In Vitro Effect of 3D Plates Used for Surgical Treatment of Condylar Fractures on Prostaglandin E2 (PGE2) and Thromboxane B2 (TXB2) Concentration in THP-1 Macrophages
Abstract
:1. Introduction
Aim of the Study
2. Results
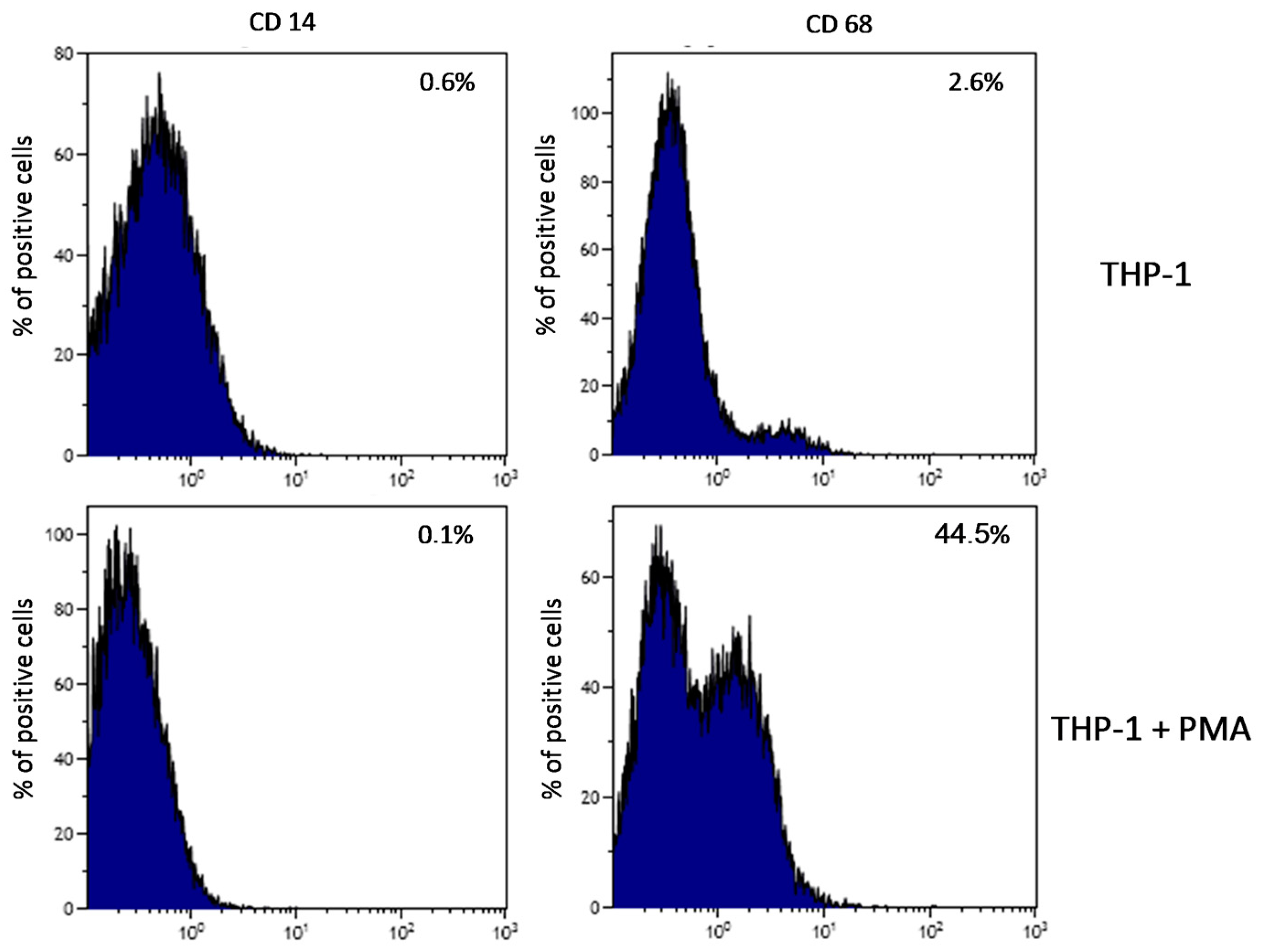
2.1. Plate-Induced Activation of THP-1 Monocytes and Macrophages
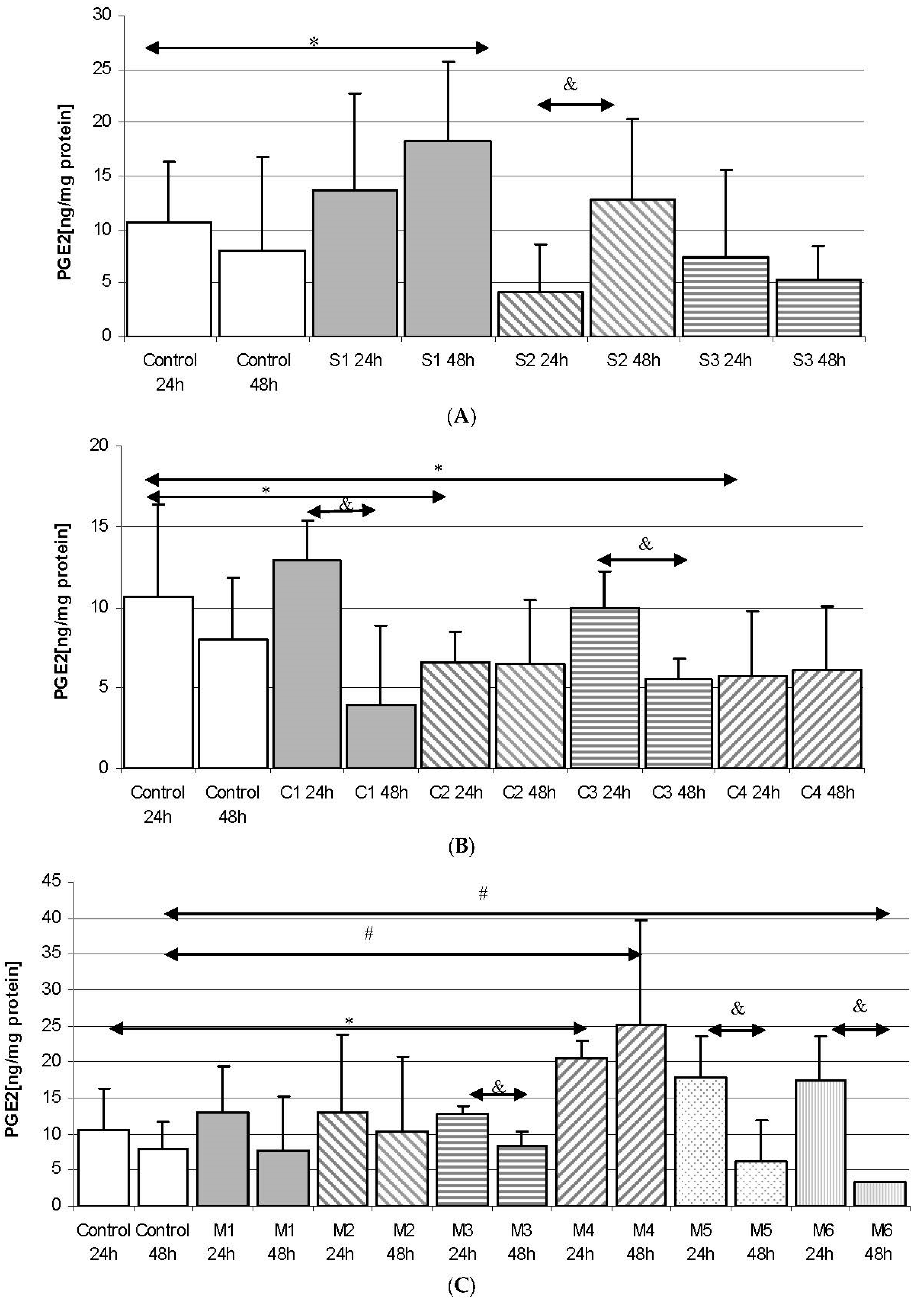
2.2. Prostaglandin E2 (PGE2) in THP-1 Monocytes
2.2.1. Incubation of THP-1 Cells with SYNTES (S) Plates
2.2.2. Incubation of THP-1 Cells with MARTIN (C) Plates
2.2.3. Incubation of THP1 Cells with MEDARTIS (M) Plates
2.3. Prostaglandin E2 (PGE2) in THP-1 Macrophages
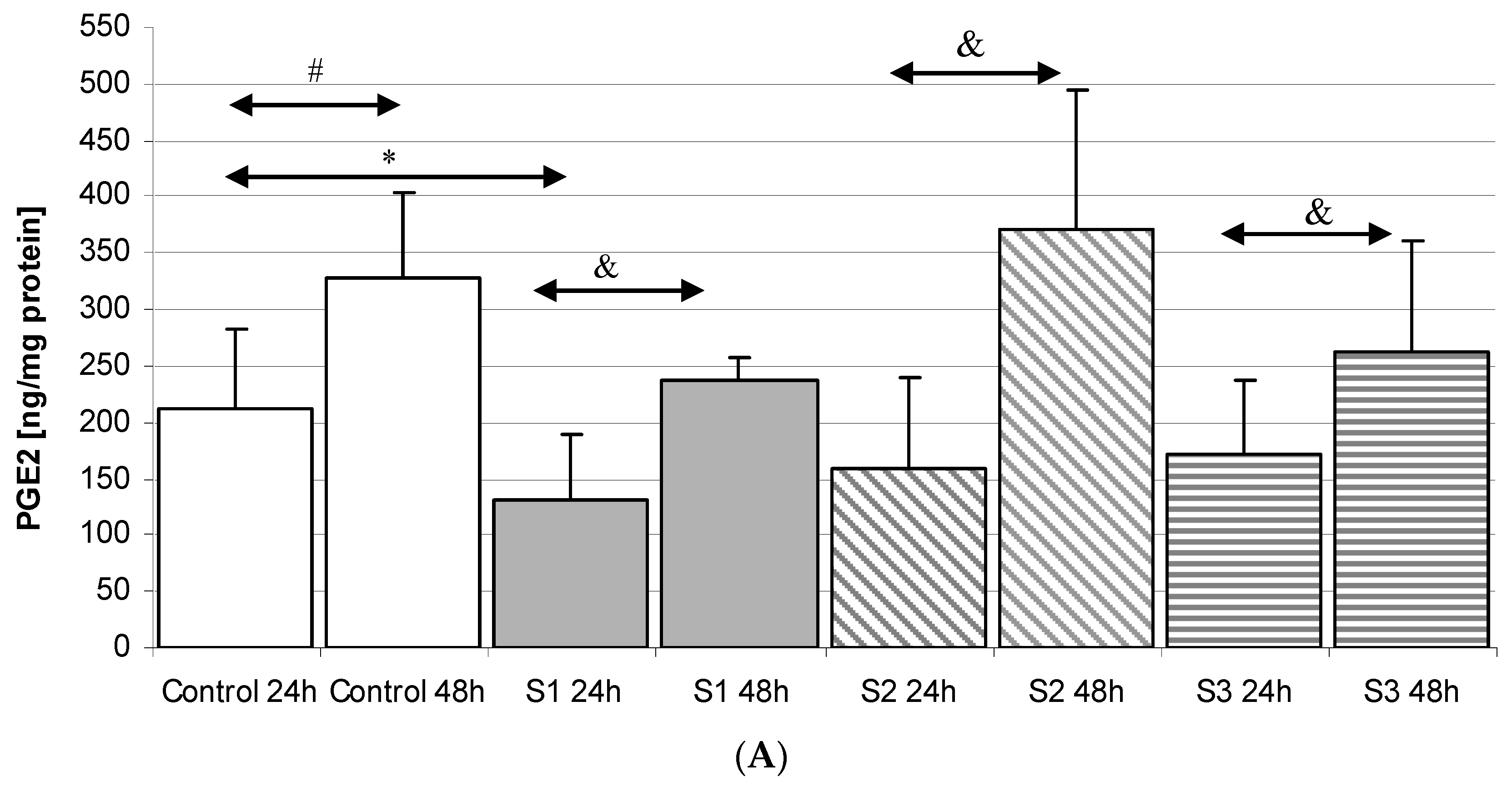
2.3.1. Incubation of Macrophages with SYNTES Plates (S)
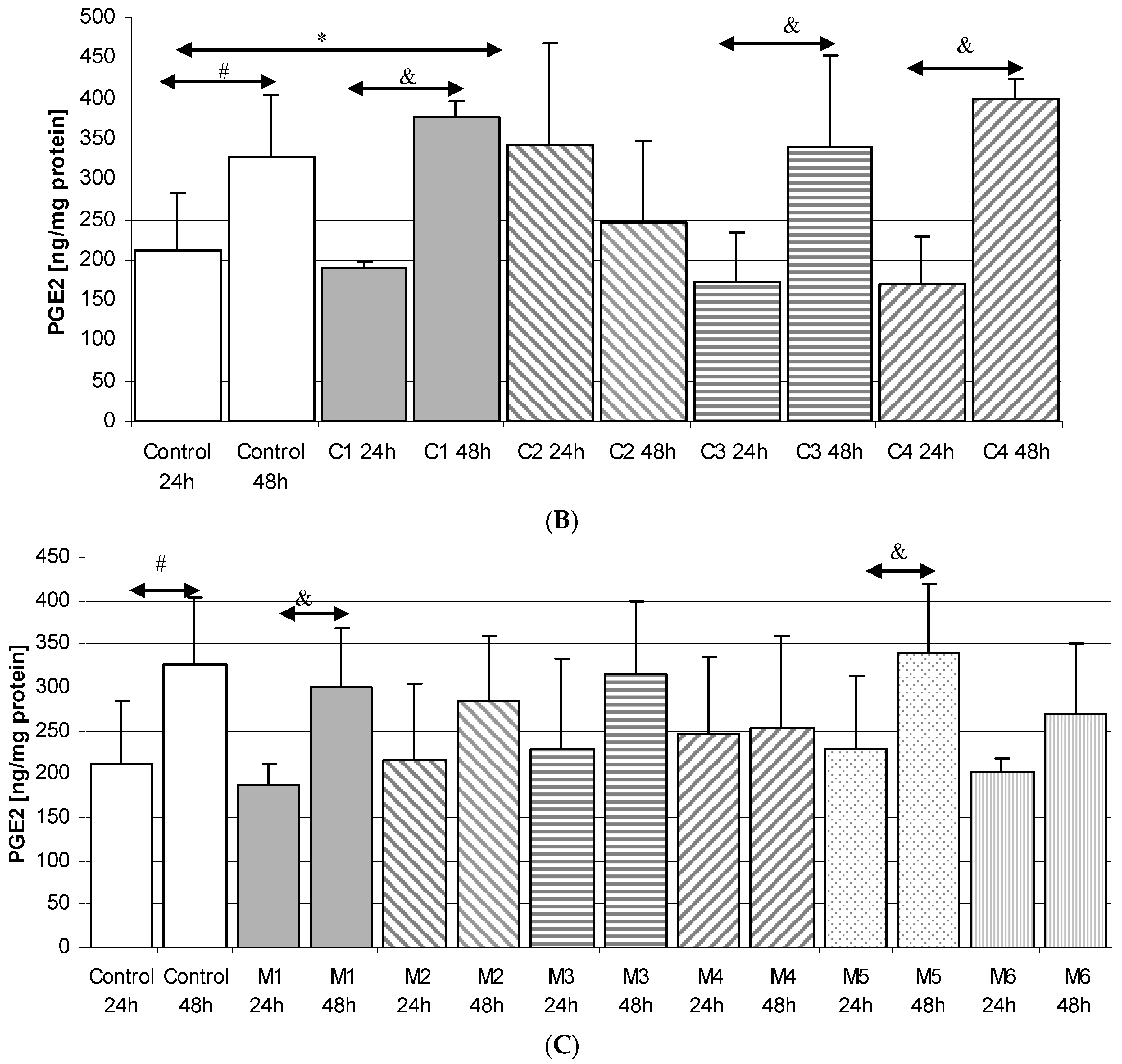
2.3.2. Incubation of Macrophages with MARTIN Plates (C)
2.3.3. Incubation of Macrophages with MEDARTIS Plates (M)
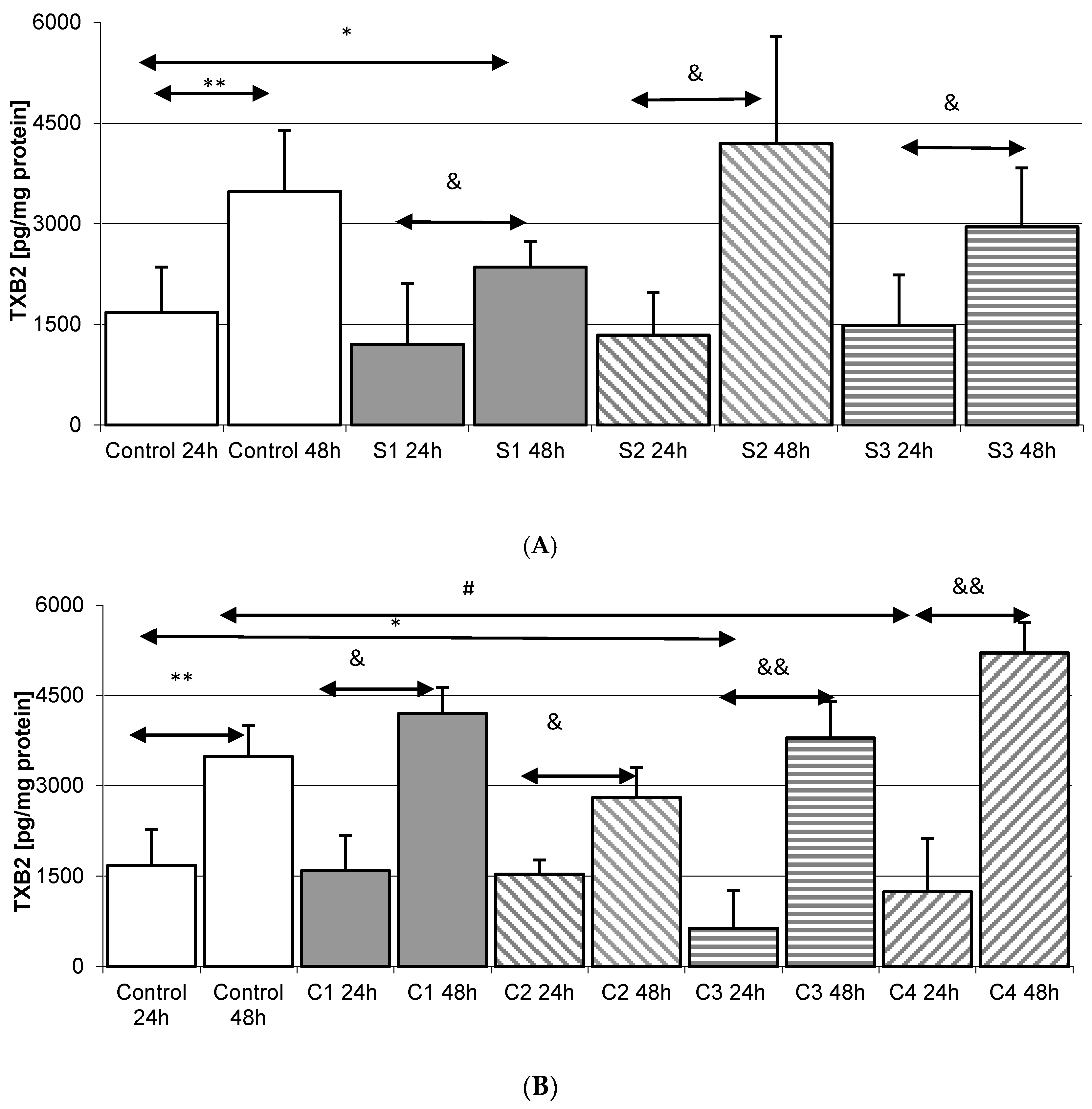
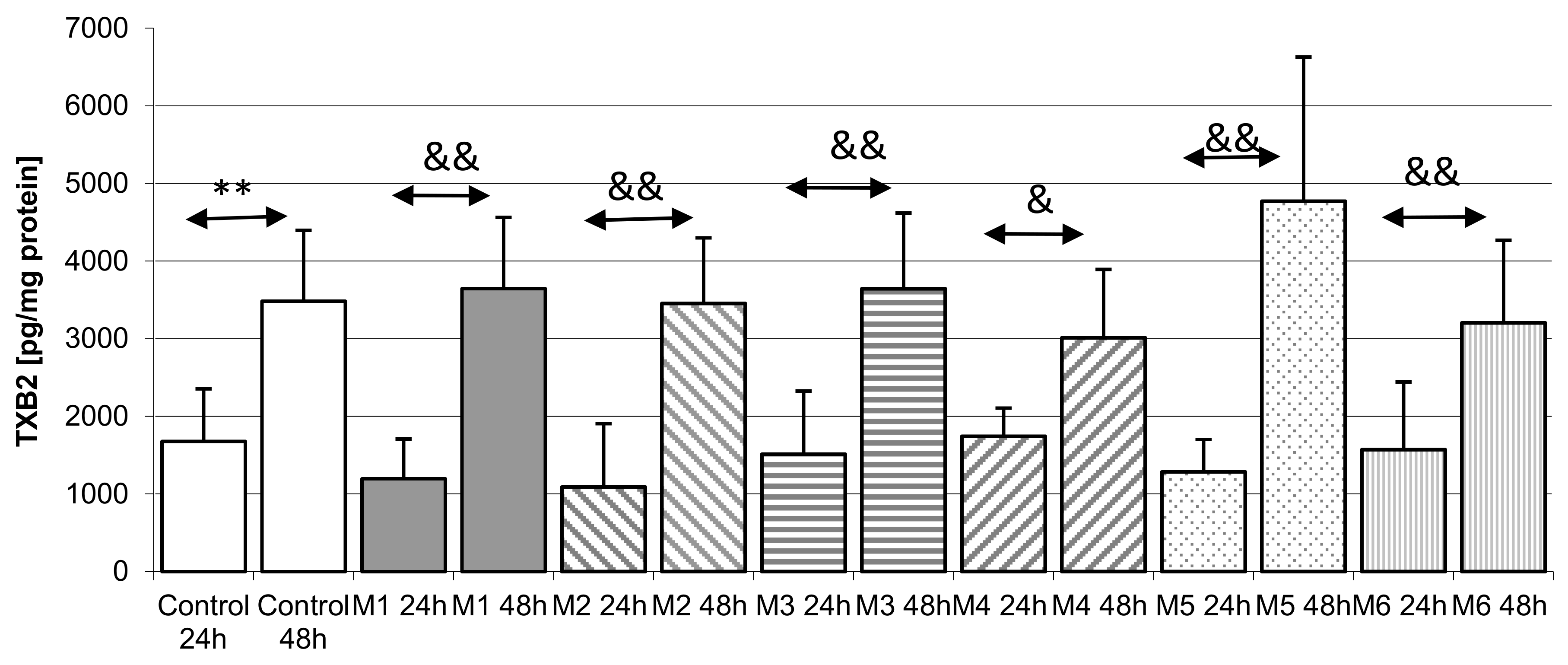
2.4. Thromboxane TXB2 in THP-1 Monocytes
2.4.1. Incubation of THP-1 Cells with SYNTES (S) Plates
2.4.2. Incubation of THP1 Cells with MARTIN Plates (C)
2.4.3. Incubation of THP1 Cells with MEDARTIS Plates (M)
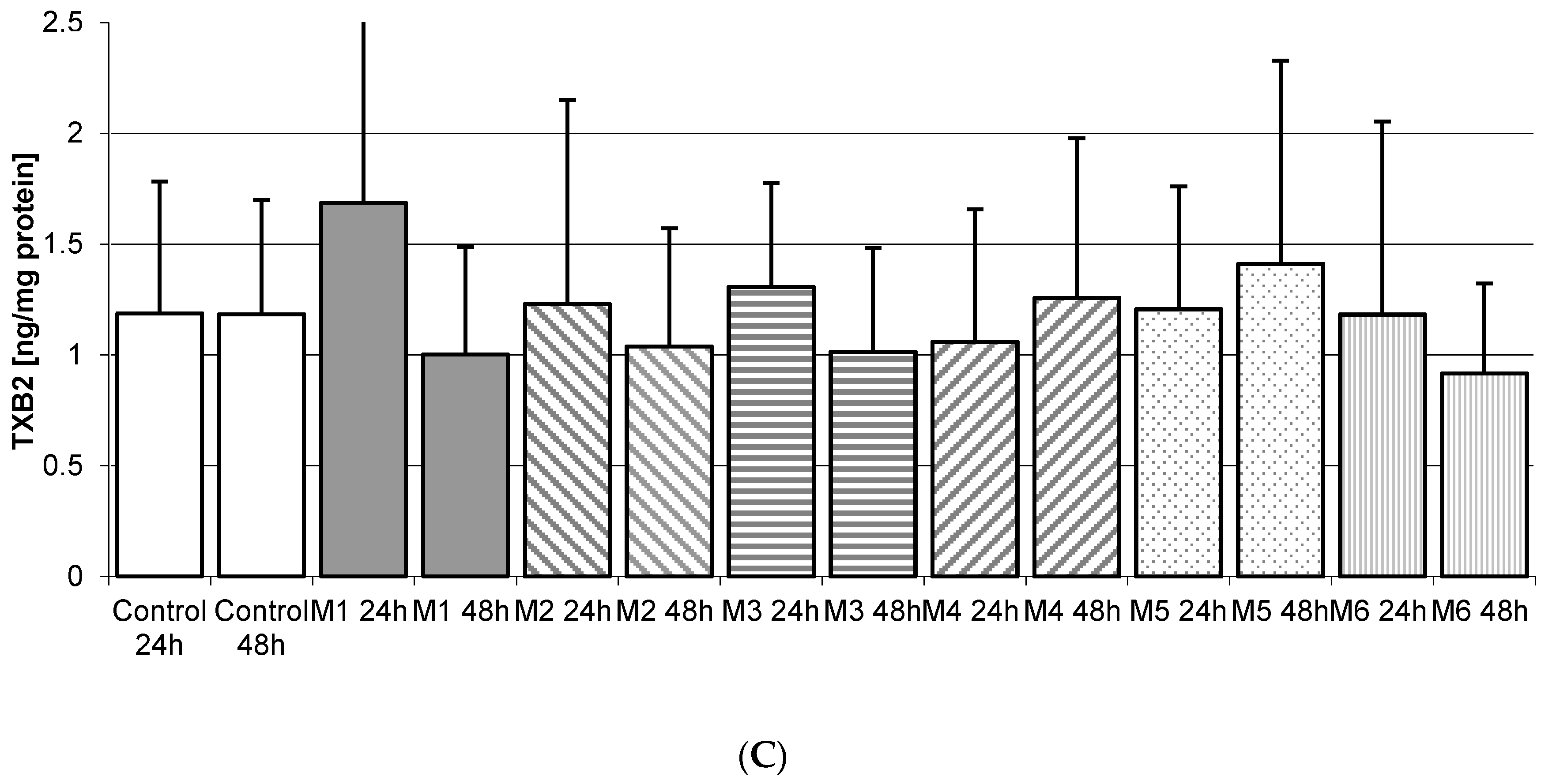
2.5. Thromboxane TXB2 in THP-1 Macrophages
2.5.1. Incubation of Macrophages with SYNTES Plates (S)
2.5.2. Incubation of Macrophages with MARTIN Plates (C)
2.5.3. Incubation of Macrophages with MEDARTIS Plates (M)
3. Discussion
4. Material and Methods
4.1. Reagents
4.2. 3D Condylar Titanium Plates
4.3. Cell Culture and Treatment
4.4. Verification of Plate-Induced Activation of THP-1 Monocytes and Initiation of Inflammatory Reaction
4.5. Flow Cytometry Measurement of THP-1 Cells Differentiation Into Macrophages
4.6. Verification of Plate-Induced Initiation of the Inflammatory Reaction in Macrophages
4.7. The Measurements of PGE2 Concentration
4.8. The Measurements of TXB2 Concentration
4.9. Protein Concentration
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Motamedi, M.H.; Dadgar, E.; Ebrahimi, A.; Shirani, G.; Haghighat, A.; Jamalpour, M.R. Pattern of maxillofacial fractures: A 5-year analysis of 8818 patients. J. Trauma Acute Care Surg. 2014, 77, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, P.; Ćwieka, K.; Adamczyk, K.; Kwaśniak, P.; Stopa, Z.; Samolczyk-Wanyura, D. Assessment of strength of titanium miniplates used for mandibular angle fractures osteosynthesis. Czas. Stomatol. 2010, 63, 749–755. [Google Scholar]
- Eckelt, U.; Schneider, M.; Erasmus, F.; Gerlach, K.L.; Kuhlisch, E.; Loukota, R.; Rasse, M.; Schubert, J.; Terheyden, H. Open versus closed treatment of fractures of the mandibular condylar process—A prospective randomized multi-centre study. J. Cranio-Maxillofac. Surg. 2006, 34, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Derfoufi, L.; Delaval, C.; Goudot, P.; Yachouh, J. Complications of condylar fracture osteosynthesis. J. Craniofac. Surg. 2011, 22, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E., 3rd; Throckmorton, G.S.; Palmieri, C. Open treatment of condylar process fractures: Assessment of adequacy of repositioning and maintenance of stability. J. Oral Maxillofac. Surg. 2000, 58, 27–34. [Google Scholar] [CrossRef]
- Sikora, M.; Sielski, M.; Stąpor, A.; Chlubek, D. Use of the Delta plate for surgical treatment of patients with condylar fractures. J. Cranio-Maxillofac. Surg. 2016, 44, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Serhir, L.; Boutemi, P. Experimental evaluation of three osteosynthesis devices used for stabilizing condylar fractures of the mandible. J. Cranio-Maxillofac Surg. 2006, 34, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Świniarski, J. “A” shape plate for open rigid internal fixation of mandible condyle neck fracture. J. Cranio-Maxillofac. Surg. 2014, 42, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Hyde, N.; Manisali, M.; Aghabeigi, B.; Sneddon, K.; Newman, L. The role of open reduction and internal fixation in unilateral fractures of the mandibular condyle: A prospective study. Br. J. Oral Maxillofac. Surg. 2002, 40, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Rallis, G.; Mourouzis, C.; Ainatzoglou, M.; Mezitis, M.; Zachariades, N. Plate osteosynthesis of condylar fractures: A retrospective study of 45 patients. Quintessence Int. 2003, 34, 45–49. [Google Scholar] [PubMed]
- Lauer, G.; Pradel, W.; Schneider, M.; Eckelt, U. A new 3-dimensional plate for transoral endoscopic-assisted osteosynthesis of condylar neck fractures. J. Oral Maxillofac. Surg. 2007, 65, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Refai, A.K.; Textor, M.; Brunette, D.M.; Waterfield, J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J. Biomed. Mater. Res. A 2004, 70, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Kzyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; Lavalle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, P.; Guo, X.; Huang, N.; Shen, R. An in vitro evaluation of inflammation response of titanium functionalized with heparyn/fibronectin complex. Cytokine 2011, 56, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotics resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Fukano, Y.; Knowles, N.G.; Usui, M.L.; Underwood, R.A.; Hauch, K.D.; Marshall, A.J.; Ratner, B.D.; Giachelli, C.; Carter, W.G.; Fleckman, P.; et al. Characterization of an in vitro model for evaluating the interface between skin and percutaneous biomaterials. Wound Repair Regen. 2006, 14, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Futami, T.; Fujii, N.; Ohnihi, H.; Taguchi, N.; Kusakari, H.; Ohshima, H.; Maeda, T. Tissue response to titanium implants in the rat maxilla: Ultrastructural and histochemical observations of the bone-titanium interface. J. Periodontol. 2000, 71, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Auger, M.J.; Ross, J.A. The biology of the macrophage. In The Macrophage; IRL Press: New York, NY, USA, 1992; pp. 3–74. [Google Scholar]
- Van Furth, R. Human monocytes and cytokines. Res. Immunol. 1998, 149, 719–720. [Google Scholar] [CrossRef]
- Gordon, S. The macrophage. Bioessays 1995, 17, 977–986. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, D.L. Ptostaglandin endoperoxide synthase: Regulation of enzyme expression. Biochim. Biophys. Acta 1991, 1083, 121–134. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Piotrowska, K.; Chlubek, D. Cyclooxygenase-1 as the main source of proinlammatory factors after sodium orthovanadate treatment. Biol. Trace Elem. Res. 2015, 163, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Morita, I. Distinct functions of COX-1 and COX-2 Prostaglandins other. Lipid Mediat. 2002, 68–69, 165–175. [Google Scholar]
- Font-Nieves, M.; Sans-Fons, G.; Gorina, R.; Bonfill-Teixidor, E.; Salas-Perdomo, A.; Marquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Induction of COX-2 enzyme and down-regualtion of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. JBC 2012, 287, 6454–6468. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Iadarola, M.; Yang, H.Y.; Dionne, R.A. Expression of COX-1and COX-2 in a clinical model of acute inflammation. J. Pain 2007, 8, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Hohg, H.; Jang, B.C. Transcriptional and translational regulation of COX-2 in a clinical model of acute inflammation. Int. J. Mol. Med. 2012, 30, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Tengvall, P.; Lundström, I.; Sjöqvist, L.; Elwing, H.; Bjursten, L.M. Titanium-hydrogen peroxide interaction: Model studies of the influence of the inflammatory response on titanium implants. Biomaterials 1989, 10, 166–175. [Google Scholar] [CrossRef]
- Overgaard, L.; Danielsen, N.; Bjursten, L.M. Anti-infalammatory properties of titanium in the joint environmental. J. Bone Jt. Surg. 1990, 80-B, 888–893. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.S.; Yin, Y.; Feng, Y.; Tan, X.W. Macrophage proinflammatory response to the titanium alloy equipment in dental implantation. Gent. Mol. Res. 2015, 14, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarette, R.; Hyzy, S.L.; Slosar, P.J.; Schneider, M.J.; Schwartz, Z.; Boyan, B.D. Implant materials Generate Different Peri-implant inflammatory factors. Spine 2015, 40, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Grutz, G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J. Leukoc. Biol. 2005, 77, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Nikura, T.; Saegusa, Y.; Nishikawa, T.; Yoshiya, K.M. Titanium-alloy particles induced cyclooxygenase-2 in human macrophage-like cells in vitro. Kobe J. Med. Sci. 2002, 48, 115–123. [Google Scholar]
- Fujishiro, T.; Nishikawa, T.; Shibanuma, N.; Akisue, T.; Takikawa, S.; Yamamoto, T.; Yoshiya, S.; Kurosaka, M. Effect of cyclic mechanical stretch and particles on prostaglandin E2 production by human macrophages in vitro. J. Biomed. Mater. Res. A 2004, 68, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Surowska, B. Metal Biomaterials and Metal-Ceramic Compositions in Stomatological Application, 1st ed.; University Publishing: Lublin, Poland, 2009; pp. 26–33. [Google Scholar]
- Auwerx, J. The human leukemia cell line, THP1: A multifacetted model for study of monocyte-macrophage differentiation. Experientia 1991, 47, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 2012, 221, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Olszowski, T.; Gutowska, I.; Baranowska-Bosiacka, I.; Piotrowska, K.; Korbecki, J.; Kurzawski, M.; Chlubek, D. The Effect of Cadmium on COX-1 and COX-2 Gene, Protein Expression, and Enzymatic Activity in THP-1 Macrophages. Biol. Trace Elem. Res. 2015, 165, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Gutowska, I.; Baranowska-Bosiacka, I.; Goschorska, M.; Kolasa, A.; Łukomska, A.; Jakubczyk, K.; Dec, K.; Chlubek, D. Fluoride as a factor initiating and potentiating inflammation in THP1 differentiated monocytes/macrophages. Toxicol. In Vitro 2015, 29, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, G.R.; De Cleen, D.M.; Cooper, S.; Knaapen, M.W.; Jans, D.M.; Martinet, W.; Herman, A.G.; Bult, H.; Kockx, M.M. Platelet phagocytosis and processing of beta-amyloid precursor protein as a mechanism of macrophage activation in atherosclerosis. Circ. Res. 2002, 90, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.L.; Coffman, T.M.; Koller, B.H. Mixed messages: Modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Investig. 2001, 108, 15–23. [Google Scholar] [CrossRef] [PubMed]

| Metal Alloy | Element (Weight) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | O | N | C | H | Al | V | Other | Ti | |
| Ti grade 2 (ASTMF 67:2000) | 0.3 | 0.25 | 0.03 | 0.08 | 0.0125 | rest | |||
| Ti6Al4V ELI grade5 (ASTM F136; ISO5832-3) (TAV) | 0.25 | 0.13 | 0.05 | 0.08 | 0.012 | 5.5–6.5 | 3.5–4.5 | rest | |
| Ti6Al7Nb (ASTM F1295; ISO:5832-11) (TAN) | 0.25 | 0.20 | 0.05 | 0.08 | 0.009 | 5.5–6.5 | Nb 6.5–7.5 Ta max. 0.5 | rest | |
| Screw/Plate | Manufacturer | Catalog Number | Symbol |
|---|---|---|---|
| Plate | SYNTHES | 04.503.834 | S1 |
| Plate | SYNTHES | 04.503.833 | S2 |
| Screw | SYNTHES | 04.503.406.01C | S3 |
| Screw | KLS MARTIN | 25-872-09 | C1 |
| Plate | KLS MARTIN | 25-283-05 | C2 |
| Plate | KLS MARTIN | 25-285-05 | C3 |
| Screw | KLS MARTIN | 25-882-09 | C4 |
| Screw | MEDARTIS | M-5240.06 | M1 |
| Screw | MEDARTIS | M-5245.06 | M2 |
| Plate | MEDARTIS | M-4318 | M3 |
| Plate | MEDARTIS | M-4852 | M4 |
| Plate | MEDARTIS | M-4894 | M5 |
| Plate | MEDARTIS | M-4658 | M6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, M.; Goschorska, M.; Baranowska-Bosiacka, I.; Chlubek, D. In Vitro Effect of 3D Plates Used for Surgical Treatment of Condylar Fractures on Prostaglandin E2 (PGE2) and Thromboxane B2 (TXB2) Concentration in THP-1 Macrophages. Int. J. Mol. Sci. 2017, 18, 2638. https://doi.org/10.3390/ijms18122638
Sikora M, Goschorska M, Baranowska-Bosiacka I, Chlubek D. In Vitro Effect of 3D Plates Used for Surgical Treatment of Condylar Fractures on Prostaglandin E2 (PGE2) and Thromboxane B2 (TXB2) Concentration in THP-1 Macrophages. International Journal of Molecular Sciences. 2017; 18(12):2638. https://doi.org/10.3390/ijms18122638
Chicago/Turabian StyleSikora, Maciej, Marta Goschorska, Irena Baranowska-Bosiacka, and Dariusz Chlubek. 2017. "In Vitro Effect of 3D Plates Used for Surgical Treatment of Condylar Fractures on Prostaglandin E2 (PGE2) and Thromboxane B2 (TXB2) Concentration in THP-1 Macrophages" International Journal of Molecular Sciences 18, no. 12: 2638. https://doi.org/10.3390/ijms18122638





