Effect of Dietary Acidolysis-Oxidized Konjac Glucomannan Supplementation on Serum Immune Parameters and Intestinal Immune-Related Gene Expression of Schizothorax prenanti
Abstract
:1. Introduction
2. Results
2.1. Visceral Index
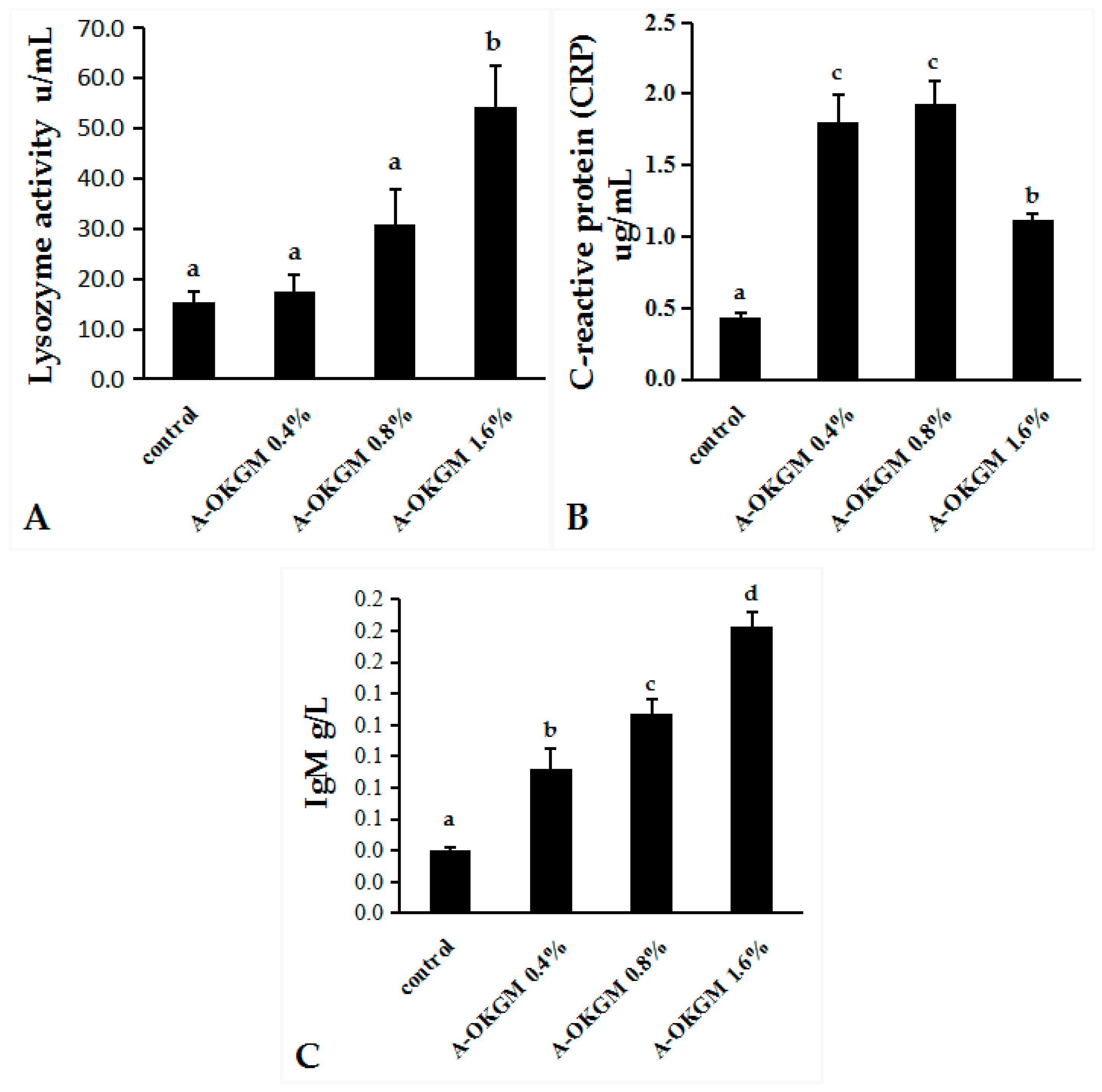
2.2. Immune Parameters
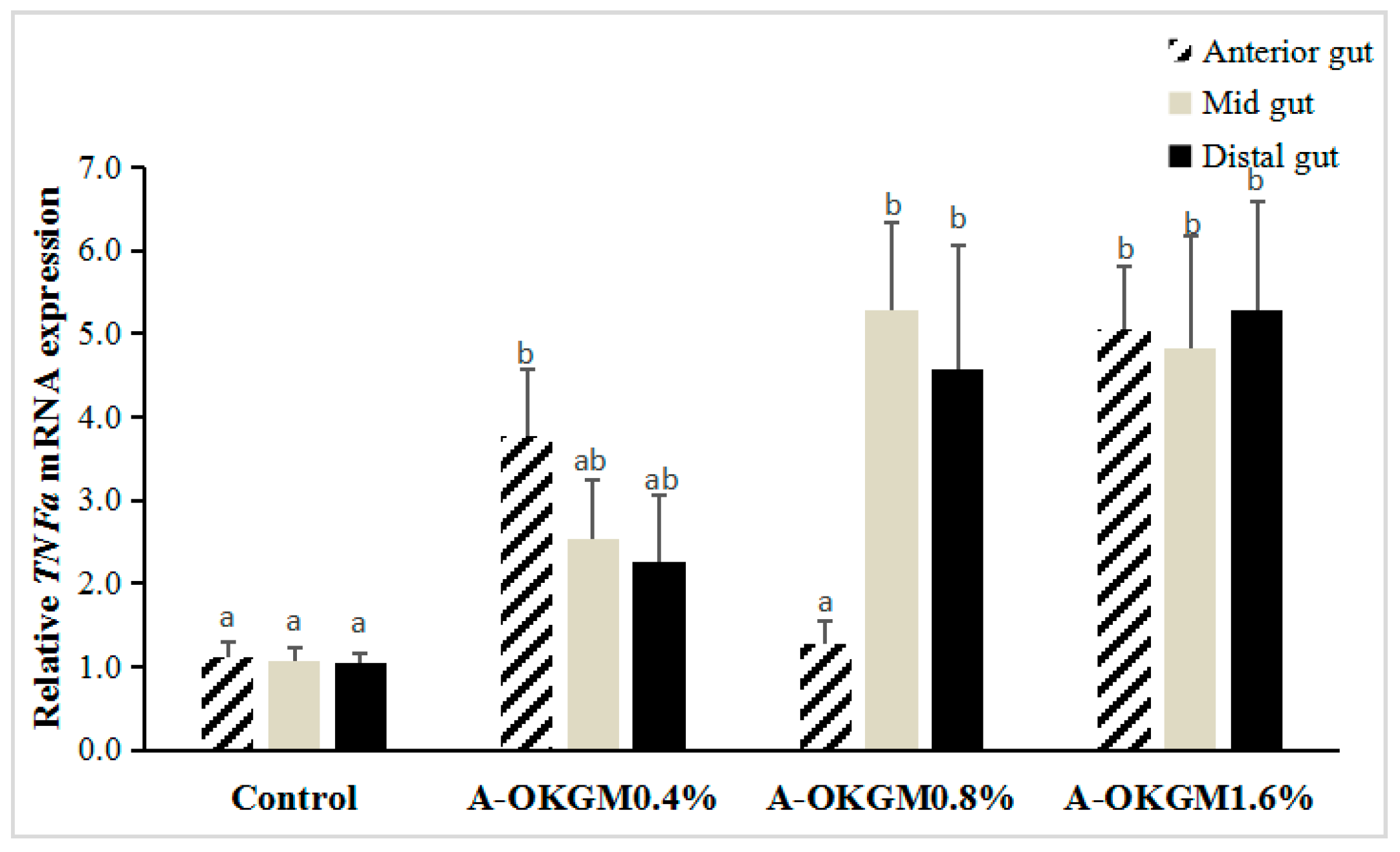
2.3. S. prenanti Tumor Necrosis Factor α (TNFα) mRNA Expression
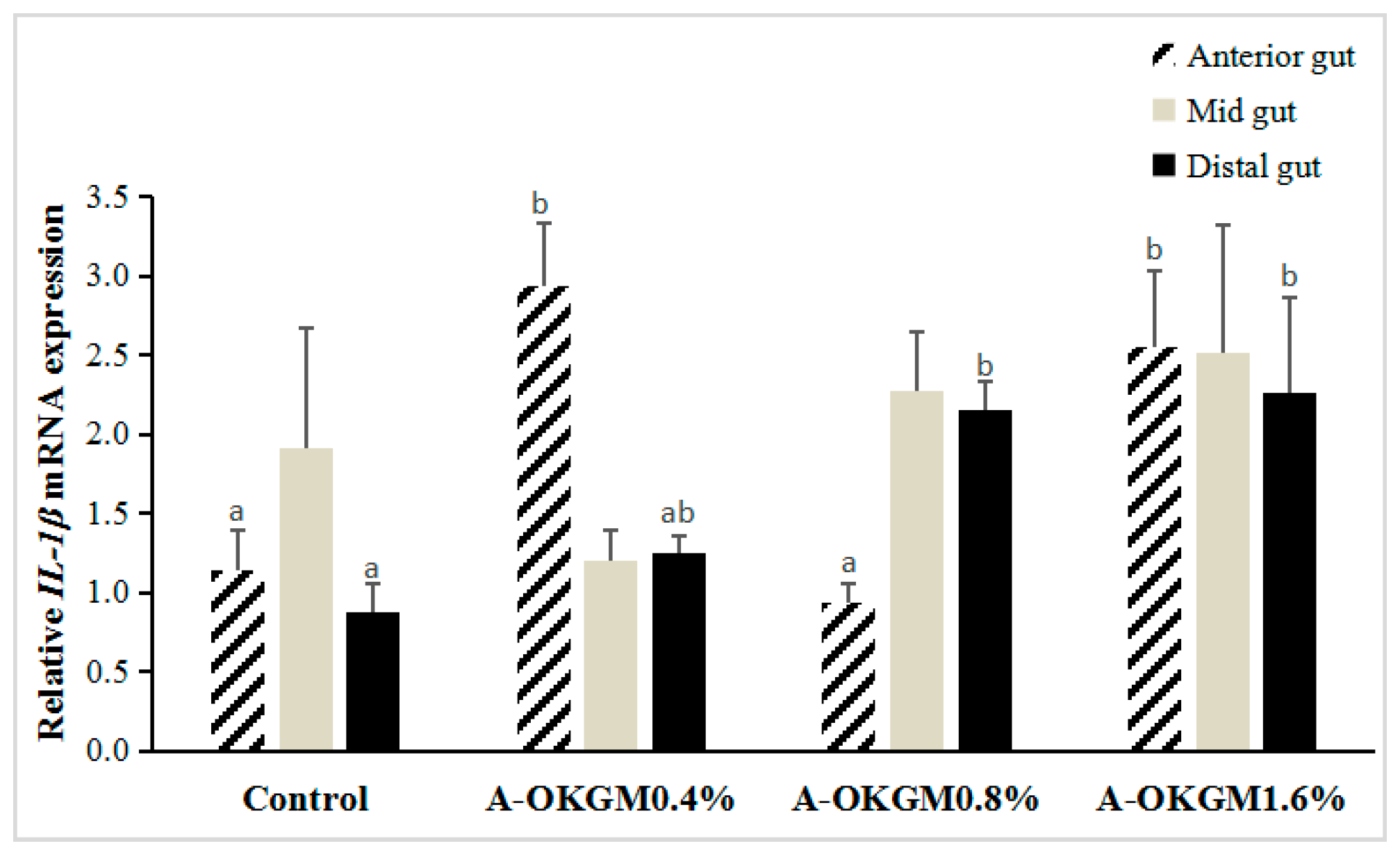
2.4. S. prenanti Interleukin (IL)-1β (IL-1β) mRNA Expression
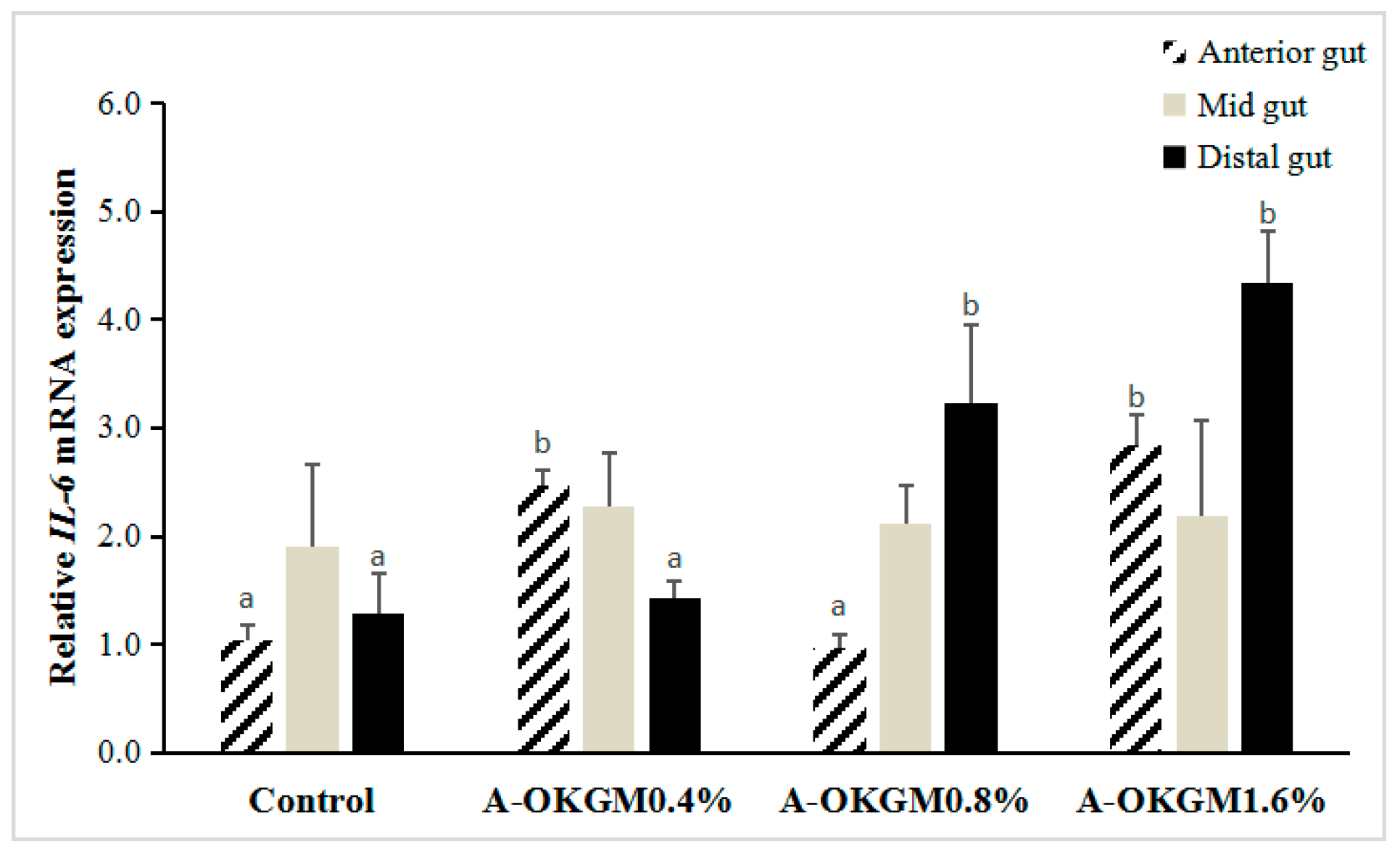
2.5. S. prenanti Interleukin-6 (IL-6) mRNA Expression
3. Discussion
4. Materials and Methods
4.1. Preparation of A-OKGM
4.2. Composition of Diets
4.3. Feeding Trials
4.4. Sampling
4.5. Biochemical Analysis
- Spleen index = weight of spleen/body weight.
- Head kidney index = weight of head kidney/body weight.
- Gut index = weight of gut/body weight.
- Mesonephros index = weight of mesonephros/body weight.
- Liver index = weight of liver/body weight.
4.6. RT-qPCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mu, X.J.; Pridgeon, J.W.; Klesius, P.H. Comparative transcriptional analysis reveals distinct expression patterns of channel catfish genes after the first infection and re-infection with Aeromonas hydrophila. Fish Shellfish Immunol. 2013, 35, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.M.; Dehler, C.E.; Król, E. Transcriptomic responses in the fish intestine. Dev. Comp. Immunol. 2016, 64, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I.; Parra, D. Fish mucosal immunity: Intestine. In Mucosal Health in Aquaculture; Beck, B.H., Peatman, E., Eds.; Academic Press: London, UK, 2015; pp. 135–171. [Google Scholar]
- Merrifield, D.L.; Rodiles, A. The fish microbiome and its interactions with mucosal tissues. In Mucosal Health in Aquaculture; Beck, B.H., Peatman, E., Eds.; Academic Press: London, UK, 2015; pp. 273–295. [Google Scholar]
- Rurangwa, E.; Sipkema, D.; Kals, J.; Ter, V.M.; Forlenza, M.; Bacanu, G.M.; Smidt, H.; Palstra, A.P. Impact of a novel protein meal on the gastrointestinal microbiota and the host transcriptome of larval zebrafish Danio rerio. Front Physiol. 2015, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, M.S.; Boutin, S.; Hoseinifar, S.H.; Derome, N. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 2014, 5, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.C.; Buentello, J.A.; Gatlin, D.M., III. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum (Sciaenops ocellatus). Aquaculture 2010, 309, 253–257. [Google Scholar] [CrossRef]
- Munir, M.B.; Hashim, R.; Chai, Y.H.; Marsh, T.L.; Nor, S.A.M. Dietary prebiotics and probiotics influence growth performance, nutrient digestibility and the expression of immune regulatory genes in snakehead (Channa striata) fingerlings. Aquaculture 2016, 460, 59–68. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Basuini, M.F.E.; Hossain, M.S. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2015, 43, 385–387. [Google Scholar] [CrossRef]
- Chen, M.R.; Wang, H.J.; Yan, Q.P.; Zheng, Q.R.; Yang, M. Effects of dietary oxidized konjac glucomannan sulfates (OKGMS) and acidolysis-oxidized konjac glucomannan (A-OKGM) on the immunity and expression of immune-related genes of Schizothorax prenanti. Fish Shellfish Immunol. 2016, 56, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Testerv, R.F.; Al-Ghazzewi, F.H. Mannans and health, with a special focus on glucomannans. Food Res. Int. 2013, 50, 384–391. [Google Scholar] [CrossRef]
- Pourabedin, M.; Zhao, X. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S. Recent advances in the role of probiotics and prebiotics in carp aquaculture: A review. Aquaculture 2016, 454, 243–251. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xie, B.J.; Xin, G. Advance in the applications of konjac glucomannan and its derivatives. Carbohydr. Polym. 2005, 60, 27–31. [Google Scholar] [CrossRef]
- Man, X.; Li, W.; Corke, H.; Yan, W.; Ni, X.; Fang, Y.; Jiang, F. Characterization of konjac glucomannan-ethyl cellulose film formation via microscopy. Int. J. Biol. Macromol. 2016, 85, 434–441. [Google Scholar]
- Zhang, L.; Wu, Y.; Wang, L.; Wang, H. Effects of Oxidized Konjac glucomannan (OKGM) on growth and immune function of Schizothorax prenanti. Fish Shellfish Immunol. 2013, 35, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Onishi, N.; Kawamoto, S.; Ueda, K.; Yamanaka, Y.; Katayama, A.; Suzuki, H.; Aki, T.; Hashimoto, K.; Hide, M.; Ono, K. Dietary pulverized konjac glucomannan prevents the development of allergic rhinitis-like symptoms and IgE response in mice. Biosci. Biotechnol. Biochem. 2007, 71, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Fan, Y.H.; Chen, M.E.; Chan, Y. Unhydrolyzed and hydrolyzed konjac glucomannans modulated cecal and fecal microflora in Balb/c mice. Nutrition 2005, 21, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.E. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef]
- Iwama, G.; Nakanishi, T. The fish immune system: Organism, pathogen, and environment. Fish Physiol. 1996, 15, 395. [Google Scholar]
- Caballero, M.J.; Montero, D. Reduced gut bacterial translocation in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS). Fish Shellfish Immunol. 2011, 30, 674–681. [Google Scholar]
- Staykov, Y.; Spring, P.; Denev, S.; Sweetman, J. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2007, 15, 153–161. [Google Scholar] [CrossRef]
- Liu, B.; Xu, L.; Ge, X.; Xie, J.; Xu, P.; Zhou, Q.; Pan, L.; Zhang, Y.Y. Effects of mannan oligosaccharide on the physiological responses, HSP70 gene expression and disease resistance of Allogynogenetic crucian carp (Carassius auratus gibelio) under Aeromonas hydrophila infection. Fish Shellfish Immunol. 2013, 34, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Misra, C.K.; Das, B.K.; Mukherjee, S.C.; Pattnaik, P. Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquaculture 2006, 255, 82–94. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and β-glucan on growth performance, digestibility and immune response of juvenile red sea bream, Pagrus major. Fish Shellfish Immunol. 2015, 45, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J.R.; Tharia, H.A.; Burns, I.; Shrive, A.K.; Hoole, D.; Greenhough, T.J. Isolation and characterisation of pentraxin-like serum proteins from the common carp Cyprinus carpio. Dev. Comp. Immunol. 2004, 28, 113–125. [Google Scholar] [CrossRef]
- Pepys, M.B.; Dash, A.C.; Fletcher, T.C.; Richardson, N.; Munn, E.A.; Feinstein, A. Analogues in other mammals and in fish of human plasma proteins, C-reactive protein and amyloid P component. Nature 1978, 273, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Gauldie, J. The acute phase response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef]
- Bayne, C.J.; Gerwick, L. The acute phase response and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 725–743. [Google Scholar] [CrossRef]
- Kodama, H.; Matsuoka, Y.; Tanaka, Y. Changes of C-reactive protein levels in rainbow trout (Oncorhynchus mykiss) sera after exposure to anti-ectoparasitic chemicals used in aquaculture. Fish Shellfish Immunol. 2004, 16, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Szalai, A.J.; Bly, J.E.; Clem, L.W. Changes in serum concentrations of channel catfish (Ictalurus punctatus Rafinesque) phosphorylcholine-reactive protein (PRP) in response to inflammatory agents, low temperature-shock and infection by the fungus Saprolegnia sp. Fish Shellfish Immunol. 1994, 4, 323–336. [Google Scholar] [CrossRef]
- Winkelhake, J.L.; Chang, R.J. Acute phase (C-reactive) protein-like macromolecules from rainbow trout (Salmo gairdneri). Dev. Comp. Immunol. 1982, 6, 481–489. [Google Scholar] [CrossRef]
- Kodama, H.; Yamada, F.; Murai, T.; Nakanishi, Y.; Mikami, T.; Izawa, H. Activation of trout macrophages and production of CRP after immunization with Vibrio angillarum. Dev. Comp. Immunol. 1989, 13, 123–132. [Google Scholar] [CrossRef]
- Pionnier, N.; Falco, A.; Miest, J.; Frost, P.; Irnazarow, I.; Shrive, A.; Hoole, D. Dietary β-glucan stimulate complement and C-reactive protein acute phase responses in common carp (Cyprinus carpio) during an Aeromonas salmonicida infection. Fish Shellfish Immunol. 2013, 34, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Chen, C.; Li, J.; Sun, L. The C-reactive protein of tongue sole Cynoglossus semilaevis is an acute phase protein that interacts with bacterial pathogens and stimulates the antibacterial activity of peripheral blood leukocytes. Fish Shellfish Immunol. 2013, 34, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Pionnier, N.; Falco, A.; Miest, J.J.; Shrive, A.K.; Hoole, D. Feeding common carp Cyprinus carpio with β-glucan supplemented diet stimulates C-reactive protein and complement immune acutephase responses following PAMPs injection. Fish Shellfish Immunol. 2014, 39, 285–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hordvik, I.; Kamil, A.; Bilal, S.; Raae, A.; Fjelldal, P.G.; Koppang, E.O. Characterization of serum IgM in teleost fish, with emphasis on salmonids. Fish Shellfish Immunol. 2013, 34, 1656. [Google Scholar] [CrossRef]
- Oppenheim, J.J.; Kovacs, E.J.; Matsushima, K.; Durum, S.K. There is more than one interleukin 1. Immunol. Today 1986, 7, 45–56. [Google Scholar] [CrossRef]
- Pleguezuelos, O.; Zou, J.; Cunningham, C.; Secombes, C.J. Cloning, sequencing, and analysis of expression of a second gene in rainbow trout (Oncorhynchus mykiss). Immunogenetics 2000, 51, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.E.; Ziemer, C.J.; Kerr, B.J. Effects of adding fibrous feedstuffs to the diet of young pigs on growth performance, intestinal cytokines, and circulating acute-phase proteins. J. Anim. Sci. 2008, 86, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Pietretti, D.; Vera-Jimenez, N.I.; Hoole, D.; Wiegertjes, G.F. Oxidative burst and nitric oxide responses in carp macrophages induced by zymosan, MacroGard® and selective dectin-1 agonists suggest recognition by multiple pattern recognition receptors. Fish Shellfish Immunol. 2013, 35, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Caipang, C.M.A.; Lazado, C.C.; Brinchmann, M.F.; Kiron, V. Transcription of selected immune-related genes in spleen cells of cod, Gadus morhua following incubation with alginic acid and β-glucan. J. Exp. Mar. Biol. Ecol. 2012, 416, 202–207. [Google Scholar] [CrossRef]
- Guzmán-Villanueva, L.T.; Tovar-Ramírez, D.; Gisbert, E.; Cordero, H.; Guardiola, F.A.; Cuesta, A.; Meseguer, J.; Ascencio-Valle, F.; Esteban, M.A. Dietary administration of β-1,3/1,6-glucan and probiotic strain Shewanella putrefaciens, single or combined, on gilthead seabream growth, immune responses and gene expression. Fish Shellfish Immunol. 2014, 39, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Naka, T.; Nishimoto, N.; Kishimoto, T. The paradigm of IL-6: From basic science to medicine. Arthritis Res. 2002, 4, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Hirano, T.; Taga, T.; Kishimoto, T. Biology of multifunctional cytokines: IL-6 and related molecules (IL-1 and TNF). FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1990, 4, 2860–2867. [Google Scholar]
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Hirano, T. Interleukin 6 and its receptor: Ten years later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Tatter, S.B.; Santhanam, U.; Helfgott, D.C.; May, L.T.; Sehgal, P.B. Regulation of expression of interleukin-6. Mol. Clin. Stud. Ann. N. Y. Acad. Sci. 1989, 557, 353–362. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Diaz, D.A.; Schmidt, J.G.; Vera-Jiménez, N.I.; Steinhagen, D.; Nielsen, M.E. β-glucan enriched bath directly stimulates the wound healing process in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2013, 35, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Raida, M.K.; Buchmann, K. Development of adaptive immunity in rainbow trout, Oncorhynchus mykiss (Walbaum) surviving an infection with Yersinia ruckeri. Fish Shellfish Immunol. 2008, 25, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, R.D.; Buchmann, K. Inflammatory response of rainbow trout Oncorhynchus mykiss (Walbaum, 1792) larvae against Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2013, 34, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.N.; Zhang, J.L.; Liu, W.B.; Wu, Q.J.; Gao, X.C.; Ren, H.T. Cloning, characterization and mRNA expression of interleukin-6 in blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2016, 54, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Kevin, O.K.; Michael, H.; Gill, D.; Leonid, H. Tumor necrosis factor alpha stimulates killing of mycobacterium tuberculosis by human neutrophils. Infect. Immun. 2002, 70, 4591–4599. [Google Scholar]
- Cunha, F.Q.; Assreuy, J.; Moss, D.W.; Rees, D.; Leal, L.M.; Moncada, S.; Carrier, M.; O’Donnell, C.A.; Liew, F.Y. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: Role of TNF-α and IL-1-β. Immunology 1994, 81, 211–215. [Google Scholar] [PubMed]
- Secombes, C.J.; Wang, T.; Hong, S.; Peddie, S.; Crampe, M.; Laing, K.J.; Cunningham, C.; Zou, J. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 713–723. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J.H.; Shen, Z.Y.; Fei, Y.; Chen, X.D. Analysis of chemical composition, structure of Grifola frondosa polysaccharides and its effect on skin TNF-α levels, lgG content, T lymphocytes rate and caspase-3 mRNA. Carbohydr. Polym. 2010, 82, 687–691. [Google Scholar] [CrossRef]
- Yuan, C.; Pan, X.; Yi, G.; Xia, A.; Wu, G.; Tang, J.; Han, X. Effects of Astragalus polysaccharides (APS) on the expression of immune response genes in head kidney, gill and spleen of the common carp, Cyprinus carpio, L. Int. Immunopharmacol. 2008, 8, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, P.Y.; Farahmand, H.; Miandare, H.K.; Mirvaghefi, A.; Hoseinifar, S.H. The effects of dietary Immunogen® on innate immune response, immune related genes expression and disease resistance of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2014, 37, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Svanborg, C.; Godaly, G.; Hedlund, M. Cytokine responses during mucosal infections: Role in disease pathogenesis and host defence. Curr. Opin. Microbiol. 1999, 2, 99–105. [Google Scholar] [CrossRef]
- Devries, M.E.; Ran, L.; Kelvin, D.J. On the edge: The physiological and pathophysiological role of chemokines during inflammatory and immunological responses. Semin. Immunol. 1999, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
| Group | Control | Acidolysis-Oxidized Konjac Glucomannan (A-OKGM) | ||
|---|---|---|---|---|
| 1 (0) | 2 (0.4%) | 3 (0.8%) | 4 (1.6%) | |
| Head kidney index | 0.0003 ± 0.00005 | 0.0004 ± 0.0001 | 0.0003 ± 0.00003 | 0.0003 ± 0.00002 |
| Mesonephros index | 0.0023 ± 0.00028 | 0.0026 ± 0.00031 | 0.0032 ± 0.00021 | 0.0027 ± 0.00044 |
| Spleen index | 0.0014 ± 0.00013 a | 0.0011 ± 0.00024 a | 0.0011 ± 0.00024 a | 0.0023 ± 0.00036 b |
| Gut index | 0.0122 ± 0.00112 | 0.0118 ± 0.00056 | 0.0127 ± 0.00111 | 0.0139 ± 0.00033 |
| Liver index | 0.0140 ± 0.0007 a | 0.0147 ± 0.00005 a | 0.0166 ± 0.00031 b | 0.0151 ± 0.00023 a |
| Diets Component | Control | A-OKGM | ||
|---|---|---|---|---|
| Formulation (%) | A 0% | A 0.4% | A 0.8% | A 1.6% |
| A-OKGM | 0 | 0.40 | 0.80 | 1.60 |
| Fish meal | 42.00 | 42.00 | 42.00 | 42.00 |
| Rapeseed oil | 3.00 | 3.00 | 3.00 | 3.00 |
| Soybean meal | 21.00 | 21.00 | 21.00 | 21.00 |
| Flour | 20.00 | 20.00 | 20.00 | 20.00 |
| Starch | 10.00 | 9.60 | 9.20 | 8.40 |
| Bran | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin premix + choline a | 0.50 | 0.50 | 0.50 | 0.50 |
| Mineral premix b | 1.00 | 1.00 | 1.00 | 1.00 |
| Ca(H2PO4)2 | 1.50 | 1.50 | 1.50 | 1.50 |
| Total | 100 | 100 | 100 | 100 |
| Nutrition level (%) | ||||
| Crude protein | 35.2 | 35.2 | 35.2 | 35.2 |
| Crude lipid | 8.19 | 8.19 | 8.19 | 8.19 |
| TE (MJ/kg) c | 16.53 | 16.46 | 16.39 | 16.31 |
| Ca | 2.02 | 2.02 | 2.02 | 2.02 |
| P | 1.52 | 1.52 | 1.52 | 1.52 |
| Lys | 2.87 | 2.87 | 2.87 | 2.87 |
| Met + Cys | 1.32 | 1.32 | 1.32 | 1.32 |
| Genes | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Product Size (bp) | Annealing Temperature |
|---|---|---|---|---|
| TNFα | TGTCTGCTTCACGCTCAACA | AATGGATGGCWGCCTTGGA | 116 | 59 °C |
| IL-1β | GGTGGTGAACATCATCATTGC | AGACGCTCTTCGATCACATTC | 120 | 55.7 °C |
| IL-6 | CCACCTGTAACCATAAGAAAAGAAC | TTGCTCAAAATCTGTCCCCAT | 120 | 59.5 °C |
| β-actin | GATTCGCTGGAGATGATGCT | CGTTGTAGAAGGTGTGATGCC | 218 | 55.8 °C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wang, S.; Liang, X.; Ma, D.; He, L.; Liu, Y. Effect of Dietary Acidolysis-Oxidized Konjac Glucomannan Supplementation on Serum Immune Parameters and Intestinal Immune-Related Gene Expression of Schizothorax prenanti. Int. J. Mol. Sci. 2017, 18, 2558. https://doi.org/10.3390/ijms18122558
Chen M, Wang S, Liang X, Ma D, He L, Liu Y. Effect of Dietary Acidolysis-Oxidized Konjac Glucomannan Supplementation on Serum Immune Parameters and Intestinal Immune-Related Gene Expression of Schizothorax prenanti. International Journal of Molecular Sciences. 2017; 18(12):2558. https://doi.org/10.3390/ijms18122558
Chicago/Turabian StyleChen, Mingrui, Shuyao Wang, Xue Liang, Donghui Ma, Li He, and Yaowen Liu. 2017. "Effect of Dietary Acidolysis-Oxidized Konjac Glucomannan Supplementation on Serum Immune Parameters and Intestinal Immune-Related Gene Expression of Schizothorax prenanti" International Journal of Molecular Sciences 18, no. 12: 2558. https://doi.org/10.3390/ijms18122558




