Lipopolysaccharide Modifies Glycerol Permeability and Metabolism in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Results
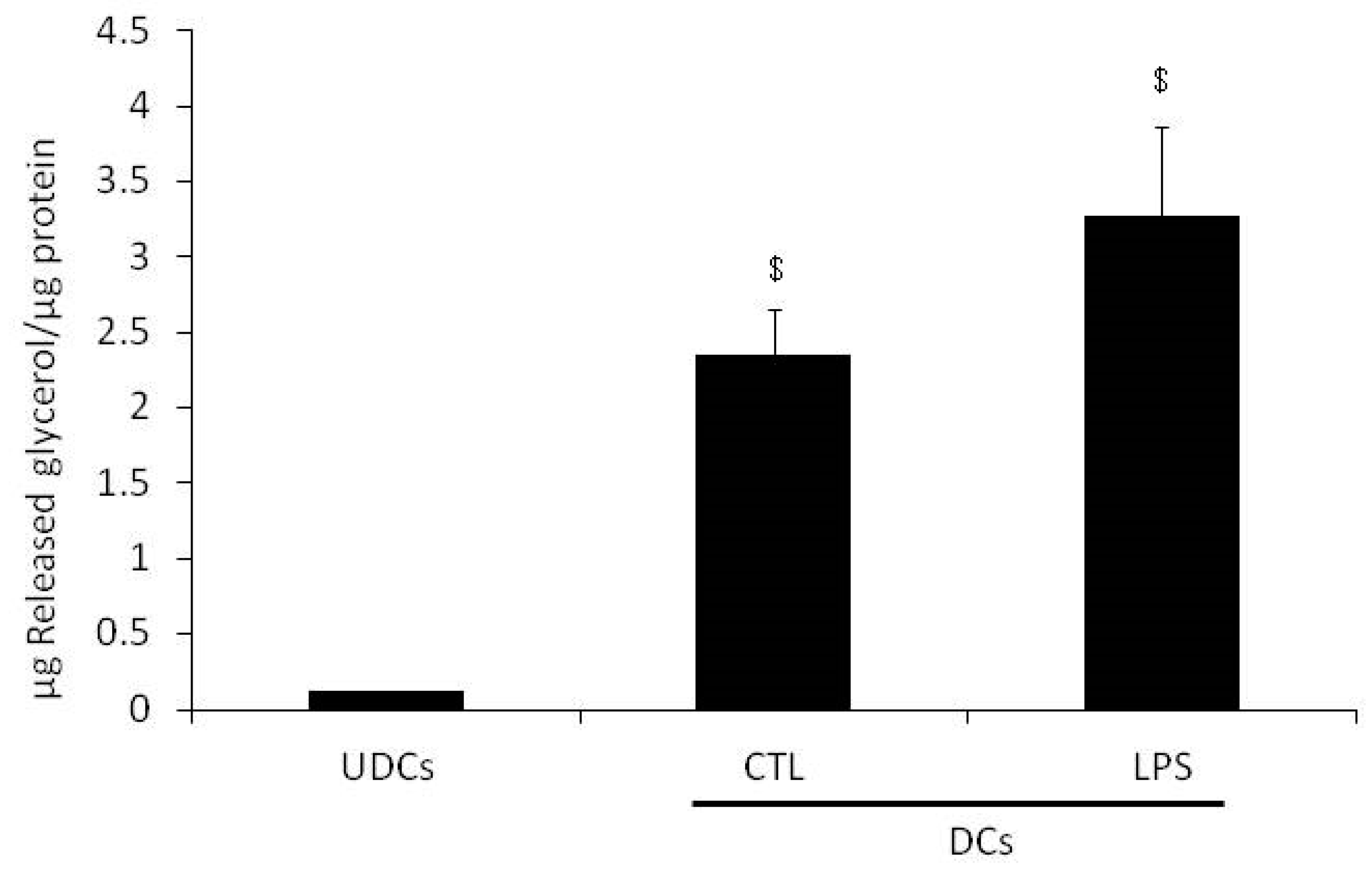
2.1. Glycerol Release and Triacylglycerols (TAGs) Content in Undifferentiated 3T3-L1 Cells (UDCs), Untreated 3T3-L1 Cells Differentiated into Adipocytes (CTL-DCs) and Lipopolysaccharide-treated 3T3-L1 Cells Differentiated into Adipocytes (LPS-DCs)
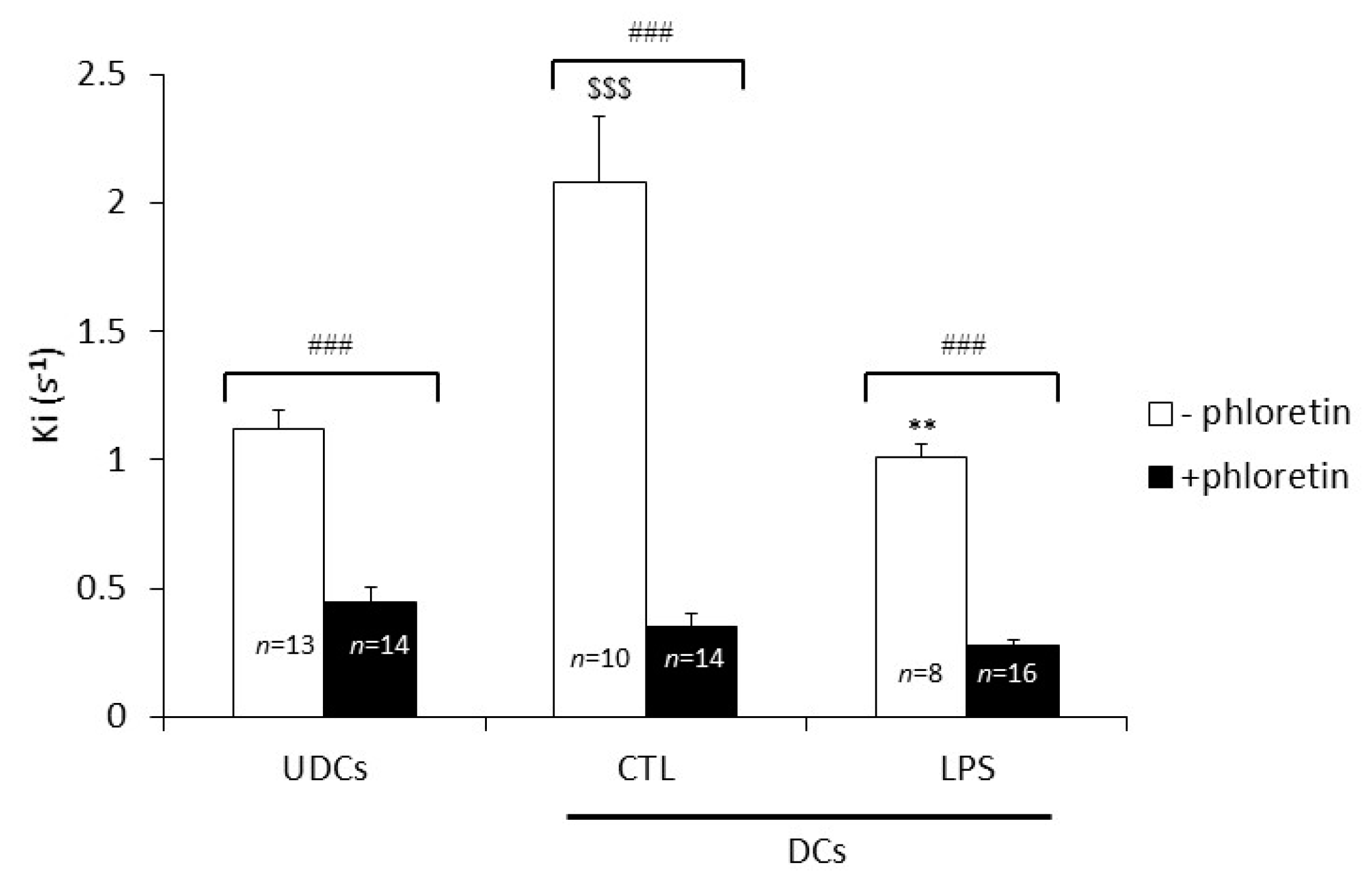
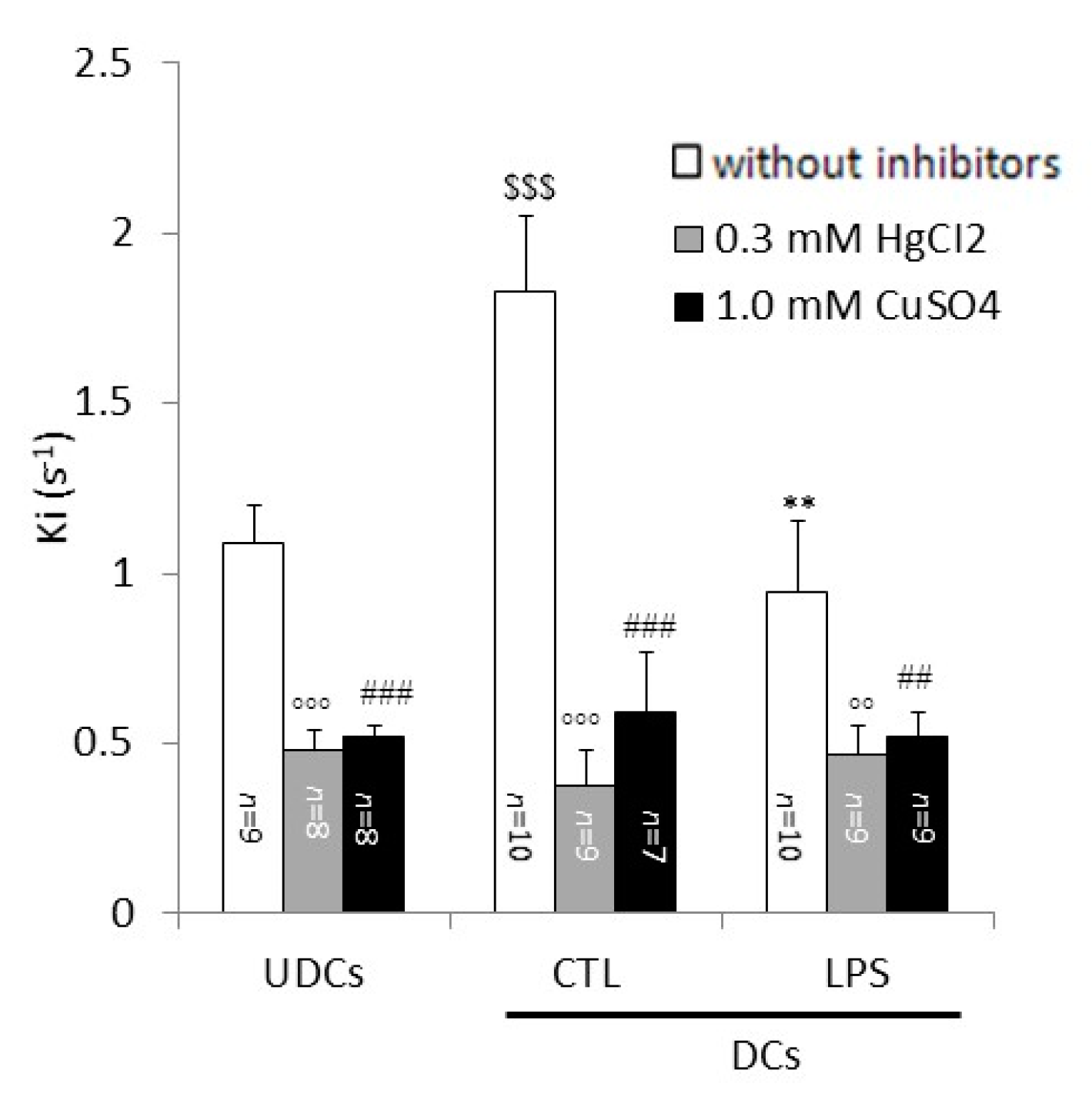
2.2. Biophysical Analysis of UDCs, Untreated DCs and LPS-Treated DCs Membrane Permeability to Glycerol
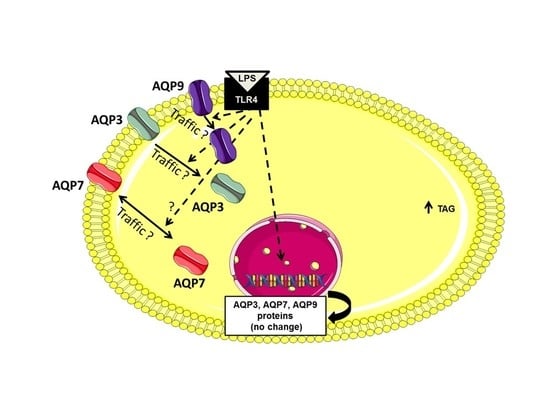
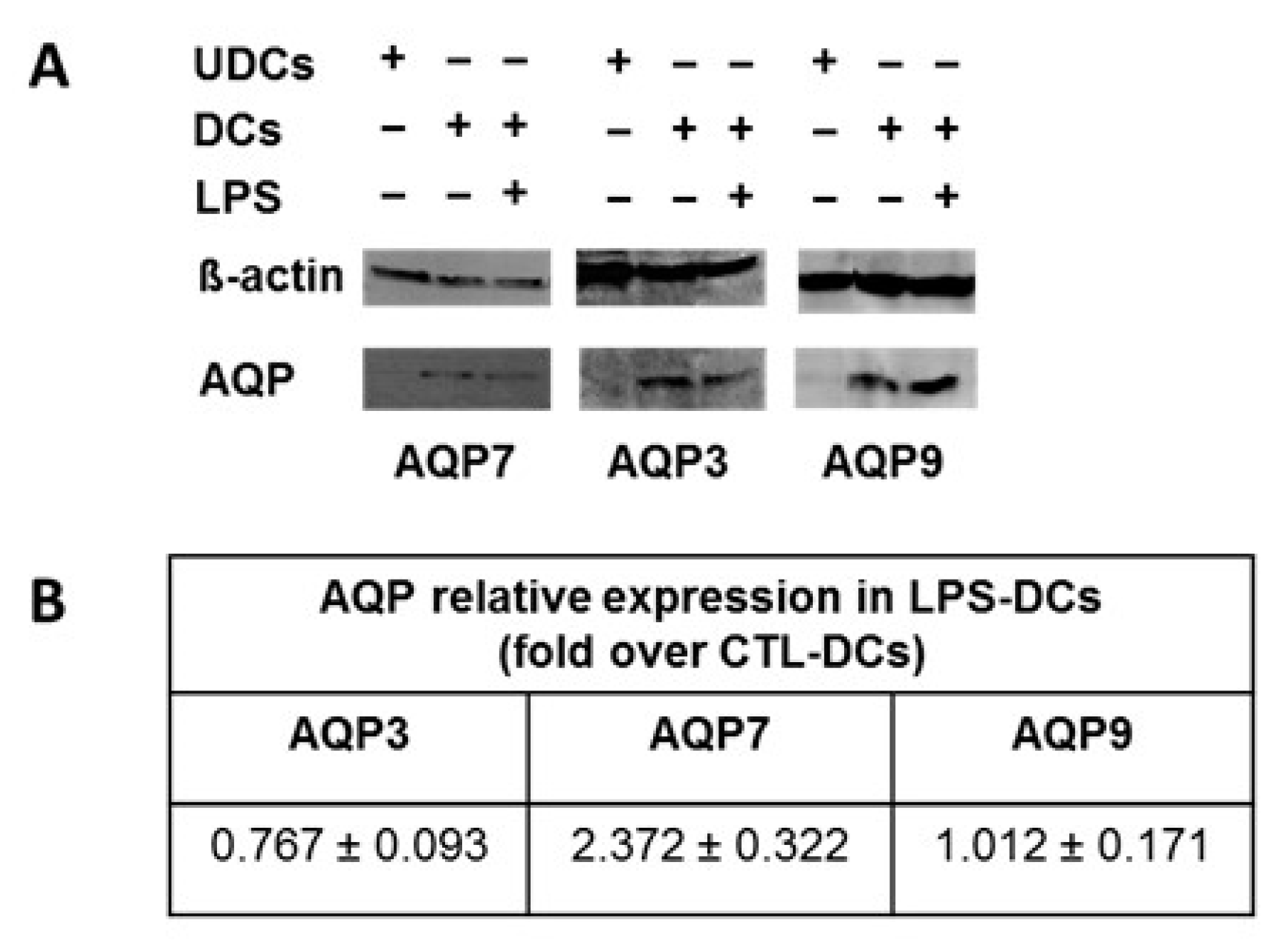
2.3. AQP7, AQP9 and AQP3 Protein Expression in UDCs, CTL-DCs and LPS-DCs
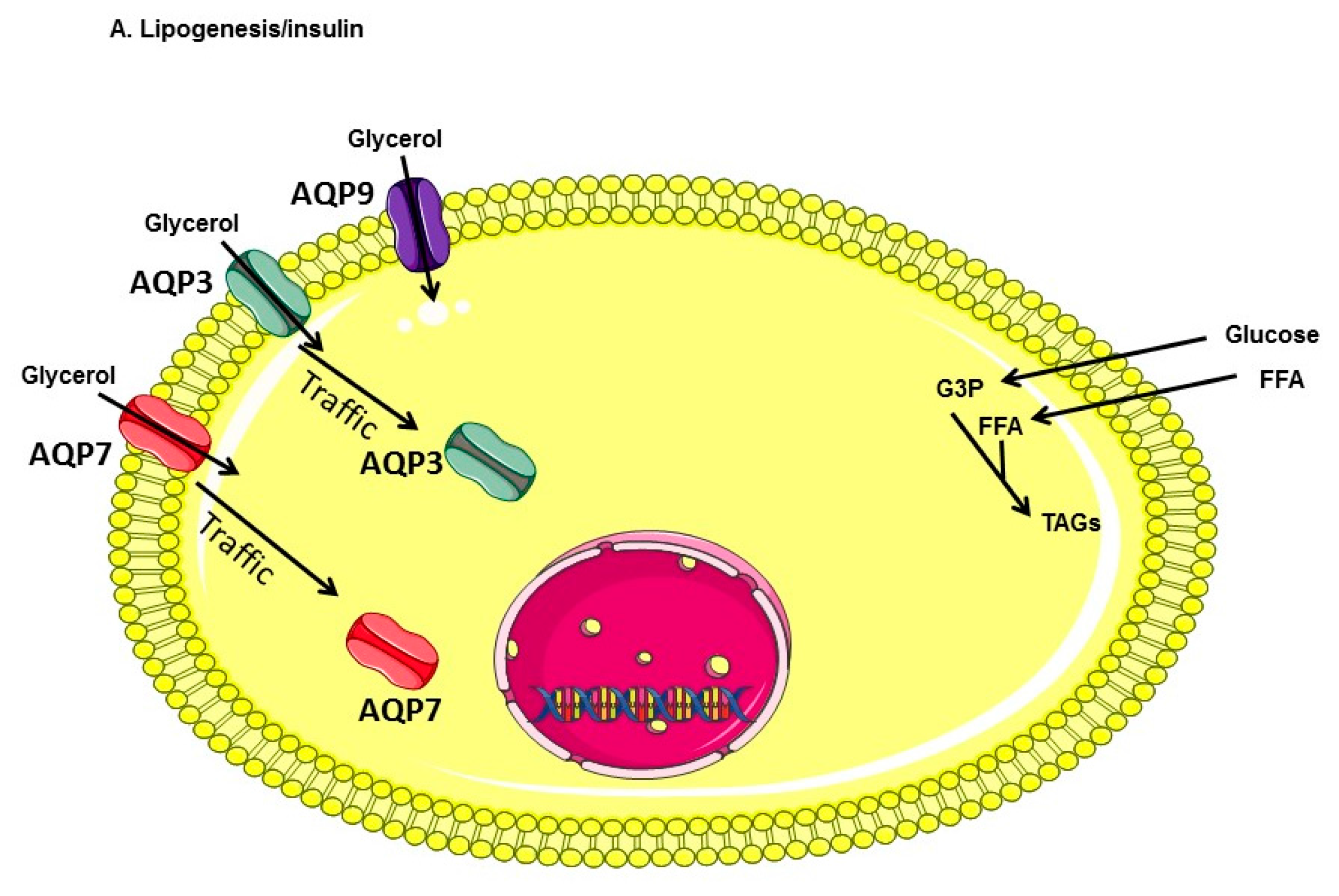
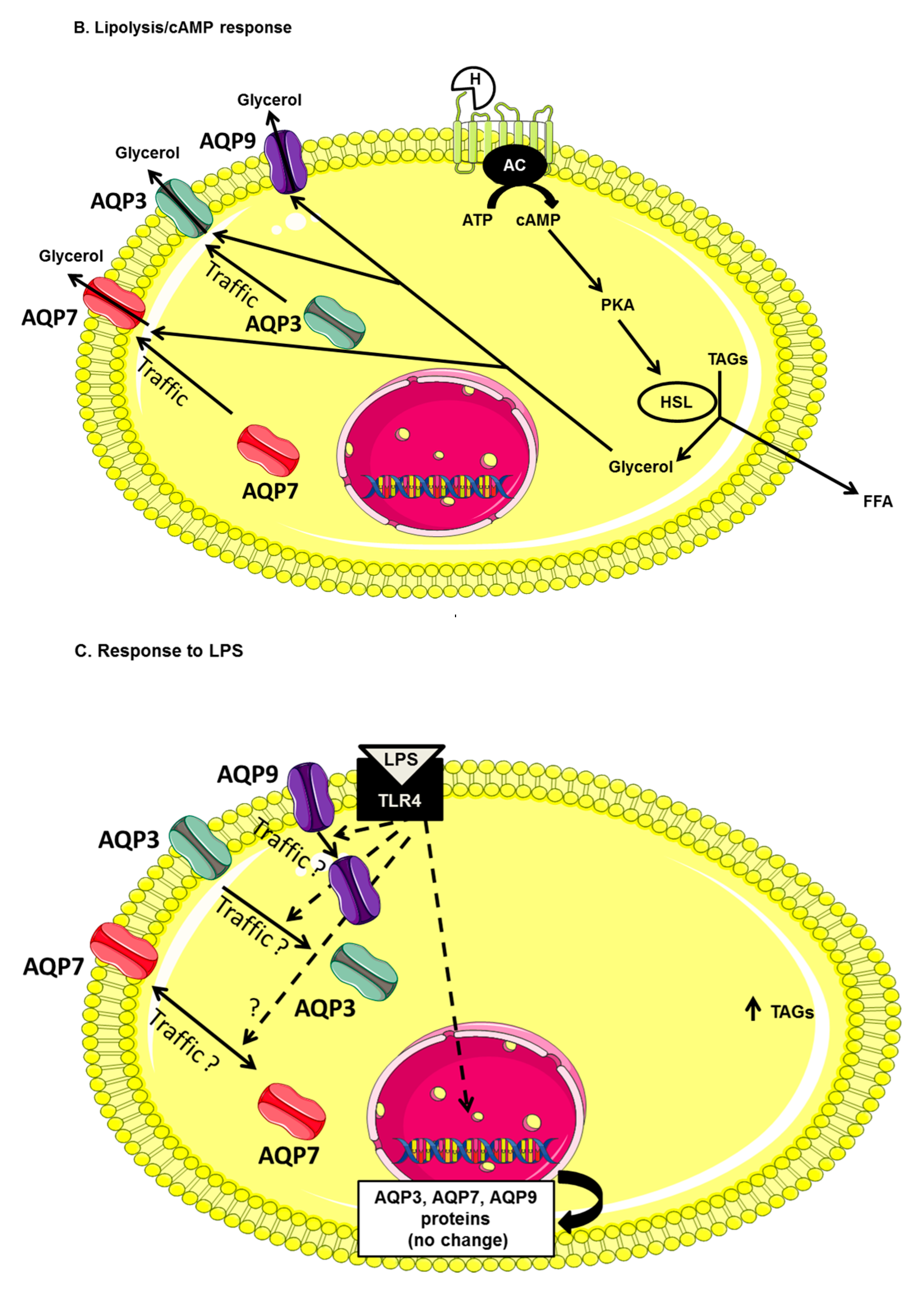
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cells Culture
4.3. Glycerol Release Measurement
4.4. Triglyceride Measurement
4.5. Western Blot Analysis
4.6. Preparation of 3T3-L1 Plasma Membrane Vesicles
4.7. Stopped-Flow Light Scattering Measurement of Glycerol Permeability
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQP | Aquaporin |
| CTL | Control |
| DCs | 3T3-L1 cells differentiated into adipocytes |
| LPS | Lipopolysaccharide |
| UDCs | Undifferentiated 3T3-L1 cells |
| TAGs | Triacylglycerols |
| cAMP | Cyclic adenosine monophosphate |
References
- Crossno, J.T.; Majka, S.M.; Grazia, T.; Gill, R.G.; Klemm, D.J. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J. Clin. Investig. 2006, 116, 3220–3228. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Human fat cell lipolysis: Biochemistry, regulation and clinical role. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G. Obesity: Aquaporin enters the picture. Nature 2005, 438, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G. Role of aquaporin-7 in the pathophysiological control of fat accumulation in mice. FEBS Lett. 2006, 580, 4771–4776. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A. Aquaporin-7 and glycerol permeability as novel obesity drug-target pathways. Trends Pharmacol. Sci. 2006, 27, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y. Therapy Insight: Adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Funahashi, T.; Shimomura, I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Hibuse, T.; Maeda, N.; Nagasawa, A.; Funahashi, T. Aquaporins and glycerol metabolism. Biochim. Biophys. Acta 2006, 1758, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Marinelli, R.A.; Tesse, A.; Frühbeck, G.; Calamita, G. Sexual Dimorphism of Adipose and Hepatic Aquaglyceroporins in Health and Metabolic Disorders. Front. Endocrinol. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2004, 43, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Bottino, C.; Gastaldi, G. Mammalian aquaglyceroporin function in metabolism. Biochim. Biophys. Acta 2016, 1858, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Praetorius, J.; Frøkiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K. New members of mammalian aquaporins: AQP10-AQP12. Handb. Exp. Pharmacol. 2009, 190, 251–262. [Google Scholar]
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafré, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obes. Silver Spring 2014, 22, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Moura, T.F.; Soveral, G. Aquaglyceroporins: Implications in adipose biology and adiposity. Cell. Mol. Life Sci. 2015, 72, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.V.; Soveral, G. Aquaporins in obesity. Adv. Exp. Med. Biol. 2017, 969, 227–238. [Google Scholar] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Physiological roles of glycerol-transporting aquaporins: The aquaglyceroporins. Cell. Mol. Life Sci. 2006, 63, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Escoté, X.; Ceperuelo-Mallafré, V.; Alcaide, M.J.; Simón, I.; Vilarrasa, N.; Wabitsch, M.; Vendrell, J. Paired subcutaneous and visceral adipose tissue aquaporin-7 expression in human obesity and type 2 diabetes: Differences and similarities between depots. J. Clin. Endocrinol. Metab. 2010, 95, 3470–3479. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, T.; Gregoire, F.; Chiadak, J.; Courtequisse, E.; Bolaky, N.; Perret, J.; Delporte, C. Pituitary adenylate cyclase activating peptide (PACAP) participates in adipogenesis by activating ERK signaling pathway. PLoS ONE 2013, 8, e72607. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Yamamoto, H.; Abe, Y.; Yoshida, G.J.; Rojek, A.; Sohara, E.; Uchida, S.; Nielsen, S.; Yasui, M. Dynamic subcellular localization of aquaporin-7 in white adipocytes. FEBS Lett. 2015, 589, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Hibuse, T.; Maeda, N.; Funahashi, T.; Yamamoto, K.; Nagasawa, A.; Mizunoya, W.; Kishida, K.; Inoue, K.; Kuriyama, H.; Nakamura, T.; et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 10993–10998. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Sohara, E.; Rai, T.; Ikawa, M.; Okabe, M.; Sasaki, S.; Uchida, S.; Verkman, A.S. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: Adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005, 280, 15493–15496. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Funahashi, T.; Hibuse, T.; Nagasawa, A.; Kishida, K.; Kuriyama, H.; Nakamura, T.; Kihara, S.; Shimomura, L.; Matsuzawa, Y. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc. Natl. Acad. Sci. USA 2004, 101, 17801–17806. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Camps, M.; Zorzano, A.; Moura, T.F.; Soveral, G. Biophysical assessment of human aquaporin-7 as a water and glycerol channel in 3T3-L1 adipocytes. PLoS ONE 2013, 8, e83442. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Kuriyama, H.; Funahashi, T.; Shimomura, I.; Kihara, S.; Ouchi, N.; Nishida, M.; Nishizawa, H.; Matsuda, M.; Takahashi, M. Aquaporin adipose, a putative glycerol channel in adipocytes. J. Biol. Chem. 2000, 275, 20896–20902. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; García-Navarro, S.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.; Salvador, J.; Burrell, M.A.; et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef] [PubMed]
- Ceperuelo-Mallafré, V.; Miranda, M.; Chacón, M.R.; Vilarrasa, N.; Megia, A.; Gutiérrez, C.; Fernández-Real, J.M.; Gómez, J.M.; Caubet, E.; Frühbeck, G.; et al. Adipose tissue expression of the glycerol channel aquaporin-7 gene is altered in severe obesity but not in type 2 diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 3640–3645. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef] [PubMed]
- Chiadak, J.D.; Arsenijevic, T.; Gregoire, F.; Bolaky, N.; Delforge, V.; Perret, J.; Delporte, C. Involvement of JNK/NFκB Signaling Pathways in the Lipopolysaccharide-Induced Modulation of Aquaglyceroporin Expression in 3T3-L1 Cells Differentiated into Adipocytes. Int. J. Mol. Sci. 2016, 17, 1742. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Song, Y.; Yang, B.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. USA 2000, 97, 4386–4391. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.M.; Skowronski, M.T.; Füchtbauer, E.-M.; Füchtbauer, A.C.; Fenton, R.A.; Agre, P.; Frøkiaer, J.; Nielsen, S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. USA 2007, 104, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Matsuzaki, T.; Hara-chikuma, M.; Andoo, A.; Shimono, M.; Matsuki, A.; Kobayashi, K.; Ikeda, M.; Yamamoto, T.; Verkman, A. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol. Cell. Biol. 2005, 25, 7770–7779. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, T.; Nakakoshi, M.; Hirao, A.; Imai, M.; Ishibashi, K. Mouse aquaporin 10 gene (AQP10) is a pseudogene. Biochem. Biophys. Res. Commun. 2002, 294, 630–634. [Google Scholar] [CrossRef]
- Calamita, G.; Gena, P.; Ferri, D.; Rosito, A.; Rojek, A.; Nielsen, S.; Marinelli, R.A.; Frühbeck, G.; Svelto, M. Biophysical assessment of aquaporin-9 as principal facilitative pathway in mouse liver import of glucogenetic glycerol. Biol. Cell 2012, 104, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.L.; Marinelli, R.A.; Maggio, A.; Gena, P.; Cataldo, I.; Alves, M.G.; Svelto, M.; Oliveira, P.; Calamita, G. Hepatocyte and sertoli cell aquaporins, recent advances and research trends. Int. J. Mol. Sci. 2016, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Boutagy, N.E.; McMillan, R.P.; Frisard, M.I.; Hulver, M.W. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 2016, 124, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chiadak, J.D.; Arsenijevic, T.; Verstrepen, K.; Gregoire, F.; Bolaky, N.; Delforge, V.; Flamand, V.; Perret, J.; Delporte, C. Forskolin inhibits lipopolysaccharide-induced modulation of MCP-1 and GPR120 in 3T3-L1 adipocytes through an inhibition of NFκB. Mediat. Inflamm. 2016, 2016, 1431789. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.V.; Madeira, A.; Lopes, P.A.; Pires, V.M.R.; Alfaia, C.M.; Prates, J.A.M.; Moura, T.; Soveral, G. Adipocyte membrane glycerol permeability is involved in the anti-adipogenic effect of conjugated linoleic acid. Biochem. Biophys. Res. Commun. 2015, 458, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lucks, A.; Gena, P.; Frascaria, D.; Altamura, N.; Svelto, M.; Beitz, E.; Calamita, G. Preparative scale production and functional reconstitution of a human aquaglyceroporin (AQP3) using a cell free expression system. New Biotechnol. 2013, 30, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Jung, J.S.; Guggino, W.B.; Agre, P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar] [PubMed]
- Zelenina, M.; Tritto, S.; Bondar, A.A.; Zelenin, S.; Aperia, A. Copper inhibits the water and glycerol permeability of aquaporin-3. J. Biol. Chem. 2004, 279, 51939–51943. [Google Scholar] [CrossRef] [PubMed]
- Marrades, M.P.; Milagro, F.I.; Martínez, J.A.; Moreno-Aliaga, M.J. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem. Biophys. Res. Commun. 2006, 339, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Prudente, S.; Flex, E.; Morini, E.; Turchi, F.; Capponi, D.; de Cosmo, S.; Tassi, V.; Guida, V.; Avogaro, A.; Folli, F.; et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes 2007, 56, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Pastor, C.; Rotellar, F.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Vendrell, J.; et al. Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes. Surg. 2008, 18, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Ceperuelo-Mallafré, V.; Lecube, A.; Hernandez, C.; Chacon, M.R.; Fort, J.M.; Gallart, L.; Baena-Fustegueras, J.; Simó, R.; Vendrell, J. Gene expression of paired abdominal adipose AQP7 and liver AQP9 in patients with morbid obesity: Relationship with glucose abnormalities. Metabolism 2009, 58, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Giménez, L.; Rodríguez, A.; Balaguer, I.; Frühbeck, G. Role of aquaglyceroporins and caveolins in energy and metabolic homeostasis. Mol. Cell. Endocrinol. 2014, 397, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Lebeck, J.; Rojek, A.; Praetorius, J.; Füchtbauer, E.-M.; Frokiaer, J.; Nielsen, S. AQP7 is localized in capillaries of adipose tissue, cardic and striated muscle: Implications in glycerol metabolism. Am. J. Physiol. Ren. Physiol. 2007, 292, F956–F965. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Skowronska, A.; Rojek, A.; Oklinski, M.K.; Nielsen, S. Prolonged starvation causes up-regulation of AQP1 in adipose tissue capillaries of AQP7 knock-out mice. Int. J. Mol. Sci. 2016, 17, 1101. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Onnockx, S.; Vandenbroere, I.; Pirson, I. Endogenous SHIP2 does not localize in lipid rafts in 3T3-L1 adipocytes. FEBS Lett. 2004, 565, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Perfield, J.W.; Obin, M.S.; Greenberg, A.S. Adipose triglyceride lipase regulates lipolysis and lipid dropler size in adipocytes. J. Cell. Biochem. 2008, 105, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.B.A.; Lamb, C.A.; Gould, G.W.; Bryant, N.J. Preparation of a Total Membrane Fraction from 3T3-L1 Adipocytes. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gena, P.; Buono, N.D.; D’Abbicco, M.; Mastrodonato, M.; Berardi, M.; Svelto, M.; Lopez, L.; Calamita, G. Dynamical modeling of liver Aquaporin-9 expression and glycerol permeability in hepatic glucose metabolism. Eur. J. Cell Biol. 2017, 96, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Gena, P.; Meleleo, D.; Ferri, D.; Svelto, M. Water permeability of rat liver mitochondria: A biophysical study. Biochim. Biophys. Acta 2006, 1758, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Ferri, D.; Gena, P.; Carreras, F.I.; Liquori, G.E.; Portincasa, P.; Marinelli, R.A.; Svelto, M. Altered expression and distribution of aquaporin-9 in the liver of rat with obstructive extrahepatic cholestasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G682–G690. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiadak, J.D.; Gena, P.; Gregoire, F.; Bolaky, N.; Delforge, V.; Perret, J.; Calamita, G.; Delporte, C. Lipopolysaccharide Modifies Glycerol Permeability and Metabolism in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2017, 18, 2566. https://doi.org/10.3390/ijms18122566
Chiadak JD, Gena P, Gregoire F, Bolaky N, Delforge V, Perret J, Calamita G, Delporte C. Lipopolysaccharide Modifies Glycerol Permeability and Metabolism in 3T3-L1 Adipocytes. International Journal of Molecular Sciences. 2017; 18(12):2566. https://doi.org/10.3390/ijms18122566
Chicago/Turabian StyleChiadak, Jeanne Durendale, Patrizia Gena, Françoise Gregoire, Nargis Bolaky, Valérie Delforge, Jason Perret, Giuseppe Calamita, and Christine Delporte. 2017. "Lipopolysaccharide Modifies Glycerol Permeability and Metabolism in 3T3-L1 Adipocytes" International Journal of Molecular Sciences 18, no. 12: 2566. https://doi.org/10.3390/ijms18122566