojas de yacon | ASTERACEAE | L | Diabetes [ 20,21,22,23], Inflammation [20], Kidneys [20], Cholesterol [20] | 5.3 | 5.5 ± 1.4 a,b | 31.9 ± 9.1 g | NA | 5.5 ± 3.0 a,b |
| 9 | P49 | Taraxacum officinale F.H. Wigg. | Diente de leon | ASTERACEAE | A, F | Inflammation [ 20] | 4.8 | NA | NA | 41.9 ± 10.8 c | 15.7 ± 1.6 b,c,d |
| 10 | A17 | Tessaria integrifolia Ruiz & Pav. | Pajaro bobo | ASTERACEAE | L | Kidneys [ 20], Liver [20], Inflammation [20] | 18.4 | 41.6 ± 1.1 d | 79.9 ± 0.1 k | 52.2 ± 3.9 c,d,e | 31.1 ± 3.3 f,g |
| 11 | P79 | Cordia lutea Lam. | Flor de overo | BORAGINACEAE | F | Inflammation [ 20,21], Liver [20,21] | 12.3 | NA | 3.6 ± 1.3 a,b | 60.2 ± 2.1 d,e,f,g,h | 36.6 ± 1.7 g |
| 12 | P36 | Tiquilia Paronychioides (Phil.) Rich. | Flor de arena | BORAGINACEAE | A | Inflammation [ 20] | 18.5 | 2.9 ± 1.1 a | 26.0 ± 0.1 f,g | 95.7 ± 0.8 m,n | 97.1 ± 0.4 m |
| 13 | A24 | Sambucus peruviana H. B. K. | Sauco | CAPRIFOLIAEAE | L | Kidneys [ 20,23], Inflammation [20] | 8.1 | 47.4 ± 4.8 d | 47.2 ± 0.9 h | 53.3 ± 1.5 c,d,e,f | 33.8 ± 6.1 g |
| 14 | A10 | Hypericum laricifolium Juss. | Hierba de la fortuna | CLUSIACEAAE | L | Inflammation [ 13,28], Infections, Musculoskeletal, Bone pain [19] | 15.9 | 97.2 ± 2.0 h | 56.9 ± 5.6 i | 81.9 ± 2.5 j,k,l,m | 58.8 ± 4.6 h,i |
| 15 | P55 | Equisetum giganteum L. | Cola de caballo | EQUISETACEAE | A | Kidneys [ 20,21], Inflammation [20,21] | 11.2 | 77.8 ± 5.9 g | 0.7 ± 0.0 a | 73.9 ± 8.9 h,i,j,k | 51.8 ± 0.3 h |
| 16 | P5 | Phyllanthus niruri L. | Chanca piedra | EUPHORBIACEAE | L | Diabetes [ 24], Kidneys [20], Liver [20], Inflammation [20] | 12.9 | 39.9 ± 1.4 d | 25.3 ± 3.7 f,g | 97.0 ± 0.1 n | 99.6 ± 0.1 m |
| 17 | P80 | Desmodium molliculum (Kunth) A.P. De Candolle | Manayupa | FABACEAE | L | Kidneys [ 20,21], Inflammation [20,21] | 23.8 | 97.9 ± 9.1 h | 10.9 ± 0.5 b,c,d | 89.9 ± 4.2 l,m,n | 73.6 ± 1.3 j,k |
| 18 | A3 | Otholobium mexicanum (L. f.) J.W. Grimes | Culen negro | FABACEAE | A | Diabetes [ 21] | 6.1 | NA | 50.4 ± 0.5 h,i | 4.5 ± 5.7 a,b | 9.6 ± 1.9 a,b,c |
| 19 | A4 | Otholobium pubescens (Poir.) J.W. Grimes | Culen blanco | FABACEAE | A | Diabetes [ 21] | 19.6 | NA | 8.8 ± 0.5 a,b,c,d | 6.1 ± 0.5 a,b,c,d | 27.4 ± 1.5 e,f,g |
| 20 | A49 | Vicia Faba | Haba | FABACEAE | Fr | Renal disorders [ 22] | 7.6 | 0.1 ± 2.7 a | NA | 4.2 ± 2.0 a,b | 13.0 ± 3.0 b,c,d |
| 21 | P7 | Gentianella tristicha (Gilg) J.S. Pringle | Hercampure | GENTIANACEAE | A | Diabetes [ 17], Cholesterol [26] | 30.3 | NA | 68.1 ± 4.2 j | 53.1 ± 0.6 cdef | 66.5 ± 4.5 i,j |
| 22 | A35 | Geranium dielsianum R. Knuth | Pasuchaca | GERANIACEAE | L | Diabetes [ 17,24] | 6.3 | 97.7 ± 1.1 h | 15.6 ± 0.2 d,e | 96.8 ± 0.3 n | 83.9 ± 4.1 k,l |
| 23 | P11 | Clinopodium brevicalyx (Epling) Harley & A. Granda | Inka muña | LAMIACEAE | L | Inflammation [ 26] | 26.8 | 1.8 ± 5.5 a | 31.9 ± 0.7 g | 95.8 ± 0.2 m,n | 94.1 ± 2.7 l,m |
| 24 | P4 | Salvia hispanica L. | Chia | LAMIACEAE | S | Diabetes [ 27], Obesity [27] | 3.5 | NA | NA | 17.3 ± 3.0 b | 21.7 ± 1.8 d,e,f |
| 25 | P40 | Peumus boldus Molina | Boldo | MONIMIACEAE | L | Inflammation [ 20], Kidneys [20], Liver [20] | 32.5 | 75.2 ± 3.0 g | 7.6 ± 0.9 a,b,c,d | 86.2 ± 3.9 k,l,m,n | 95.7 ± 0.5 m |
| 26 | P39 | Eucalyptus globolus Labill. | Eucalipto | MYRTACEAE | L | Burn fat [ 20] | 18.0 | 62.3 ± 0.9 e,f | 11.2 ± 0.8 b,c,d | 77.5 ± 0 i,j,k,l | 99.5 ± 0.3 m |
| 27 | A2 | Argyrochosma nivea (Poir.) Windham | Cuti - Cuti hembra blanca | PTERIDACEAE | A | Diabetes [ 17] | 2.3 | 50.5 ± 3.2 d,e | 59.1 ± 0.3 i,j | 71.2 ± 0.7 g,h,i,j | 34.7 ± 7.3 g |
| 28 | P17 | Cheilanthes pilosa Goldm. | Cuti Cuti | PTERIDACEAE | A | Diabetes [ 26], Liver [26] | 14.3 | 72.4 ± 0.6 f,g | 9.6 ± 0.9 b,c,d | 58.9 ± 4.8 d,e,f,g | 56.4 ± 4.3 h,i |
| 29 | A1 | Cheilanthes pruinata Kaulf. | Cuti - Cuti marron macho | PTERIDACEAE | A | Diabetes [ 17] | 30.3 | 73.4 ± 2.9 f,g | 41.6 ± 1.1 h | 53.2 ± 0.3 c,d,e,f | 31.1 ± 4.1 f,g |
| 30 | A19 | Buddleja Americana L. | Flor blanca | SCROPHULARIA CEAE | F | Inflammation [ 26] | 8.5 | 16.1 ± 5.6 b,c | 3.7 ± 0.1 a,b,c | 2.4 ± 1.0 a | 19.6 ± 1.2 c,d,e |
| Extracts | α-Glucosidase 1 | AR 2 | DPPH 3 | ABTS 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Inhibition (%) | IC50 (μg/mL) 5 | Inhibition (%) | IC50 (μg/mL) | Inhibition (%) | IC50 (μg/mL) | Inhibition (%) | IC50 (μg/mL) | |
| MC | 11.65 ± 2.1 * | - | 28.9 ± 7.4 * | - | 17.5 ± 0.3 * | - | 14.4 ± 1.3 * | - |
| 70% MeOH | 92.36 ± 1.1 * | 56.6 ± 2.5 | 64.51 ± 1.3 * | 3.3 ± 52 | 93.0 ± 0.1 * | 42.5 ± 0.6 | 78.9 ± 0.6 * | 14.4 ± 1.3 |
| Acarbose | 55.82 ± 2.3 | 367.4 ± 2.1 | - | - | - | - | - | - |
| Quercetin | - | - | 83.7 ± 2.6 | 1.3 ± 3.6 | - | - | - | - |
| L-Ascorbic | - | - | - | - | 99.1 ± 0 | 17.6 ± 0.1 | - | - |
| Trolox | - | - | - | - | - | - | 100 ± 0.1 | 4.6 ± 0.1 |
| Compounds | α-glucosidase 1 | AR 2 | Antioxidants | |||||
|---|---|---|---|---|---|---|---|---|
| DPPH 3 | ABTS 4 | |||||||
| Conc. (μg/mL) | IC50 (μM) 5 | Conc. (μg/mL) | IC50 (μM) | Conc. (μg/mL) | IC50 (μM) | Conc. (μg/mL) | IC50 (μM) | |
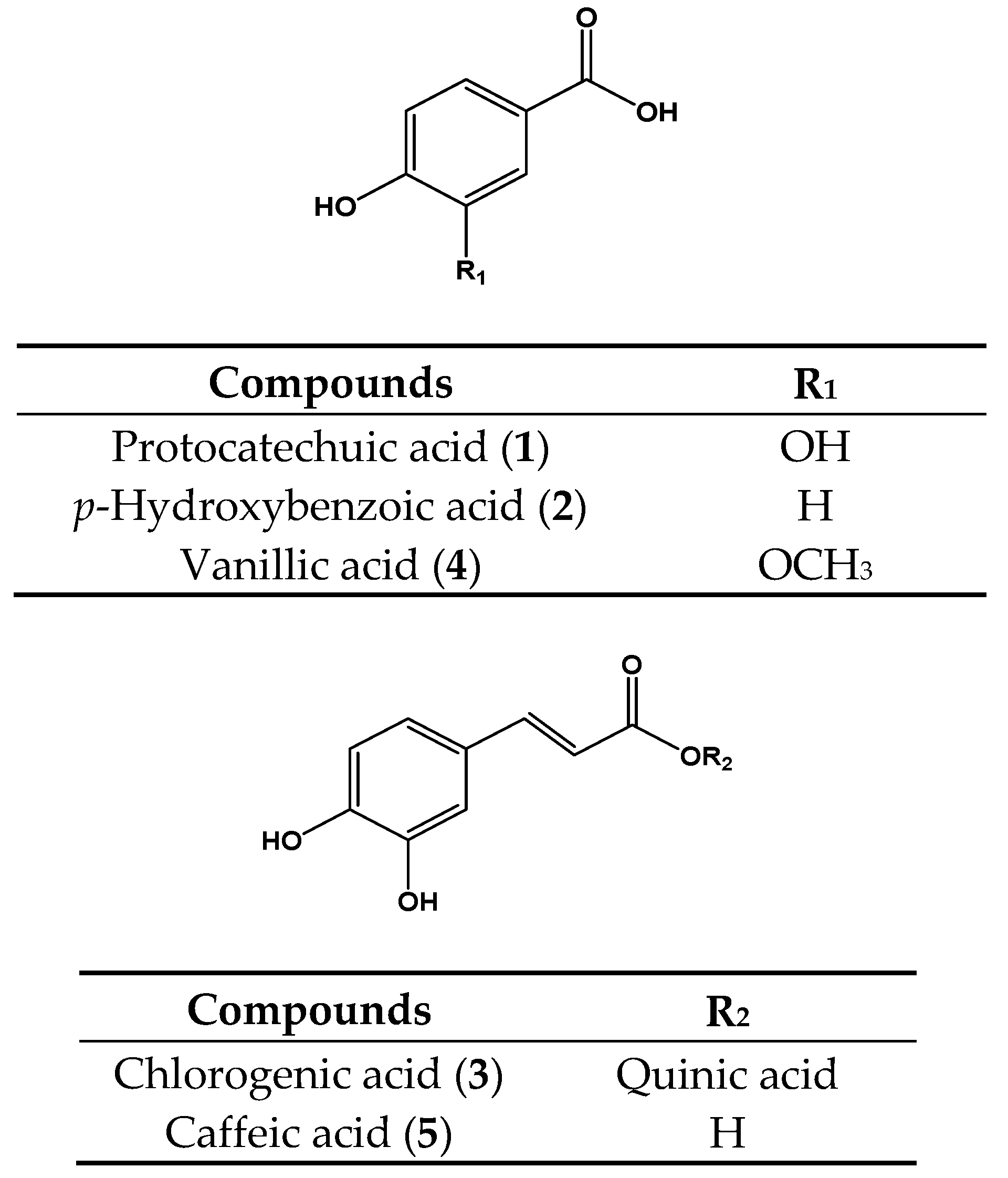
| Protocatechuic acid (1) | 50 | NA 6 | 10 | 16.9 ± 1.9 e | 143 | 263.4 ± 0.2 e | 17 | 9.7 ± 0.9 c |
| p-Hydroxybenzoic acid (2) | 50 | NA | 10 | NA | 143 | NA | 33 | NA |
| Chlorogenic acid (3) | 50 | NA | 10 | 0.23 ± 1.8 a | 143 | 123.1 ± 2.9 d | 33 | 23.1 ± 3.5 f |
| Vanilic acid (4) | 50 | NA | 10 | NA | 143 | NA | 33 | NA |
| Caffeic acid (5) | 50 | NA | 10 | 28.3 ± 0.9 f | 143 | 47.2 ± 0.1 b | 17 | 7.2 ± 1.1 b |
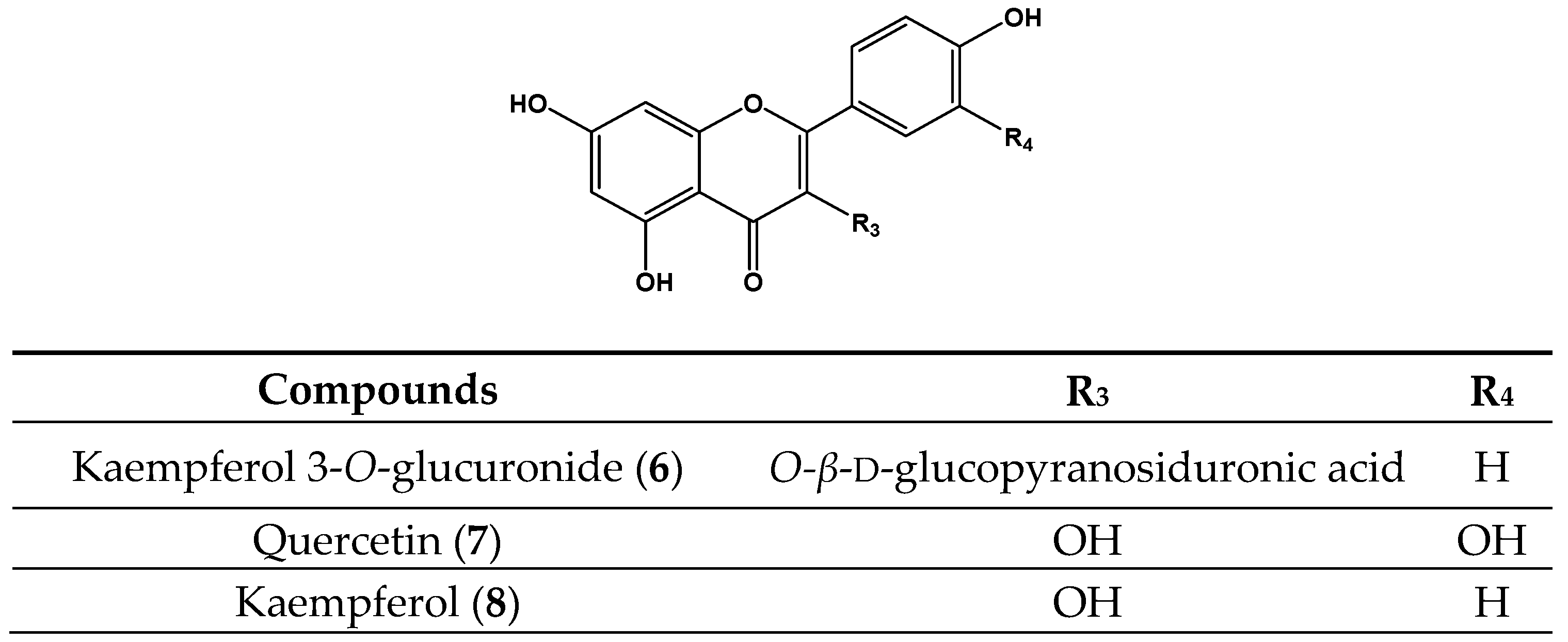
| Kaempferol 3-O-glucuronide (6) | 50 | NA | 10 | 6.0 ± 1.1 c | 143 | 58.8 ± 2.1 c | 17 | 11.0 ± 0.2 d |
| Quercetin (7) | 50 | 15.9 ± 1.2 a | 10 | 2.5 ± 0.8 b | 71 | 0.33 ± 1.4 a | 17 | 0.33 ± 0.6 a |
| Kaempferol (8) | 50 | NA | 10 | 9.7 ± 0.5 d | 143 | 326.4 ± 3.3 f | 33 | 191.8 ± 1.1 g |
| Acarbose | 500 | 439.9 ± 8.9 b | - | - | - | - | - | - |
| Quercetin | - | - | 10 | 2.4 ± 1.2 b | - | - | - | - |
| l-Ascorbic | - | - | - | - | 71 | 0.57 ± 0.4 a | - | - |
| Trolox | - | - | - | - | - | - | 17 | 18.8 ± 0.5 e |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillen Quispe, Y.N.; Hwang, S.H.; Wang, Z.; Zuo, G.; Lim, S.S. Screening In Vitro Targets Related to Diabetes in Herbal Extracts from Peru: Identification of Active Compounds in Hypericum laricifolium Juss. by Offline High-Performance Liquid Chromatography. Int. J. Mol. Sci. 2017, 18, 2512. https://doi.org/10.3390/ijms18122512
Guillen Quispe YN, Hwang SH, Wang Z, Zuo G, Lim SS. Screening In Vitro Targets Related to Diabetes in Herbal Extracts from Peru: Identification of Active Compounds in Hypericum laricifolium Juss. by Offline High-Performance Liquid Chromatography. International Journal of Molecular Sciences. 2017; 18(12):2512. https://doi.org/10.3390/ijms18122512
Chicago/Turabian StyleGuillen Quispe, Yanymee N., Seung Hwan Hwang, Zhiqiang Wang, Guanglei Zuo, and Soon Sung Lim. 2017. "Screening In Vitro Targets Related to Diabetes in Herbal Extracts from Peru: Identification of Active Compounds in Hypericum laricifolium Juss. by Offline High-Performance Liquid Chromatography" International Journal of Molecular Sciences 18, no. 12: 2512. https://doi.org/10.3390/ijms18122512