Structural Masquerade of Plesiomonas shigelloides Strain CNCTC 78/89 O-Antigen—High-Resolution Magic Angle Spinning NMR Reveals the Modified d-galactan I of Klebsiella pneumoniae
Abstract
:1. Introduction
2. Results
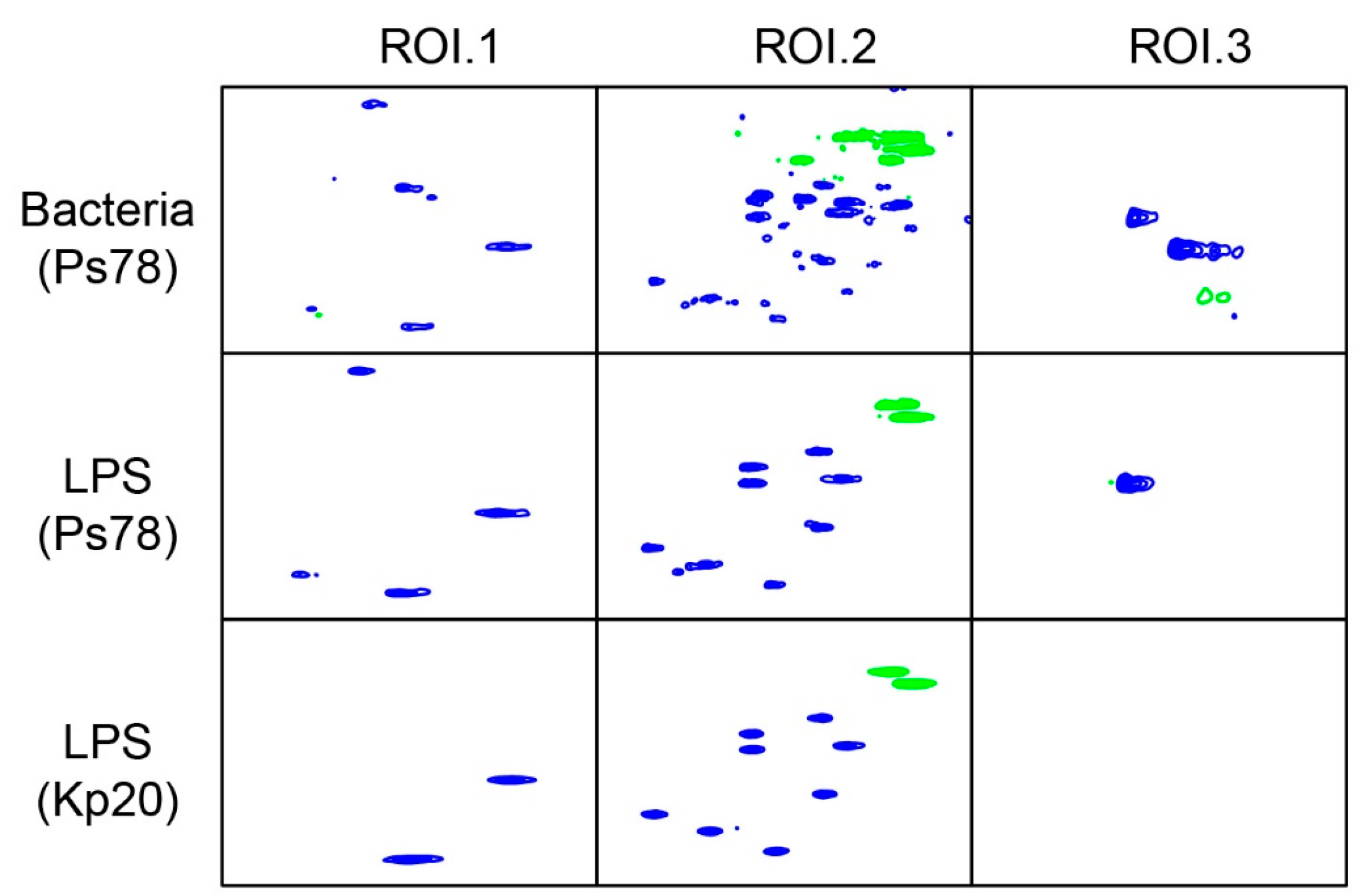
2.1. HR-MAS NMR Analysis of P. shigelloides 78/89 Bacteria and LPS
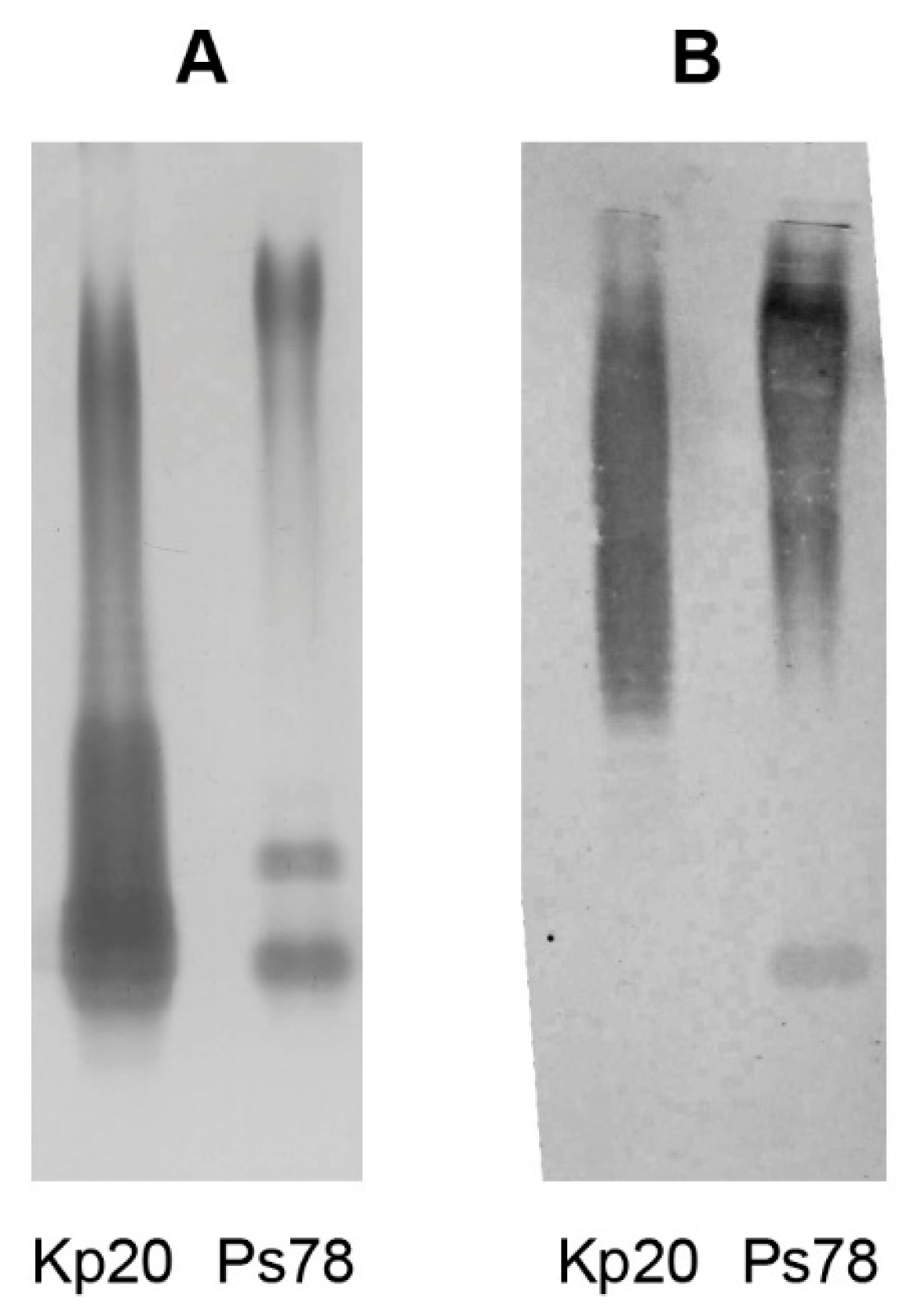
2.2. Isolation of LPS and O-Antigen Fractions
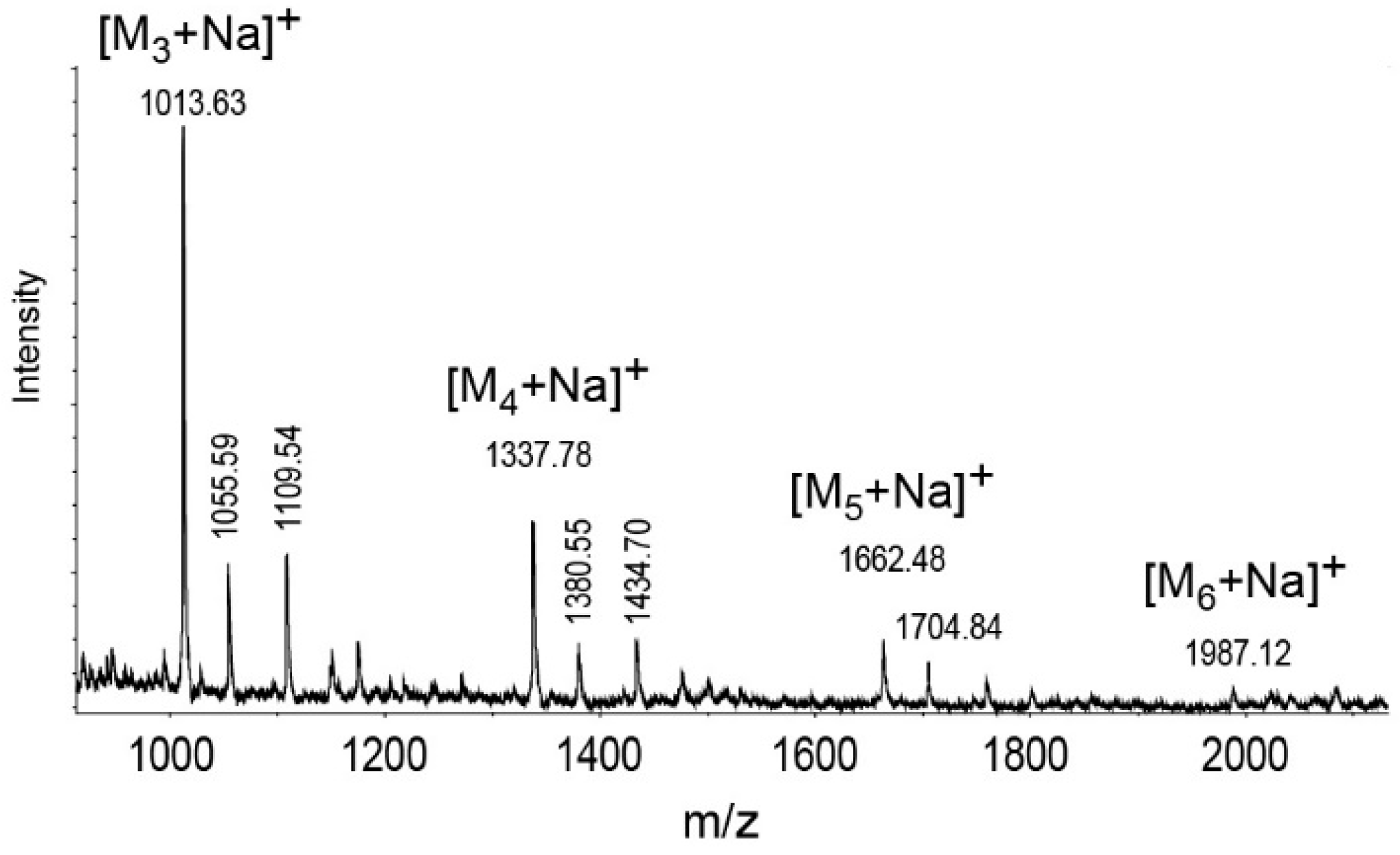
2.3. Structure Analysis of the O-Specific Polysaccharide

2.4. Serological Analysis
3. Discussion
4. Materials and Methods
4.1. Bacteria
4.2. Lipopolysaccharides and O-Specific Polysaccharide Fractions
4.3. Analytical Procedures
4.4. O-Deacetylation of Polysaccharide
4.5. Partial Acid Hydrolysis
4.6. SDS-PAGE and Serological Analysis
4.7. Mass Spectrometry
4.8. NMR Spectroscopy
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LPS | Lipopolysaccharide |
| PS | O-specific polysaccharide |
| OS | Oligosaccharide |
| HR-MAS | High-Resolution Magic Angle Spinning |
| NMR | Nuclear Magnetic Resonance |
| COSY | Correlation spectroscopy |
| TOCSY | Total correlation spectroscopy |
| NOESY | Nuclear Overhouser Effect spectroscopy |
| HSQC | Heteronuclear Single Quantum Coherence |
| DEPT | Distortionless Enhancement by polarization transfer |
| HMBC | Heteronuclear Multiple Bond Correlation |
| GC | Gas chromatography |
| MS | Mass spectrometry |
| MALDI-TOF | Matrix-assisted laser desorption ionization time-of-flight |
| SDS | Sodium dodecyl sulfate |
References
- Garrity, G.M.; Bell, J.A.; Lilburn, T.G. Taxonomic Outline of the Prokaryotes. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2004; pp. 1–399. [Google Scholar]
- Farmer, J.; Arduino, M.J.; Hickman-Brenner, F.W. The genera Aeromonas and Plesiomonas. In The Prokaryotes; Springer: New York, NY, USA, 1992; Volume 3, pp. 3012–3043. [Google Scholar]
- Yamada, S.; Matsushita, S.; Dejsirilert, S.; Kudoh, Y. Incidence and clinical symptoms of Aeromonas-associated travellers’ diarrhoea in Tokyo. Epidemiol. Infect. 1997, 119, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Rautelin, H.; Sivonen, A.; Kuikka, A.; Renkonen, O.V.; Valtonen, V.; Kosunen, T.U. Enteric Plesiomonas shigelloides infections in Finnish patients. Scand. J. Infect. Dis. 1995, 27, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Plesiomonas shigelloides and Salmonella serotype Hartford infections associated with a contaminated water supply—Livingston County, New York, 1996. Morb. Mortal. Wkly. Rep. 1998, 47, 394–396. [Google Scholar]
- Levy, D.; Bens, M.; Craun, G.; Calderon, R.; Herwaldt, B. Surveillance for waterborne-disease outbreaks—United States, 1995–1996. MMWR CDC Surveill. Summ. 1998, 47, 1–34. [Google Scholar] [PubMed]
- McNeeley, D.; Ivy, P.; Craft, J.C.; Cohen, I. Plesiomonas: Biology of the organism and diseases in children. Pediatr. Infect. Dis. 1984, 3, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Whale, K.; Morson, B.C. Acute colitis due to Plesiomonas shigelloides. Br. Med. J. (Clin. Res. Ed.) 1982, 285, 1539–1540. [Google Scholar] [CrossRef]
- Brenden, R.A.; Miller, M.A.; Janda, J.M. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev. Infect. Dis. 1988, 10, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Munford, R.S. Sensing Gram-Negative Bacterial Lipopolysaccharides: A Human Disease Determinant? Infect. Immun. 2008, 76, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Holst, O.; di Lorenzo, F.; Callaghan, M.; Nurisso, A.; D’Errico, G.; Zamyatina, A.; Peri, F.; Berisio, R.; Jerala, R.; et al. Chemistry of lipid A: At the heart of innate immunity. Chem. Eur. J. 2015, 21, 500–519. [Google Scholar] [CrossRef] [PubMed]
- Caroff, M.; Karibian, D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003, 338, 2431–2447. [Google Scholar] [CrossRef] [PubMed]
- Aldová, E.; Shimada, T. New O and H antigens of the international antigenic scheme for Plesiomonas shigelloides. Folia Microbiol. (Praha) 2000, 45, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Linnerborg, M.; Widmalm, G.; Weintraub, A.; Albert, M.J. Structural elucidation of the O-antigen lipopolysaccharide from two strains of Plesiomonas shigelloides that share a type-specific antigen with Shigella flexneri 6, and the common group 1 antigen with Shigella flexneri spp and Shigella dysenteriae 1. Eur. J. Biochem. 1995, 231, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Czaja, J.; Jachymek, W.; Niedziela, T.; Lugowski, C.; Aldova, E.; Kenne, L. Structural studies of the O-specific polysaccharide from Plesiomonas shigelloides strain CNCTC 113/92. Eur. J. Biochem. 2000, 267, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, T.; Lukasiewicz, J.; Jachymek, W.; Dzieciatkowska, M.; Lugowski, C.; Kenne, L. Core oligosaccharides of Plesiomonas shigelloides O54:H2 (strain CNCTC 113/92): Structural and serological analysis of the lipopolysaccharide core region, the O-antigen biological repeating unit, and the linkage between them. J. Biol. Chem. 2002, 277, 11653–11663. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, T.; Dag, S.; Lukasiewicz, J.; Dzieciatkowska, M.; Jachymek, W.; Lugowski, C.; Kenne, L. Complete lipopolysaccharide of Plesiomonas shigelloides O74:H5 (strain CNCTC 144/92). 1. Structural analysis of the highly hydrophobic lipopolysaccharide, including the O-antigen, its biological repeating unit, the core oligosaccharide, and the linkage between them. Biochemistry 2006, 45, 10422–10433. [Google Scholar] [CrossRef] [PubMed]
- Kubler-Kielb, J.; Schneerson, R.; Mocca, C.; Vinogradov, E. The elucidation of the structure of the core part of the LPS from Plesiomonas shigelloides serotype O17 expressing O-polysaccharide chain identical to the Shigella sonnei O-chain. Carbohydr. Res. 2008, 343, 3123–3127. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, G.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Canals, R.; Merino, S.; Tomás, J.M. Structural Studies of the O-Chain Polysaccharide from Plesiomonas shigelloides Strain 302–73 (Serotype O1). Eur. J. Org. Chem. 2008, 2008, 3149–3155. [Google Scholar] [CrossRef]
- Pieretti, G.; Carillo, S.; Lindner, B.; Lanzetta, R.; Parrilli, M.; Jimenez, N.; Regué, M.; Tomás, J.M.; Corsaro, M.M. The complete structure of the core of the LPS from Plesiomonas shigelloides 302–73 and the identification of its O-antigen biological repeating unit. Carbohydr. Res. 2010, 345, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, A.; Lukasiewicz, J.; Kaszowska, M.; Man-Kupisinska, A.; Jachymek, W.; Lugowski, C. Core oligosaccharide of Plesiomonas shigelloides PCM 2231 (Serotype O17) lipopolysaccharide—Structural and serological analysis. Mar. Drugs 2013, 11, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Kaszowska, M.; Jachymek, W.; Lukasiewicz, J.; Niedziela, T.; Kenne, L.; Lugowski, C. The unique structure of complete lipopolysaccharide isolated from semi-rough Plesiomonas shigelloides O37 (strain CNCTC 39/89) containing (2S)-O-(4-oxopentanoic acid)-α-d-Glcp (α-d-Lenose). Carbohydr. Res. 2013, 378, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kaszowska, M.; Jachymek, W.; Niedziela, T.; Koj, S.; Kenne, L.; Lugowski, C. The novel structure of the core oligosaccharide backbone of the lipopolysaccharide from the Plesiomonas shigelloides strain CNCTC 80/89 (serotype O13). Carbohydr. Res. 2013, 380, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, L.C.E.; Kaszowska, M.; Sandström, C. NMR study of the O-specific polysaccharide and the core oligosaccharide from the lipopolysaccharide produced by Plesiomonas shigelloides O24:H8 (strain CNCTC 92/89). Molecules 2015, 20, 5729–5739. [Google Scholar] [CrossRef] [PubMed]
- Batta, G.; Lipták, A.; Schneerson, R.; Pozsgay, V. Conformational stabilization of the altruronic acid residue in the O-specific polysaccharide of Shigella sonnei/Plesiomonas shigelloides. Carbohydr. Res. 1997, 305, 93–99. [Google Scholar] [CrossRef]
- Kenne, L.; Lindberg, B.; Petersson, K.; Katzenellenbogen, E.; Romanowska, E. Structural studies of the O-specific side-chains of the Shigella sonnei phase I lipopolysaccharide. Carbohydr. Res. 1980, 78, 119–126. [Google Scholar] [CrossRef]
- Albert, M.J.; Ansaruzzaman, M.; Qadri, F.; Hossain, A.; Kibriya, A.K.; Haider, K.; Nahar, S.; Faruque, S.M.; Alam, A.N. Characterisation of Plesiomonas shigelloides strains that share type-specific antigen with Shigella flexneri 6 and common group 1 antigen with Shigella flexneri spp. and Shigella dysenteriae 1. J. Med. Microbiol. 1993, 39, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ansaruzzaman, M.; Albert, M.J.; Holme, T.; Jansson, P.-E.; Rahman, M.M.; Widmalm, G. A Klebsiella pneumoniae Strain that Shares a Type-Specific Antigen with Shigella flexneri Serotype 6. Eur. J. Biochem. 1996, 237, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Nestor, G.; Lukasiewicz, J.; Sandström, C. Structural Analysis of the Core Oligosaccharide and the O-Specific Polysaccharide from the Plesiomonas shigelloides O33:H3 (Strain CNCTC 34/89) Lipopolysaccharide. Eur. J. Org. Chem. 2013, 2014, 1241–1252. [Google Scholar] [CrossRef]
- Maciejewska, A. Structural and Serological Studies of Bacterial Endotoxins of Plesiomonas Shigelloides O17 and O51. Ph.D. Thesis, Hirszfeld Institute of Immunology & Experimental Therapy, Wroclaw, Poland, 2008. [Google Scholar]
- Kelly, R.F.; Severn, W.B.; Richards, J.C.; Perry, M.B.; MacLean, L.L.; Tomás, J.M.; Merino, S.; Whitfield, C. Structural variation in the O-specific polysaccharides of Klebsiella pneumoniae serotype O1 and O8 lipopolysaccharide: Evidence for clonal diversity in rfb genes. Mol. Microbiol. 1993, 10, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Gorin, P.A.J.; Mazurek, M. Further Studies on the Assignment of Signals in 13C Magnetic Resonance Spectra of aldoses and derived Methyl Glycosides. Can. J. Chem. 1975, 1212–1223. [Google Scholar] [CrossRef]
- Szijártó, V.; Guachalla, L.M.; Hartl, K.; Varga, C.; Banerjee, P.; Stojkovic, K.; Kaszowska, M.; Nagy, E.; Lukasiewicz, J.; Nagy, G. Both clades of the epidemic KPC-producing Klebsiella pneumoniae clone ST258 share a modified galactan O-antigen type. Int. J. Med. Microbiol. 2016, 306, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Richards, J.C.; Perry, M.B.; Clarke, B.R.; MacLean, L.L. Expression of two structurally distinct d-galactan O antigens in the lipopolysaccharide of Klebsiella pneumoniae serotype O1. J. Bacteriol. 1991, 173, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, G.; Ning, J. First synthesis of β-d-Galf-(1→3)-d-Galp—The repeating unit of the backbone structure of the O-antigenic polysaccharide present in the lipopolysaccharide (LPS) of the genus Klebsiella. Carbohydr. Res. 2003, 338, 1033–1037. [Google Scholar] [CrossRef]
- Hansen, D.S.; Mestre, F.; Albertí, S.; Hernández-Allés, S.; Álvarez, D.; Doménech-Sánchez, A.; Gil, J.; Merino, S.; Tomás, J.M.; Benedí, V.J. Klebsiella pneumoniae Lipopolysaccharide O Typing: Revision of Prototype Strains and O-Group Distribution among Clinical Isolates from Different Sources and Countries. J. Clin. Microbiol. 1999, 37, 56–62. [Google Scholar] [PubMed]
- Stojkovic, K.; Szijártó, V.; Kaszowska, M.; Niedziela, T.; Hartl, K.; Nagy, G.; Lukasiewicz, J. Identification of d-Galactan-III as Part of the Lipopolysaccharide of Klebsiella pneumoniae Serotype O1. Front. Microbiol. 2017, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- Westphal, O.; Jann, K. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 1965, 5, 83–89. [Google Scholar]
- Petersson, C.; Niedziela, T.; Jachymek, W.; Kenne, L.; Zarzecki, P.; Lugowski, C. Structural Studies of the O-Specific Polysaccharide of Hafnia alvei Strain PCM 1206 Lipopolysaccharide Containing d-Allothreonine. Eur. J. Biochem. 1997, 244, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. A Rapid Permethylation of Glycolipid, and Polysaccharide Catalyzed by Methylsulfinyl Carbanion in Dimethyl Sulfoxide. J. Biochem. 1964, 55, 205–208. [Google Scholar] [PubMed]
- Gerwig, G.J.; Kamerling, J.P.; Vliegenthart, J.F. Determination of the absolute configuration of mono-saccharides in complex carbohydrates by capillary G.L.C. Carbohydr. Res. 1979, 77, 10–17. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef]
- Jennings, H.J.; Lugowski, C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J. Immunol. 1981, 127, 1011–1018. [Google Scholar] [PubMed]
- Voller, A.; Draper, C.; Bidwell, D.E.; Bartlett, A. Microplate enzyme-linked immunosorbent assay for Chagas’ disease. Lancet 1975, 305, 426–428. [Google Scholar] [CrossRef]
- Jachymek, W.; Czaja, J.; Niedziela, T.; Lugowski, C.; Kenne, L. Structural studies of the O-specific polysaccharide of Hafnia alvei strain PCM 1207 lipopolysaccharide. Eur. J. Biochem. 1999, 266, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jachymek, W.; Niedziela, T.; Petersson, C.; Lugowski, C.; Czaja, J.; Kenne, L. Structures of the O-specific polysaccharides from Yokenella regensburgei (Koserella trabulsii) strains PCM 2476, 2477, 2478, and 2494: High-resolution magic-angle spinning NMR investigation of the O-specific polysaccharides in native lipopolysaccharides and directly on the surface of living bacteria. Biochemistry 1999, 38, 11788–11795. [Google Scholar] [CrossRef] [PubMed]
- Goddard, T.D.; Kneller, D.G. Sparky, 3rd ed.; University of California: San Francisco, CA, USA, 2001. [Google Scholar]
- Lewis, I.A.; Schommer, S.C.; Markley, J.L. rNMR: Open source software for identifying and quantifying metabolites in NMR spectra. Magn. Reson. Chem. 2009, 47, S123–S126. [Google Scholar] [CrossRef] [PubMed]
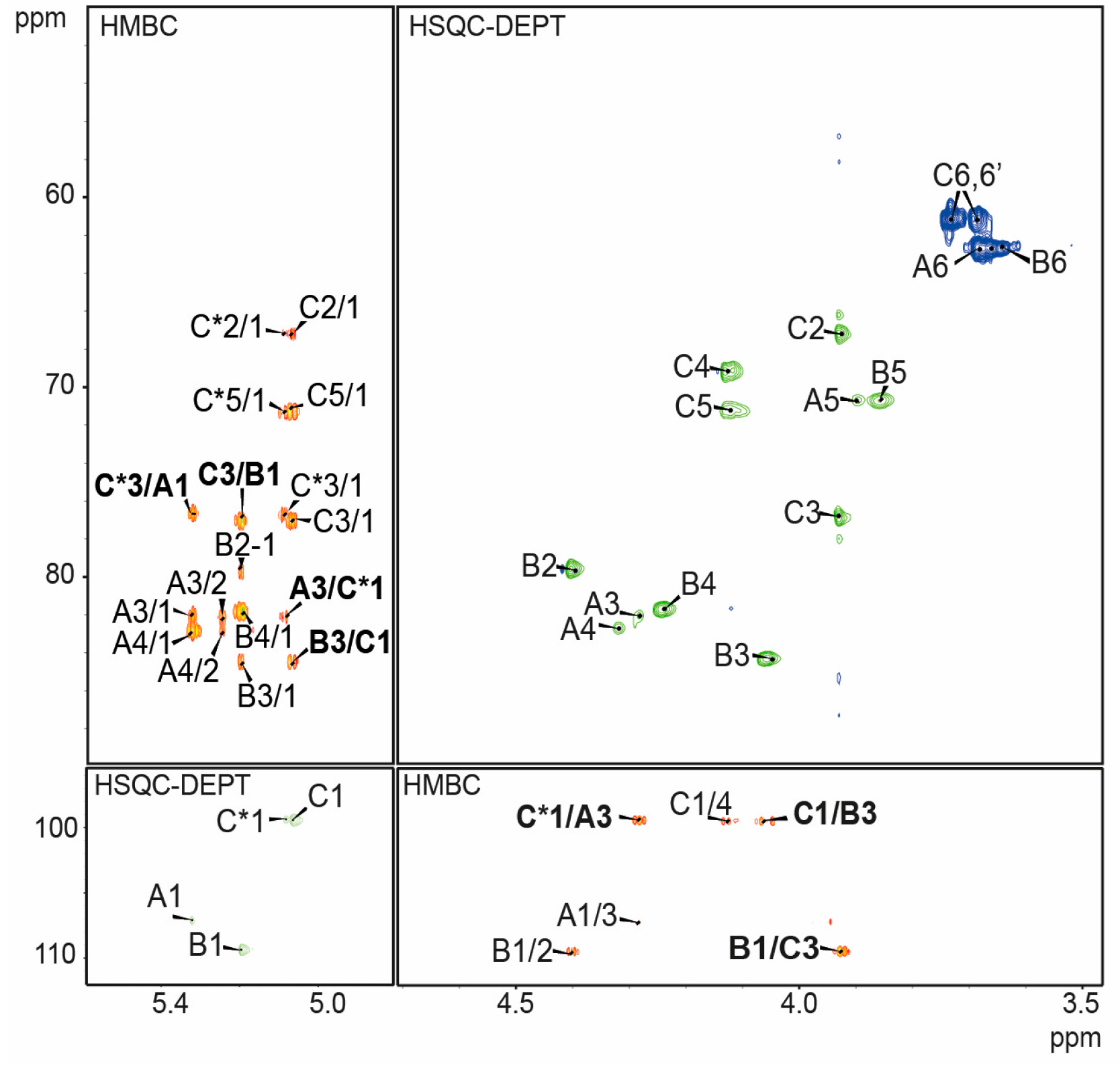

| Residue | Chemical Shifts (ppm) | |||||
|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6, H-6′ | |
| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | |
| A →3)-β-d-Galf2OAc-(1→ | 5.32 | 5.25 b | 4.28 | 4.32 | 3.89 | 3.68, 3.65 |
| 107.1 | 81.8 | 82.1 | 82.9 | 70.8 | 62.7 | |
| B →3)-β-d-Galf-(1→ | 5.20 | 4.40 | 4.06 | 4.24 | 3.86 | 3.66, 3.68 |
| 109.4 | 79.8 | 84.5 | 81.8 | 70.8 | 62.7 | |
| C →3)-α-d-Galp-(1→ | 5.07 | 3.93 | 3.93 | 4.12 | 4.12 | 3.73, 3.68 |
| 99.5 | 67.2 | 76.9 | 69.2 | 71.3 | 61.1 | |
| C′ α-d-Galp-(1→ c | 5.11 | 3.90 | 4.01 | 4.12 | 4.27 | 3.8, 3.75 |
| 95.4 | 68.5 | 69.6 | 70.3 | 71.2 | 60.7 | |
| C* →3)-α-d-Galp-(1→ | 5.08 | 3.94 | 3.95 | 4.11 | 4.11 | 3.69, 3.62 |
| 99.4 | 67.2 | 76.7 | 69.1 | 71.3 | 60.9 | |
| Residue | Atom H-1/C-1 | Connectivities to | Inter-Residue | |
|---|---|---|---|---|
| (ppm) | δC | δH | Atom/Residue | |
| A →3)-β-d-Galf2OAc-(1→ | 5.32/107.1 | 76.7 | 3.95 | C-3, H-3 of C* |
| B →3)-β-d-Galf-(1→ | 5.20/109.4 | 76.9 | 3.93 | C-3, H-3 of C |
| C →3)-α-d-Galp-(1→ | 5.07/99.5 | 84.5 | 4.06 | C-3, H-3 of B |
| C* →3)-α-d-Galp-(1→ | 5.08/99.4 | 82.1 | 4.28 | C-3, H-3 of A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ucieklak, K.; Koj, S.; Pawelczyk, D.; Niedziela, T. Structural Masquerade of Plesiomonas shigelloides Strain CNCTC 78/89 O-Antigen—High-Resolution Magic Angle Spinning NMR Reveals the Modified d-galactan I of Klebsiella pneumoniae. Int. J. Mol. Sci. 2017, 18, 2572. https://doi.org/10.3390/ijms18122572
Ucieklak K, Koj S, Pawelczyk D, Niedziela T. Structural Masquerade of Plesiomonas shigelloides Strain CNCTC 78/89 O-Antigen—High-Resolution Magic Angle Spinning NMR Reveals the Modified d-galactan I of Klebsiella pneumoniae. International Journal of Molecular Sciences. 2017; 18(12):2572. https://doi.org/10.3390/ijms18122572
Chicago/Turabian StyleUcieklak, Karolina, Sabina Koj, Damian Pawelczyk, and Tomasz Niedziela. 2017. "Structural Masquerade of Plesiomonas shigelloides Strain CNCTC 78/89 O-Antigen—High-Resolution Magic Angle Spinning NMR Reveals the Modified d-galactan I of Klebsiella pneumoniae" International Journal of Molecular Sciences 18, no. 12: 2572. https://doi.org/10.3390/ijms18122572