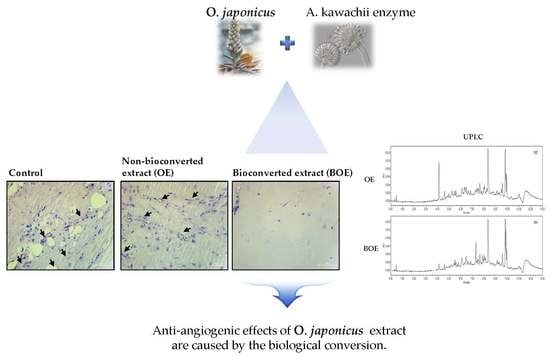
Bioconverted Orostachys japonicas Extracts Suppress Angiogenic Activity of Ms-1 Endothelial Cells
Abstract
:1. Introduction
2. Results and Discussion
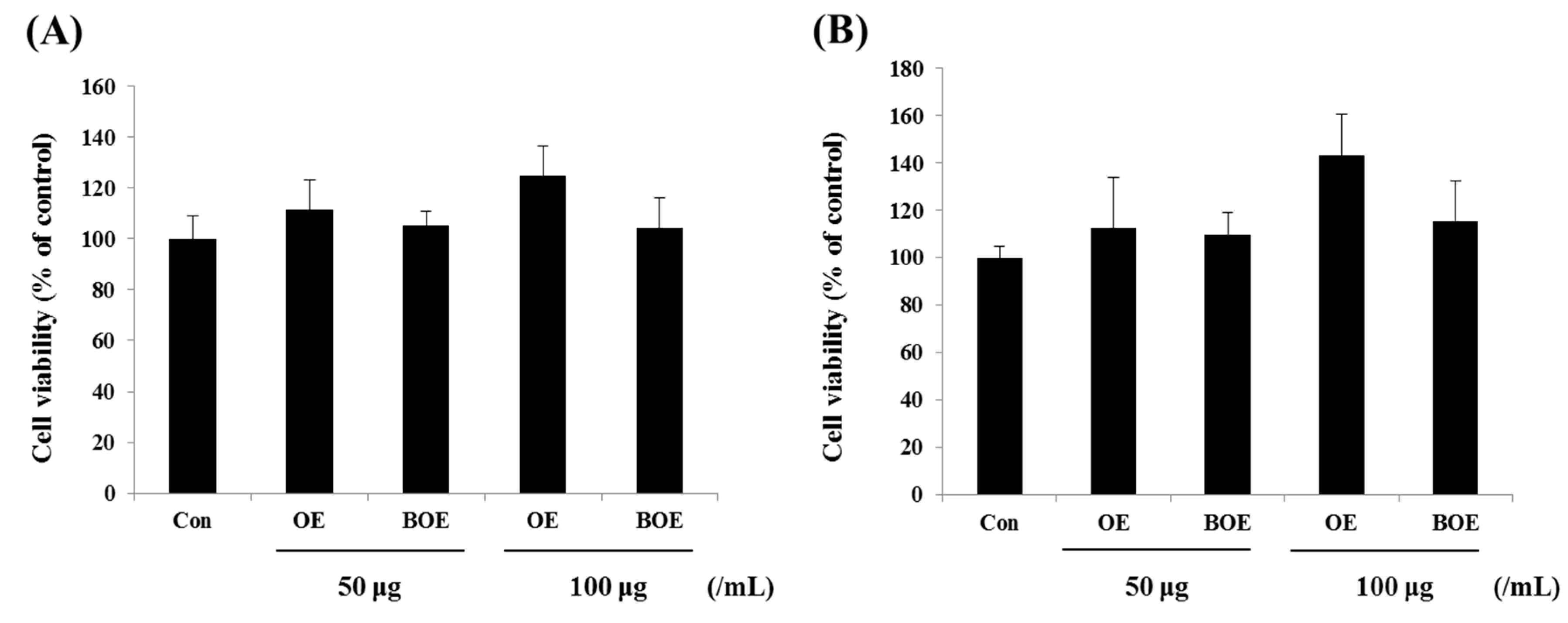
2.1. O. japonicus Extract (OE) and Bioconverted O. japonicus Extract (BOE) Did Not Affect the Viability of Ms-1 Cells
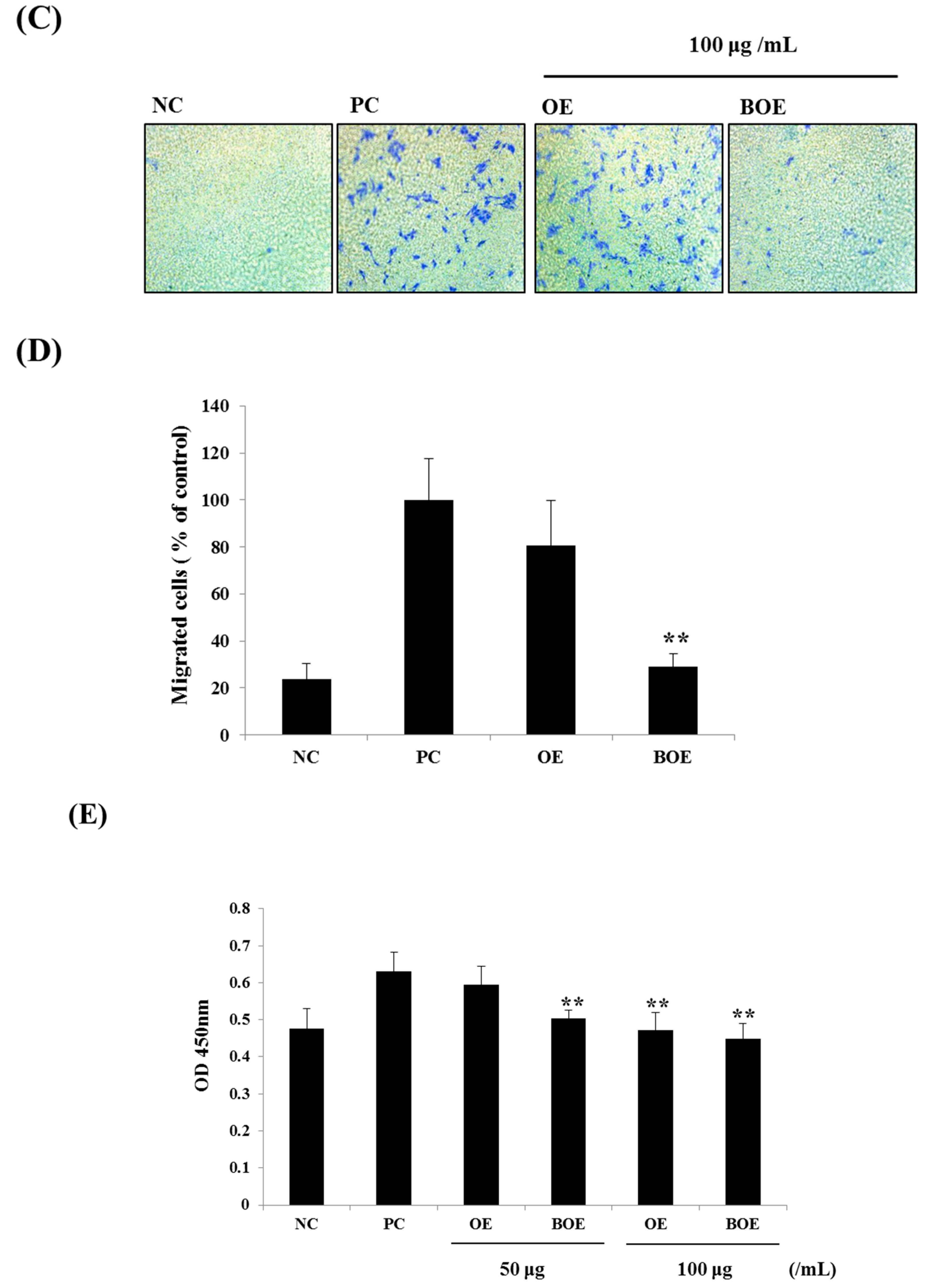
2.2. BOE Suppress the Migration and Adhesion Abilities of Ms-1 Cells
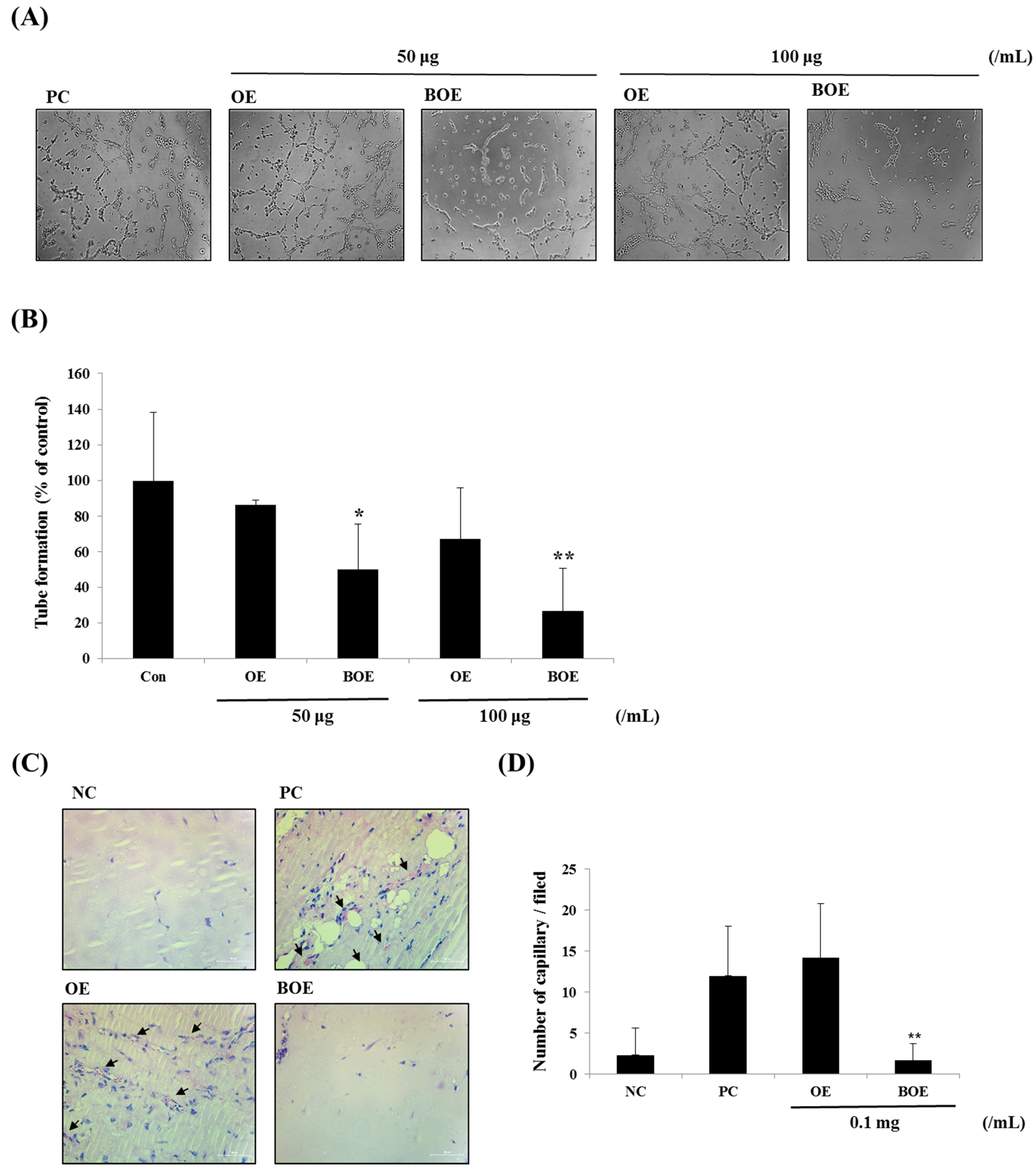
2.3. BOE Inhibits Angiogenesis of Ms-1 Cells
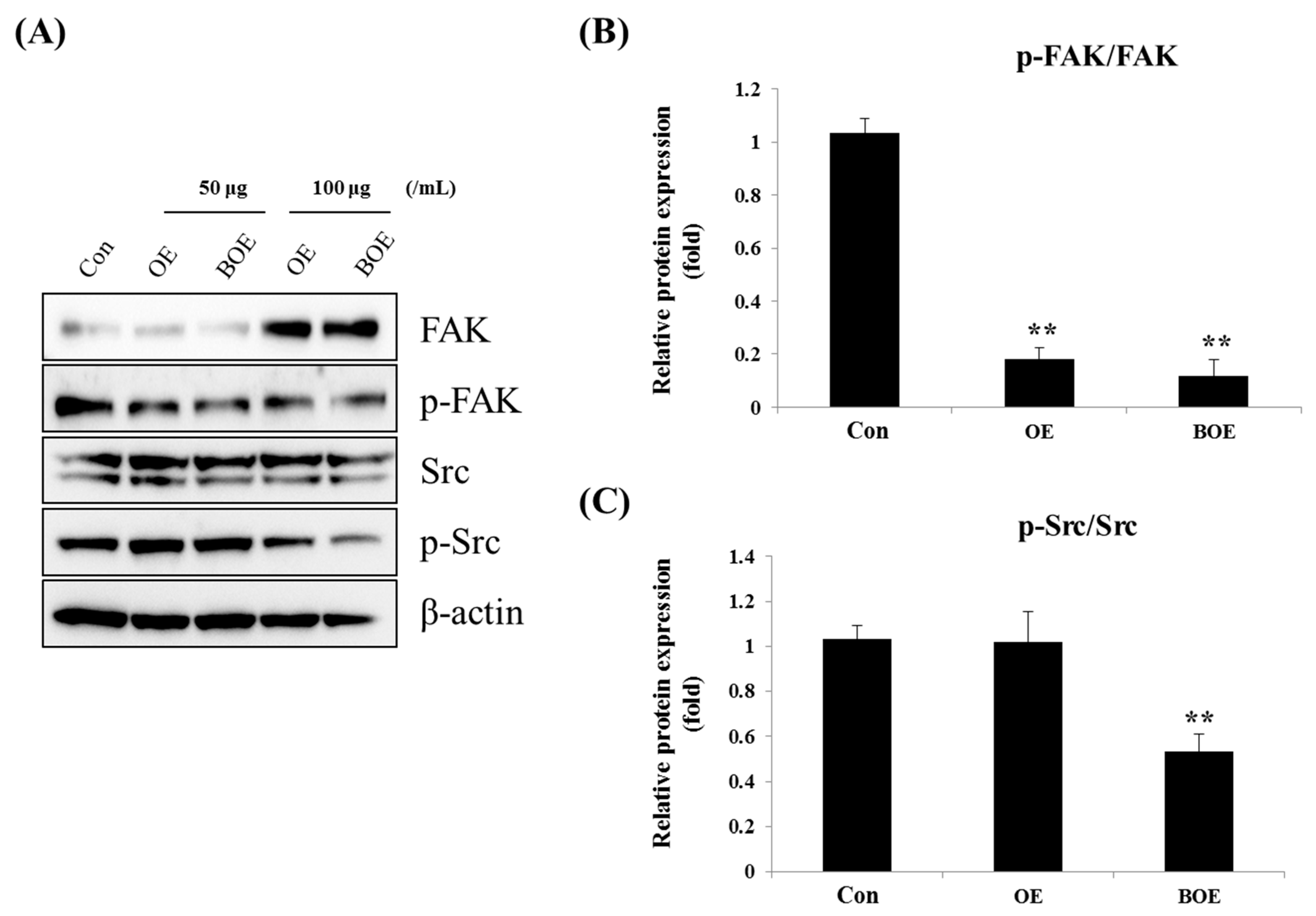
2.4. BOE Inhibited FAK (Focal Adhesion Kinase) and Src Activation
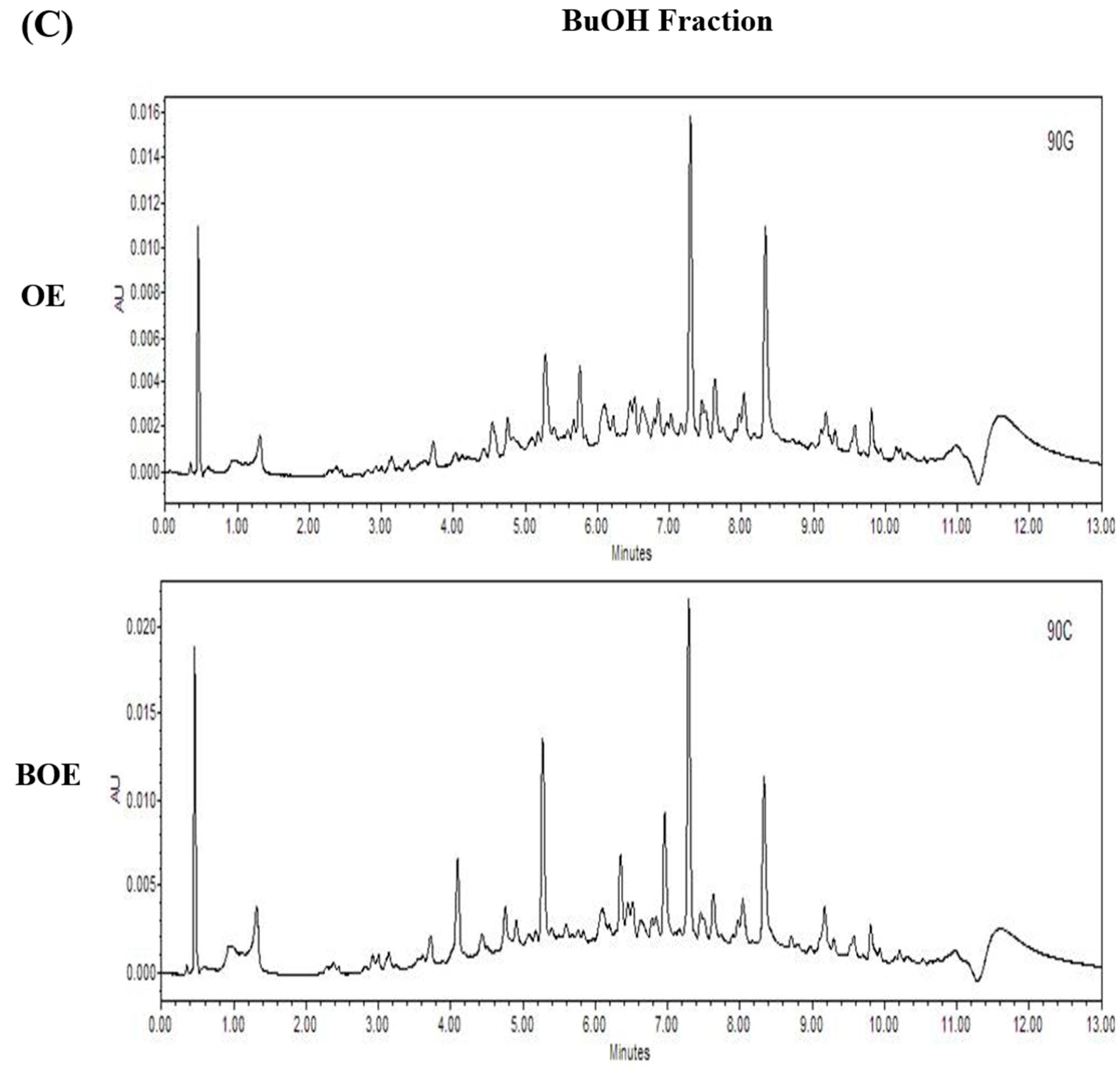
2.5. Effects of Bioconversion on Changing Ultra Performance Liquid Chromatography (UPLC)Patterns of O. japonicus
3. Materials and Methods
3.1. Sample Preparation and Cell Culture
3.2. Preparation of A. kawachii Enzyme
3.3. UPLC Analysis
3.4. MTT (3-(4,5)-Dimethylthiazo(-2-y1)-2,5-diphenytetrazolium bromide) Assay
3.5. Adhesion Assay
3.6. Wound Healing and Migration Assay
3.7. Tube Formation Assay
3.8. Matrigel Plug Assay
3.9. Western Blotting
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jung, H.J.; Choi, J.; Nam, J.H.; Park, H.J. Anti-ulcerogenic effects of the flavonoid-rich fraction from the extract of Orostachys japonicus in mice. J. Med. Food 2007, 10, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Yang, H.J.; Kim, K.H.; Kim, S.H. Aqueous extract of Orostachys japonicus A. Berger exerts immunostimulatory activity in RAW 264.7 macrophages. J. Ethnopharmacol. 2015, 170, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Song, E.J.; Lee, S.Y.; Kim, K.B.W.R.; Kim, S.Y.; Yoon, S.Y.; Lee, C.J. Antioxidant Activity of Leaf, Stem and Root Extracts from Orostachys japonicus and Their Heat and pH Stabilities. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1571–1579. [Google Scholar] [CrossRef]
- Ryu, D.S.; Kim, S.H.; Kwon, J.H.; Lee, D.S. Orostachys japonicus induces apoptosis and cell cycle arrest through the mitochondria-dependent apoptotic pathway in AGS human gastric cancer cells. Int. J. Oncol. 2014, 45, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.D.; Shin, G.H.; Kim, J.M.; Oh, J.W.; Choi, S.I.; Lee, J.H.; Cho, M.L.; Lee, S.J.; Heo, I.J.; Park, S.J.; et al. Changes in Lignan Content and Antioxidant Activity of Fermented Sesame (Sesame indicum L.) by Cultivars. J. Korean Soc. Food Sci. Nutr. 2016, 45, 143–148. [Google Scholar] [CrossRef]
- Im, A.R.; Song, J.H.; Lee, M.Y.; Yeon, S.H.; Um, K.A.; Chae, S. Anti-wrinkle effects of fermented and non-fermented Cyclopia Intermedia in hairless mice. BMC Complement. Altern. Med. 2014, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Seo, J.; Song, E.; Choi, H.J.; Shim, E.; Lee, O.; Hwang, J. Bioconverted Jeju Hallabong tangor (Citrus Kiyomi × Ponkan) peel extracts by cytolase enhance antioxidant and anti-inflammatory capacity in RAW 264.7 cells. Nutr. Res. Pract. 2016, 10, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Yuan, F. Numerical simulations of angiogenesis in the cornea. Microvasc. Res. 2001, 61, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ueda, H.; Zhou, H.; Stokol, T.; Shen, T.L.; Alcaraz, A.; Nagy, T.; Vassalli, J.D.; Guan, J.L. Overexpression of focal adhesion kinase in vascular endothelial cells promotes angiogenesis in transgenic mice. Cardiovasc. Res. 2004, 64, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Harmey, J.H.; Bouchier-Hayes, D. Vascular endothelial growth factor (VEGF), a survival factor for tumour cells: Implications for anti-angiogenic therapy. BioEssays 2002, 24, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Schimmenti, L.A.; Yan, H.C.; Madri, J.A.; Albelda, S.M. Platelet endothelial cell adhesion molecule, PECAM-1, modulates cell migration. J. Cell. Physiol. 1992, 153, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Annabi, B.; Lee, Y.T.; Turcotte, S.; Naud, E.; Desrosiers, R.R.; Champagne, M.; Eliopoulos, N.; Galipeau, J.; Beliveau, R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells 2003, 21, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Haskell, H.; Natarajan, M.; Hecker, T.P.; Ding, Q.; Stewart, J., Jr.; Grammer, J.R.; Gladson, C.L. Focal adhesion kinase is expressed in the angiogenic blood vessels of malignant astrocytic tumors in vivo and promotes capillary tube formation of brain microvascular endothelial cells. Clin. Cancer Res. 2003, 9, 2157–2165. [Google Scholar] [PubMed]
- Bolos, V.; Gasent, J.M.; Lopez-Tarruella, S.; Grande, E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. OncoTargets Ther. 2010, 3, 83–97. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, S.I.; Park, S.Y.; Bang, H.Y.; Jeong, J.H.; So, J.H.; Rhee, I.K.; Song, K.S. Fermentation enhances the in vitro antioxidative effect of onion (Allium Cepa) via an increase in quercetin content. Food Chem. Toxicol. 2012, 50, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Malinda, K.M. In vivo matrigel migration and angiogenesis assay. Methods Mol. Biol. 2009, 467, 287–294. [Google Scholar] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.G.; Kim, J.S.; Lee, H.-S.; Lim, Y.-M.; So, J.-H.; Hahn, D.; Ha, Y.S.; Nam, J.-O. Bioconverted Orostachys japonicas Extracts Suppress Angiogenic Activity of Ms-1 Endothelial Cells. Int. J. Mol. Sci. 2017, 18, 2615. https://doi.org/10.3390/ijms18122615
Lee SG, Kim JS, Lee H-S, Lim Y-M, So J-H, Hahn D, Ha YS, Nam J-O. Bioconverted Orostachys japonicas Extracts Suppress Angiogenic Activity of Ms-1 Endothelial Cells. International Journal of Molecular Sciences. 2017; 18(12):2615. https://doi.org/10.3390/ijms18122615
Chicago/Turabian StyleLee, Seul Gi, Jin Soo Kim, Han-Saem Lee, Yu-Mi Lim, Jai-Hyun So, Dongyup Hahn, Yu Shin Ha, and Ju-Ock Nam. 2017. "Bioconverted Orostachys japonicas Extracts Suppress Angiogenic Activity of Ms-1 Endothelial Cells" International Journal of Molecular Sciences 18, no. 12: 2615. https://doi.org/10.3390/ijms18122615