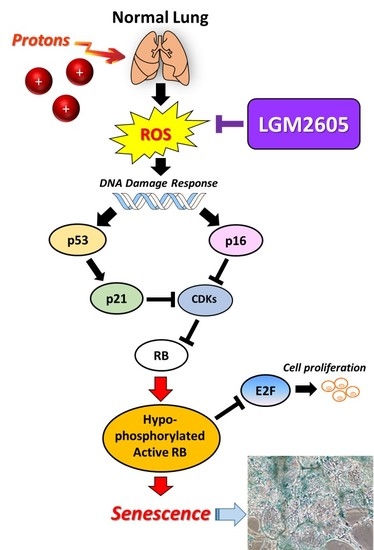
Synthetic Secoisolariciresinol Diglucoside (LGM2605) Protects Human Lung in an Ex Vivo Model of Proton Radiation Damage
Abstract
:1. Introduction
2. Results
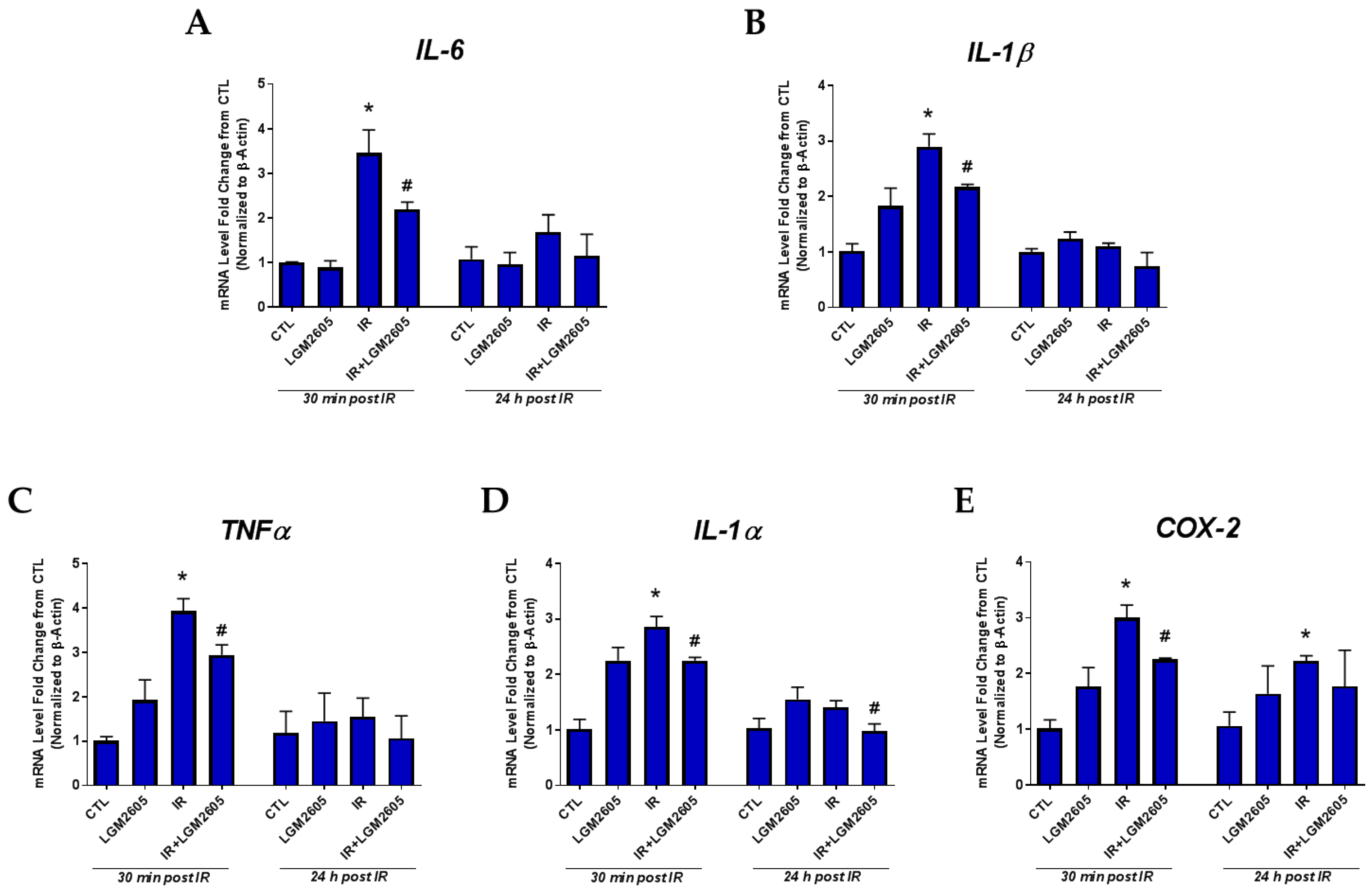
2.1. LGM2605 Prevents the Expression of Proinflammatory Cytokine Gene Levels and Reduces the Induction COX-2 by Proton Radiation in huPCLS
2.2. LGM2605 Boosts Antioxidant Gene Levels by Proton Radiation in huPCLS
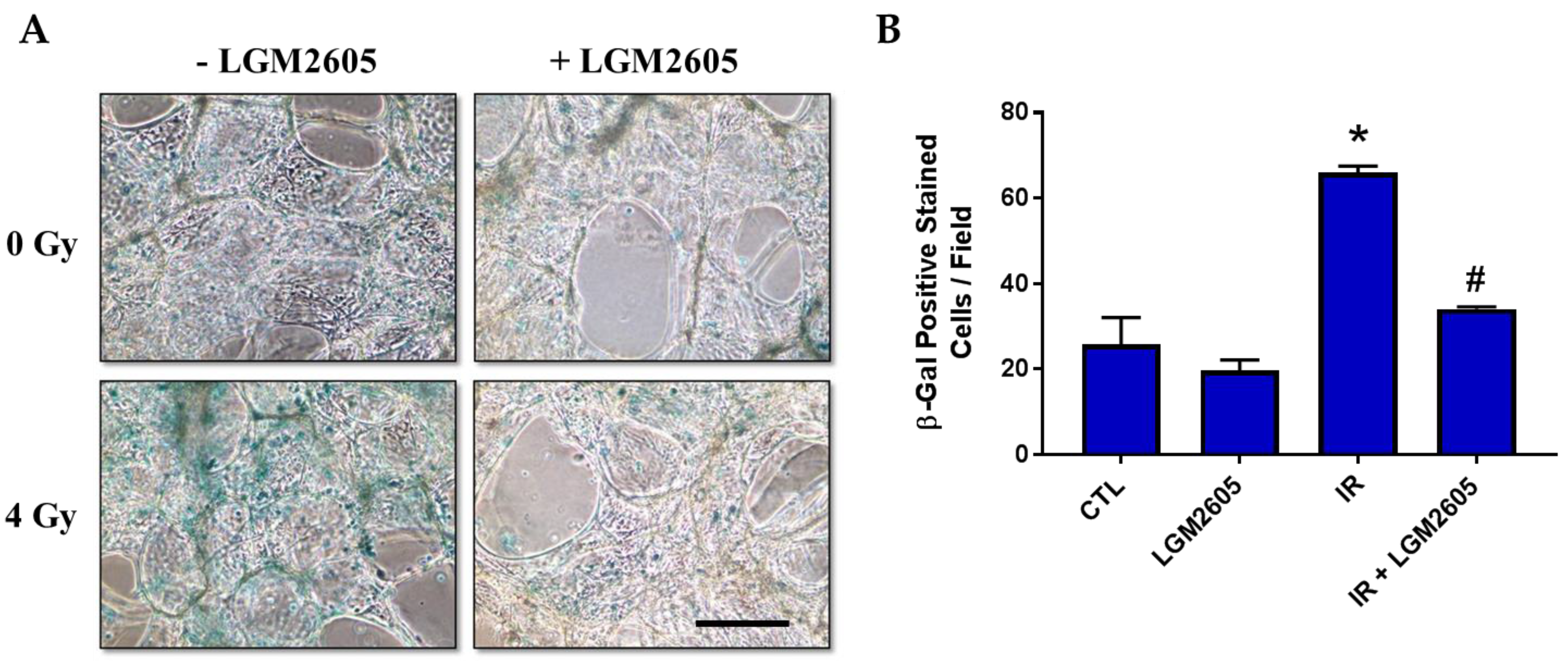
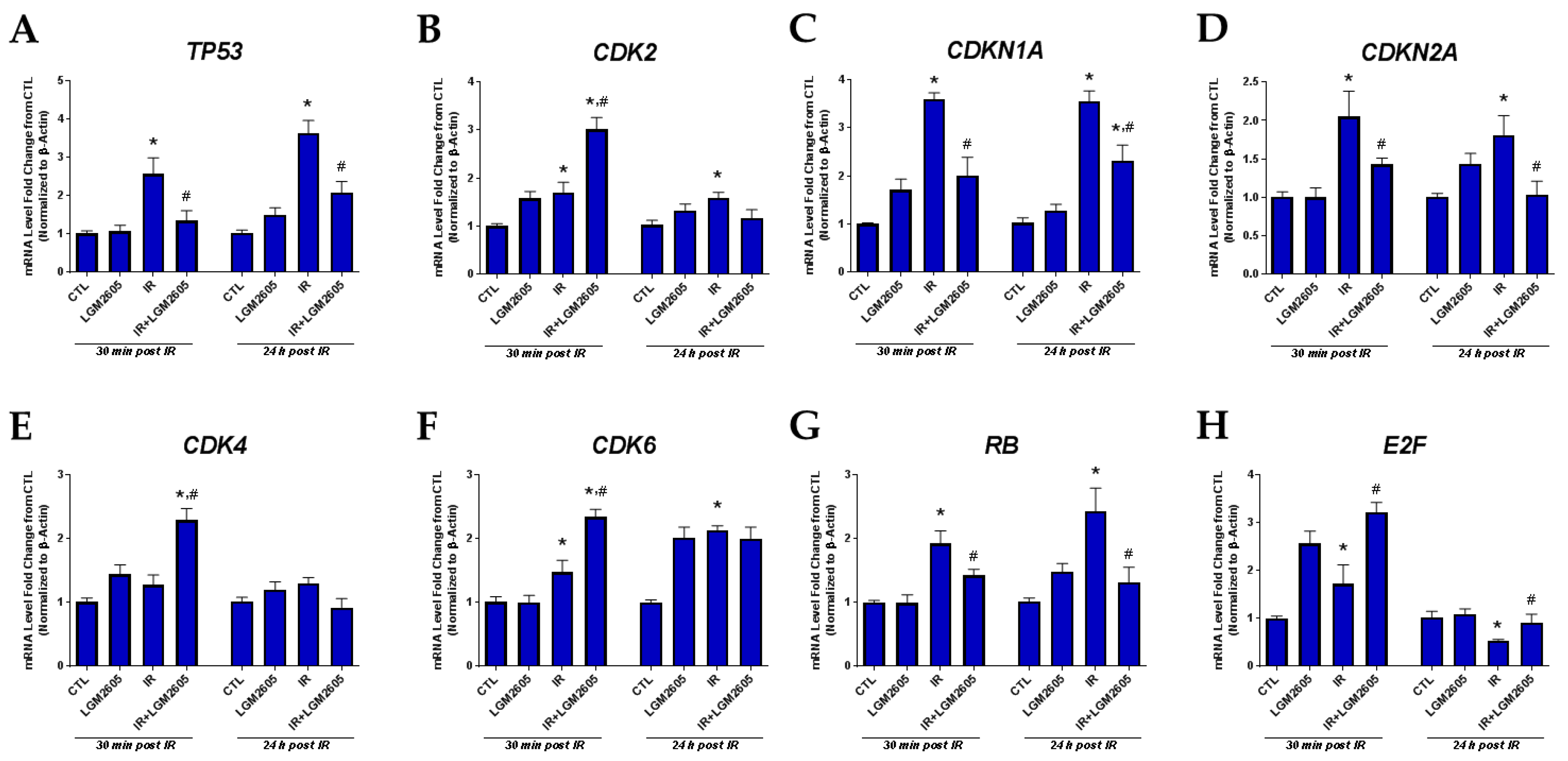
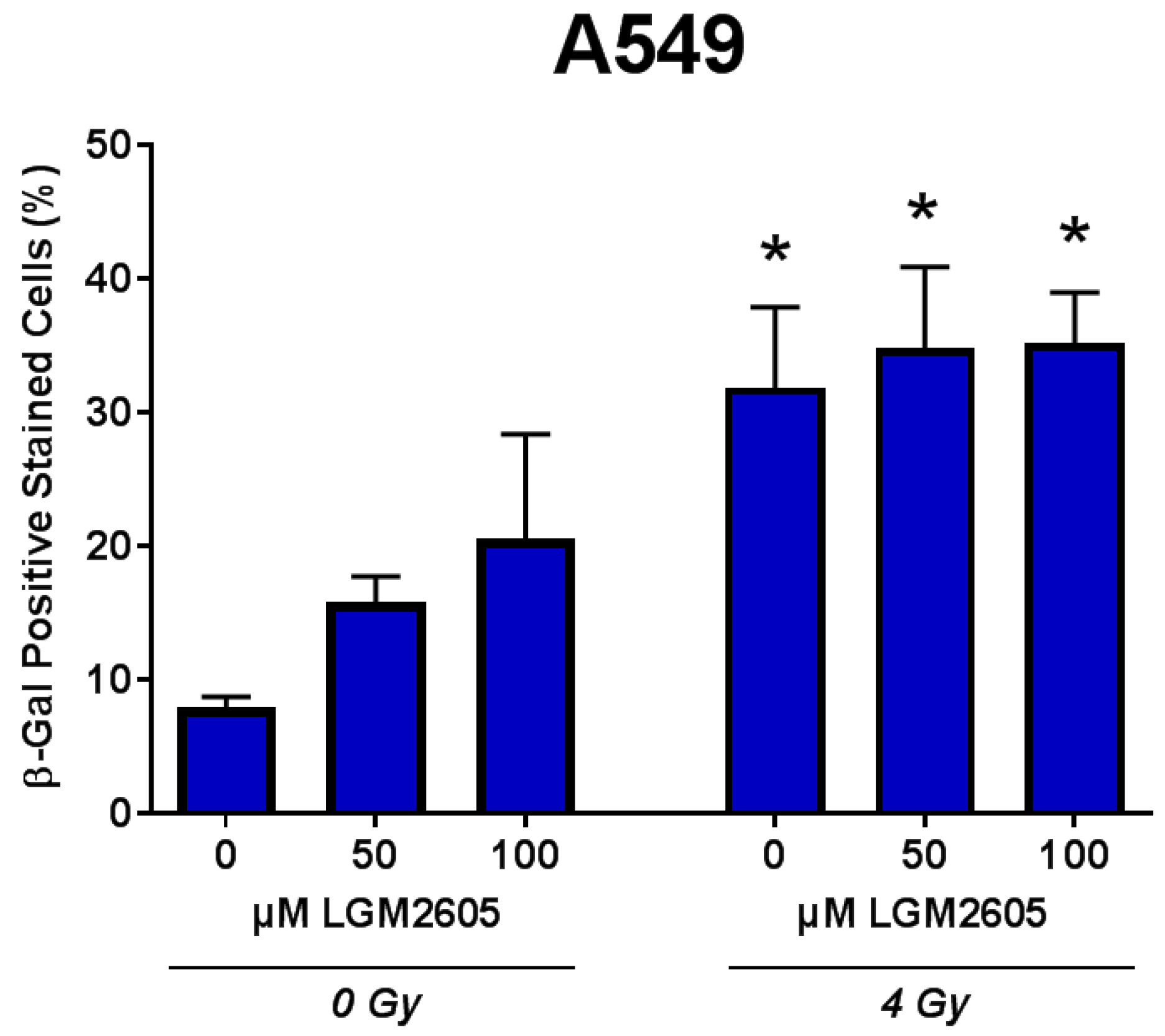
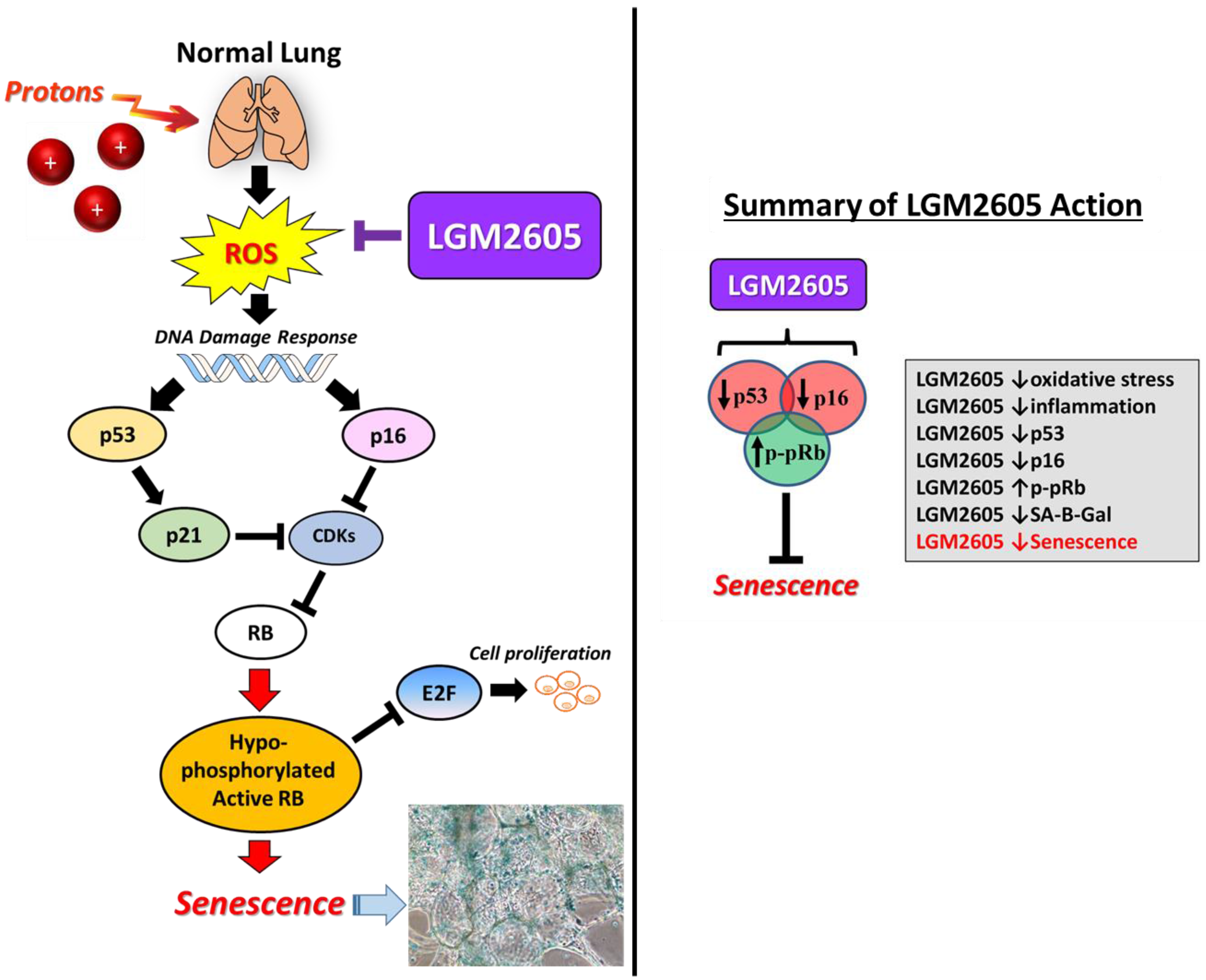
2.3. LGM2605 Decreases Proton Radiation-Induced Senescence and Biomarkers of Cellular Senescence in huPCLS
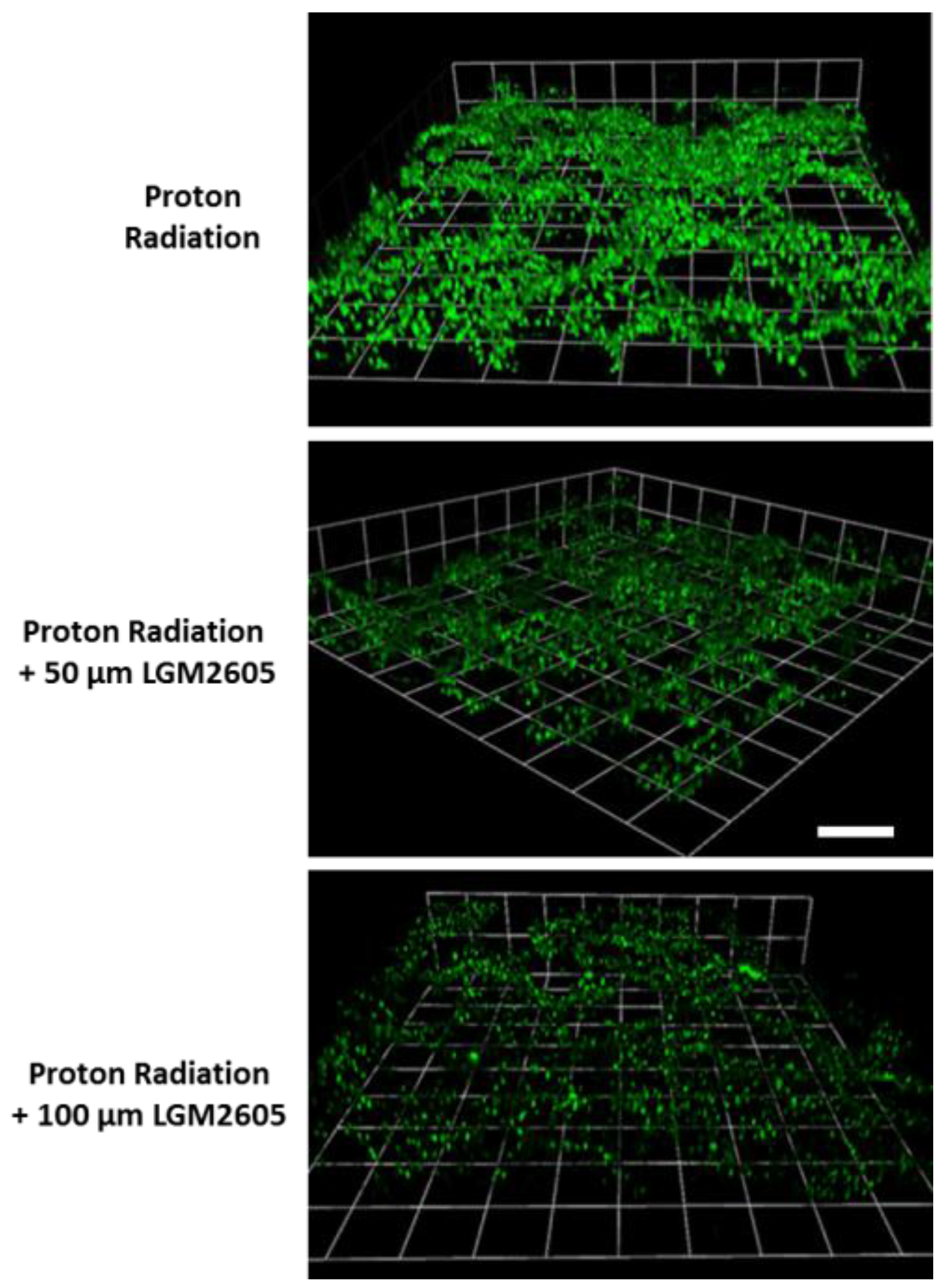
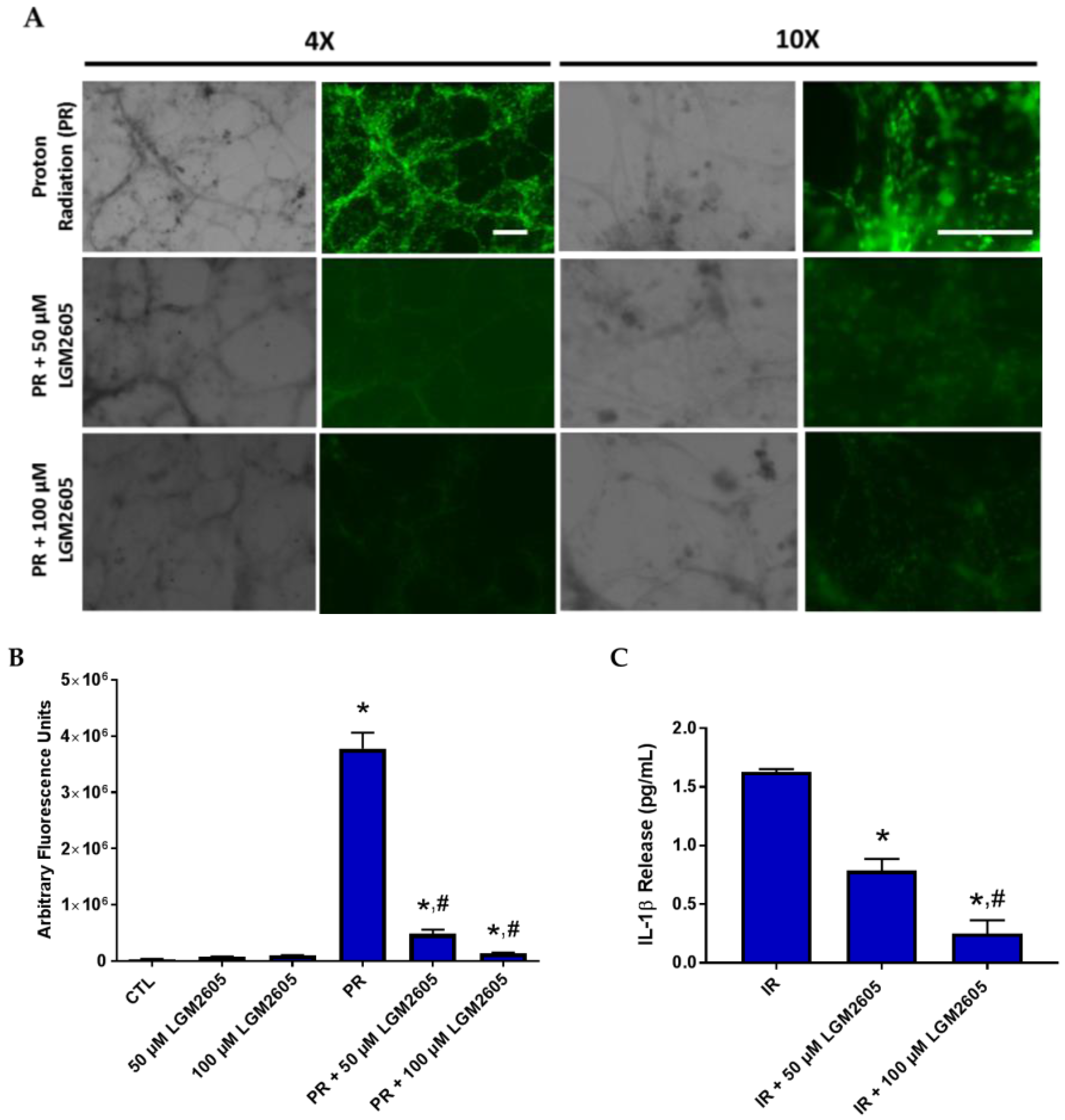
2.4. LGM2605 Reduces Proton Radiation-Induced Oxidative Stress in huPCLS
2.5. LGM2605 Reduces a Proinflammatory Phenotype in Proton-Irradiated huPCLS
2.6. LGM2605 Does Not Impair Tumor Cell Eradication by Proton Radiation
3. Discussion
4. Materials and Methods
4.1. Human, Precision-Cut Lung Slices (huPCLS)
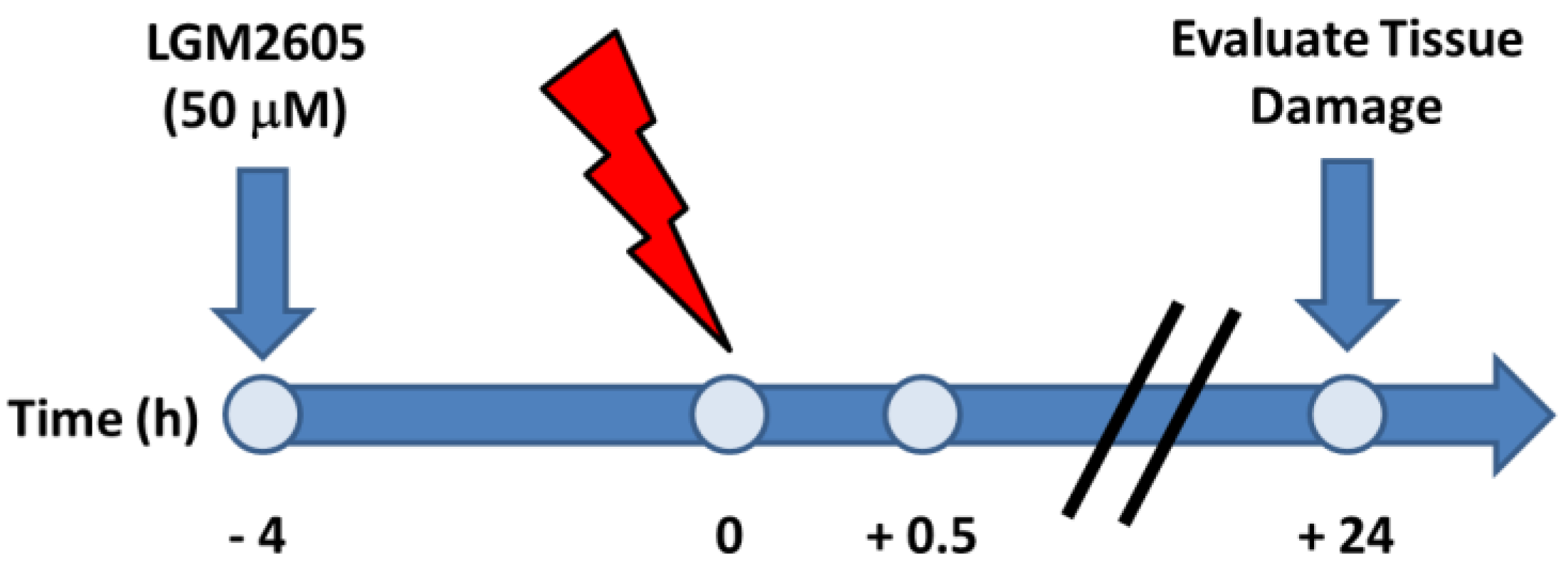
4.2. Irradiation Procedure
4.3. LGM2605 Treatment
4.4. Western Blots
4.5. Oxidative Stress
4.6. Images in Z-Stack
4.7. RNA Isolation and Gene Expression Analysis
4.8. Senescence-Associated β-Galactosidase Staining
4.9. Intercellular Adhesion Molecule (ICAM) Quantification
4.10. IL-1β Cytokine Release
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CDK | cyclin-dependent kinase |
| COPD | chronic obstructive pulmonary disease |
| COX-2 | cyclooxygenase-2 |
| EAR | endogenous antioxidant response |
| ELISA | enzyme-linked immunosorbent assay |
| GCR | galactic cosmic radiation |
| HMOX1 | heme oxygenase-1 |
| huPCLS | human, precision-cut lung slices |
| ICAM | intercellular adhesion molecule |
| IL-1α | interleukin-1α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| IMPT | intensity modulated proton therapy |
| IMRT | intensity modulated photon beam radiotherapy |
| LGM2605 | synthetic SDG |
| Nrf2 | nuclear factor (erythroid-derived 2)-like 2 |
| NQO1 | NADPH: quinone oxidoreductase-1 |
| OAR | organs at risk |
| PBS | phosphate-buffered saline |
| qPCR | quantitative polymerase chain reaction |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SA-β-gal | senescence-associated β-galactosidase |
| S1P | sphingosine-1 phosphate |
| SDG | secoisolariciresinol diglucoside |
| SphK1 | sphingosine kinase 1 |
| SphK2 | sphingosine kinase 2 |
| SpHL | sphingosine lyase |
| TNFα | tumor necrosis factor α |
References
- Feliciano, J.; Feigenberg, S.; Mehta, M. Chemoradiation for definitive, preoperative, or postoperative therapy of locally advanced non-small cell lung cancer. Cancer J. 2013, 19, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, K.E.; Gomez, J.E. Concurrent chemotherapy and radiation therapy for inoperable locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2017, 35, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Cotter, S.E.; McBride, S.M.; Yock, T.I. Proton radiotherapy for solid tumors of childhood. Technol. Cancer Res. Treat. 2012, 11, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Brodin, N.P.; Munck Af Rosenschold, P.; Aznar, M.C.; Kiil-Berthelsen, A.; Vogelius, I.R.; Nilsson, P.; Lannering, B.; Bjork-Eriksson, T. Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol. 2011, 50, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Lee, A.K.; Newhauser, W.D. Risk of secondary malignant neoplasms from proton therapy and intensity-modulated X-ray therapy for early-stage prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Pietrofesa, R.A.; Arguiri, E.; Schweitzer, K.S.; Berdyshev, E.V.; McCarthy, M.; Corbitt, A.; Alwood, J.S.; Yu, Y.; Globus, R.K.; et al. Space radiation-associated lung injury in a murine model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L416–L428. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.; Turowski, J.; Tyagi, S.; Dukes, F.; Arguiri, E.; Busch, T.M.; Gallagher-Colombo, S.M.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer 2013, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Turowski, J.B.; Arguiri, E.; Milovanova, T.N.; Solomides, C.C.; Thom, S.R.; Christofidou-Solomidou, M. Oxidative lung damage resulting from repeated exposure to radiation and hyperoxia associated with space exploration. J. Pulm. Respir. Med. 2013, 3, 1000158. [Google Scholar]
- Pietrofesa, R.A.; Velalopoulou, A.; Arguiri, E.; Menges, C.W.; Testa, J.R.; Hwang, W.T.; Albelda, S.M.; Christofidou-Solomidou, M. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis 2015, 37, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Pietrofesa, R.A.; Velalopoulou, A.; Lehman, S.L.; Arguiri, E.; Solomides, P.; Koch, C.J.; Mishra, O.P.; Koumenis, C.; Goodwin, T.J.; Christofidou-Solomidou, M. Novel double-hit model of radiation and hyperoxia-induced oxidative cell damage relevant to space travel. Int. J. Mol. Sci. 2016, 17, 953. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Simmons, N.; Tyagi, S.; Pietrofesa, R.; Shuvaev, V.V.; Valiulin, R.A.; Heretsch, P.; Nicolaou, K.C.; Christofidou-Solomidou, M. Synthesis and antioxidant evaluation of (S,S)- and (R,R)-secoisolariciresinol diglucosides (SDGs). Bioorg. Med. Chem. Lett. 2013, 23, 5325–5328. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Pietrofesa, R.; Christofidou-Solomidou, M. Novel synthetic (S,S) and (R,R)-secoisolariciresinol diglucosides (SDGs) protect naked plasmid and genomic DNA from γ radiation damage. Radiat. Res. 2014, 182, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Lamb, R.; Day, N.D.; Branigan, P.J.; Kajekar, R.; San Mateo, L.; Hornby, P.J.; Panettieri, R.A., Jr. TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L530–L537. [Google Scholar] [CrossRef] [PubMed]
- Koziol-White, C.J.; Jia, Y.; Baltus, G.A.; Cooper, P.R.; Zaller, D.M.; Crackower, M.A.; Sirkowski, E.E.; Smock, S.; Northrup, A.B.; Himes, B.E.; et al. Inhibition of spleen tyrosine kinase attenuates IgE-mediated airway contraction and mediator release in human precision cut lung slices. Br. J. Pharmacol. 2016, 173, 3080–3087. [Google Scholar] [CrossRef] [PubMed]
- Velalopoulou, A.; Tyagi, S.; Pietrofesa, R.A.; Arguiri, E.; Christofidou-Solomidou, M. The flaxseed-derived lignan phenolic secoisolariciresinol diglucoside (SDG) protects non-malignant lung cells from radiation damage. Int. J. Mol. Sci. 2015, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, J.C.; Scott, G.B.; Sutton, J.P. Space radiation: The number one risk to astronaut health beyond low earth orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, P.; Sanderson, M.J. Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices. Am. J. Respir. Cell Mol. Biol. 2006, 35, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Yanagawa, J.; Peebles, K.A.; Sharma, S.; Mao, J.T.; Dubinett, S.M. Inflammation in lung carcinogenesis: New targets for lung cancer chemoprevention and treatment. Crit. Rev. Oncol. Hematol. 2008, 66, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. Cytoplasmic functions of the tumour suppressor p53. Nature 2009, 458, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Oren, M. The first 30 years of p53: Growing ever more complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Chandler, H.; Peters, G. Stressing the cell cycle in senescence and aging. Curr. Opin. Cell Biol. 2013, 25, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Moritake, T.; Tsuchida, Y.; Tokuuye, K.; Matsumura, A.; Ando, K. Cell cycle checkpoint and apoptosis induction in glioblastoma cells and fibroblasts irradiated with carbon beam. J. Radiat. Res. 2007, 48, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, L.; Schulte, B.A.; Yang, A.; Tang, S.; Wang, G.Y. Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells. Int. J. Oncol. 2013, 43, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, A.; McDonald, D.G.; Riemer, E.C.; Vanek, K.N.; Schulte, B.A.; Wang, G.Y. MiR-34a modulates ionizing radiation-induced senescence in lung cancer cells. Oncotarget 2017, 8, 69797–69807. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Turowski, J.; Grieshaber, P.A.; Solomides, C.C.; Cengel, K.A. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG). Radiat. Res. 2012, 178, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Wilson, P.; Thieberger, P.; Lowenstein, D.; Wang, M.; Cucinotta, F.A. Biological characterization of low-energy ions with high-energy deposition on human cells. Radiat. Res. 2014, 182, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, M.F.; Welsh, J.; Godoy, M.C.; Betancourt, S.L.; Mawlawi, O.R.; Munden, R.F. New era of radiotherapy: An update in radiation-induced lung disease. Clin. Radiol. 2013, 68, e275–e290. [Google Scholar] [CrossRef] [PubMed]
- Remick, J.S.; Schonewolf, C.; Gabriel, P.; Doucette, A.; Levin, W.P.; Kucharczuk, J.C.; Singhal, S.; Pechet, T.T.V.; Rengan, R.; Simone, C.B., II; et al. First clinical report of proton beam therapy for postoperative radiotherapy for non-small-cell lung cancer. Clin. Lung Cancer 2017, 18, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Rwigema, J.M.; Verma, V.; Lin, L.; Berman, A.T.; Levin, W.P.; Evans, T.L.; Aggarwal, C.; Rengan, R.; Langer, C.; Cohen, R.B.; et al. Prospective study of proton-beam radiation therapy for limited-stage small cell lung cancer. Cancer 2017, 123, 4244–4251. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Krochak, R.; Blouin, A.; Kanterakis, S.; Chatterjee, S.; Arguiri, E.; Vachani, A.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol. Ther. 2009, 8, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Tan, K.S.; Hagan, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Heitjan, D.F.; Solomides, C.C.; Cengel, K.A. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer 2011, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Lafargue, A.; Degorre, C.; Corre, I.; Alves-Guerra, M.C.; Gaugler, M.H.; Vallette, F.; Pecqueur, C.; Paris, F. Ionizing radiation induces long-term senescence in endothelial cells through mitochondrial respiratory complex II dysfunction and superoxide generation. Free Radic. Biol. Med. 2017, 108, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Boerma, M.; Zhou, D. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat. Res. 2016, 186, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.T.; Teo, B.K.; Dolney, D.; Swisher-McClure, S.; Shahnazi, K.; Both, S.; Rengan, R. An in-silico comparison of proton beam and IMRT for postoperative radiotherapy in completely resected stage IIIA non-small cell lung cancer. Radiat. Oncol. 2013, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Andreo, P.; Burns, D.T.; Hohlfeld, K.; Huq, M.S.; Kanai, T.; Laitano, F.; Smyth, V.; Vynckier, S. Absorbed Dose Determination in External Beam Radiotherapy: An international Code of Practice for Dosimetry Based on Standards of Absorbed Dose to Water; Technical Report; IAEA: Vienna, Austria, 2000. [Google Scholar]
- Pietrofesa, R.A.; Velalopoulou, A.; Albelda, S.M.; Christofidou-Solomidou, M. Asbestos induces oxidative stress and activation of Nrf2 signaling in murine macrophages: Chemopreventive role of the synthetic lignan secoisolariciresinol diglucoside (LGM2605). Int. J. Mol. Sci. 2016, 17, 322. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velalopoulou, A.; Chatterjee, S.; Pietrofesa, R.A.; Koziol-White, C.; Panettieri, R.A.; Lin, L.; Tuttle, S.; Berman, A.; Koumenis, C.; Christofidou-Solomidou, M. Synthetic Secoisolariciresinol Diglucoside (LGM2605) Protects Human Lung in an Ex Vivo Model of Proton Radiation Damage. Int. J. Mol. Sci. 2017, 18, 2525. https://doi.org/10.3390/ijms18122525
Velalopoulou A, Chatterjee S, Pietrofesa RA, Koziol-White C, Panettieri RA, Lin L, Tuttle S, Berman A, Koumenis C, Christofidou-Solomidou M. Synthetic Secoisolariciresinol Diglucoside (LGM2605) Protects Human Lung in an Ex Vivo Model of Proton Radiation Damage. International Journal of Molecular Sciences. 2017; 18(12):2525. https://doi.org/10.3390/ijms18122525
Chicago/Turabian StyleVelalopoulou, Anastasia, Shampa Chatterjee, Ralph A. Pietrofesa, Cynthia Koziol-White, Reynold A. Panettieri, Liyong Lin, Stephen Tuttle, Abigail Berman, Constantinos Koumenis, and Melpo Christofidou-Solomidou. 2017. "Synthetic Secoisolariciresinol Diglucoside (LGM2605) Protects Human Lung in an Ex Vivo Model of Proton Radiation Damage" International Journal of Molecular Sciences 18, no. 12: 2525. https://doi.org/10.3390/ijms18122525