Identifying the Long-Term Role of Inducible Nitric Oxide Synthase after Contusive Spinal Cord Injury Using a Transgenic Mouse Model
Abstract
:1. Introduction
2. Results
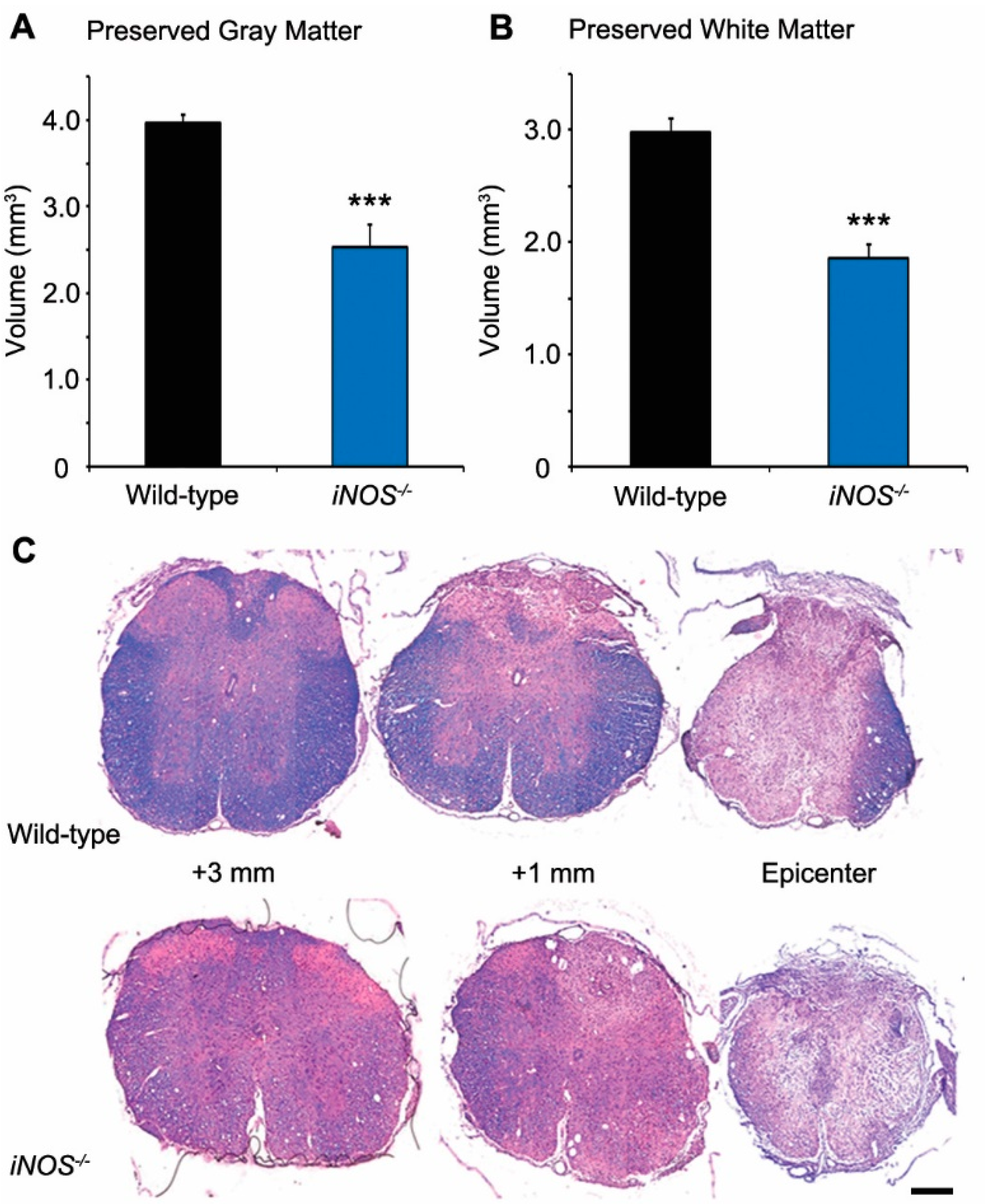
2.1. White and Gray Matter Preservation Was Significantly Less in Inducible Nitric Oxide Synthase (iNOS−/−) Mice after Spinal Cord Injury (SCI)
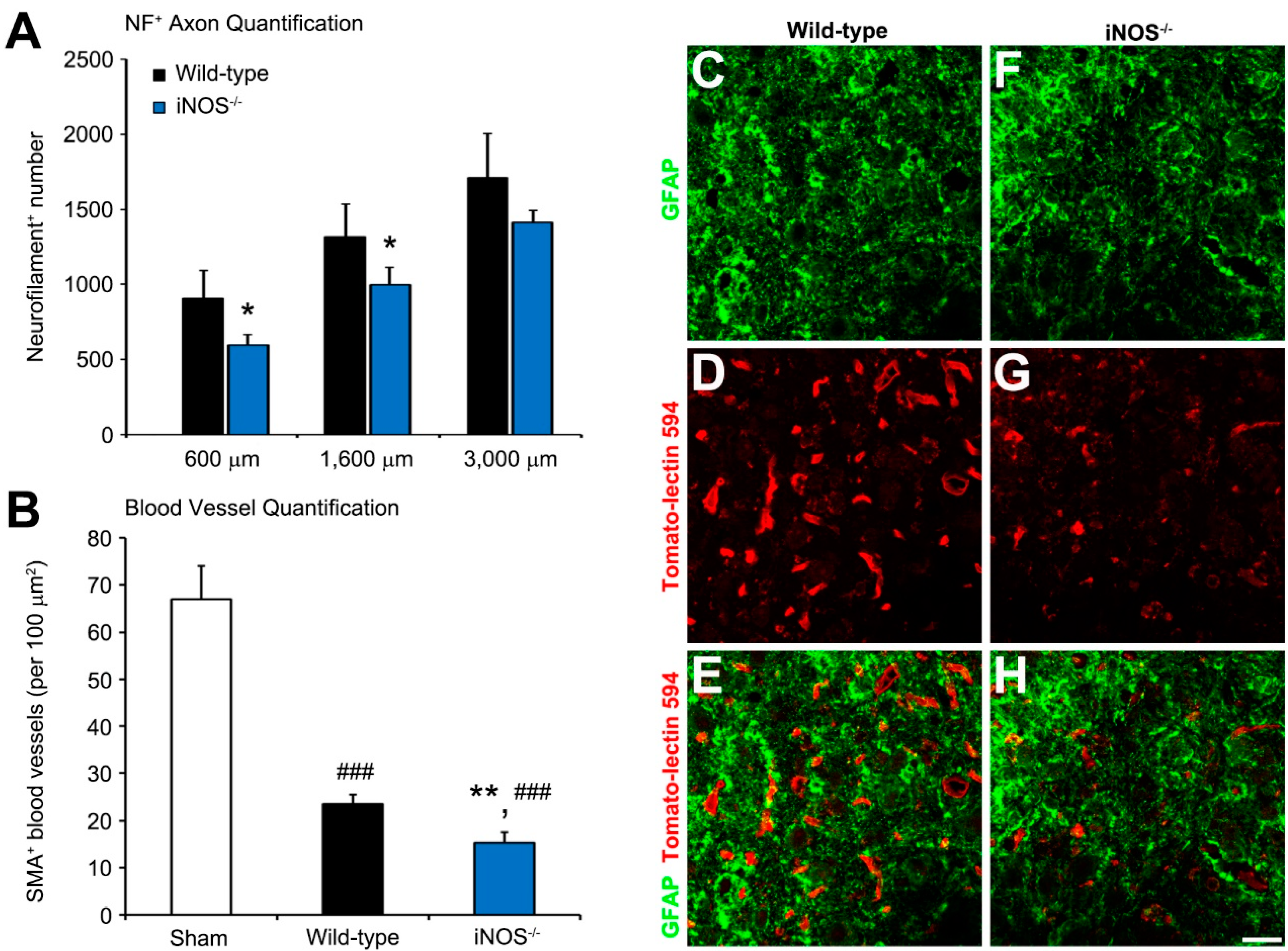
2.2. Fewer Dorsal Column Neurofilament (NF)+ Axon Profiles and Perilesional Blood Vessels Were Present Rostral to the Lesion in Inducible Nitric Oxide Synthase (iNOS−/−) Mice after Spinal Cord Injury (SCI)
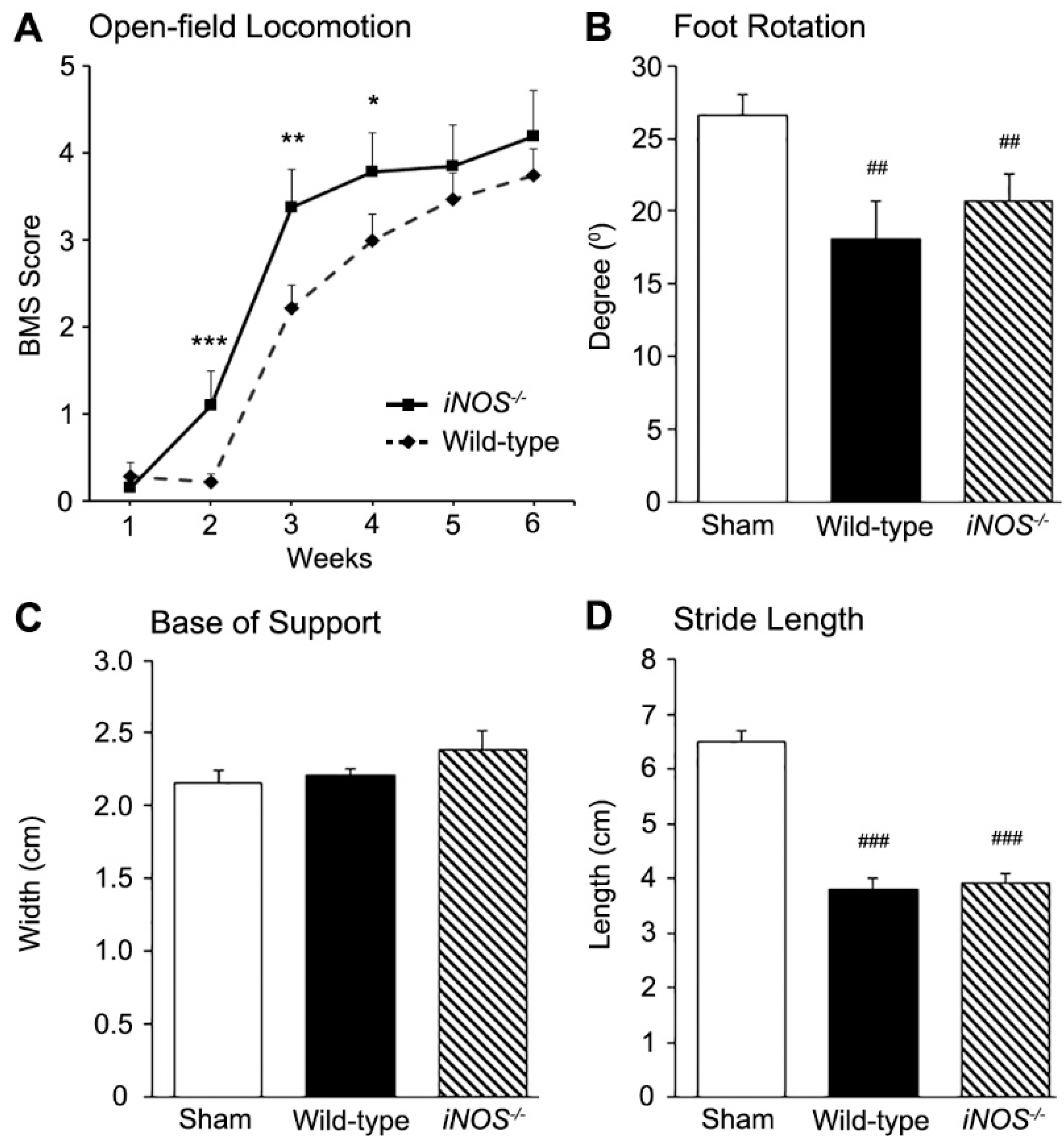
2.3. Inducible Nitric Oxide Synthase (iNOS−/−) Mice Showed an Acute Improvement in Functional Recovery over Wild-Type (WT) Controls after Spinal Cord Injury (SCI) That Did Not Persist Long-Term
3. Discussion
4. Experimental Procedures
4.1. Animals
4.2. Moderate Thoracic Contusion Injury
4.3. Histology
4.4. Estimation of Tissue Volumes
4.5. Immunohistochemistry and Quantification of Stained Profiles
4.6. Behavioral Testing
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Pereira, F.C.; Marcillo, A.E.; Bates, M.L.; Berrocal, Y.A.; Filbin, M.T.; Bunge, M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Bunge, M.B. Designing cell- and gene-based regeneration strategies to repair the injured spinal cord. J. Neurotrauma 2006, 23, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Bethea, J.R.; Dietrich, W.D. Targeting the host inflammatory response in traumatic spinal cord injury. Curr. Opin. Neurol. 2002, 15, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Liu, D. Peroxynitrite generated in the rat spinal cord induces neuron death and neurological deficits. Neuroscience 2002, 115, 839–849. [Google Scholar] [CrossRef]
- Bao, F.; Chen, Y.; Dekaban, G.A.; Weaver, L.C. An anti-CD11d integrin antibody reduces cyclooxygenase-2 expression and protein and DNA oxidation after spinal cord injury in rats. J. Neurochem. 2004, 90, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Lee, Y.S.; Lin, C.Y.; Lin, V.W.; Sindhu, R.K. NAD(P)H oxidase, superoxide dismutase, catalase, glutathione peroxidase and nitric oxide synthase expression in subacute spinal cord injury. Brain Res. 2004, 995, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Baud, O.; Vartanian, T.; Volpe, J.J.; Rosenberg, P.A. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 9936–9941. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bao, F. Hydrogen peroxide administered into the rat spinal cord at the level elevated by contusion spinal cord injury oxidizes proteins, DNA and membrane phospholipids, and induces cell death: Attenuation by a metalloporphyrin. Neuroscience 2015, 285, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Kamencic, H.; Griebel, R.W.; Lyon, A.W.; Paterson, P.G.; Juurlink, B.H. Promoting glutathione synthesis after spinal cord trauma decreases secondary damage and promotes retention of function. FASEB J. 2001, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Lewen, A.; Gasche, Y.; Yu, F.; Chan, P.H. Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release. FASEB J. 2002, 16, 1997–1999. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Chatzipanteli, K.; Marcillo, A.E.; Bunge, M.B.; Dietrich, W.D. Comparison of iNOS inhibition by antisense and pharmacological inhibitors after spinal cord injury. J. Neuropathol. Exp. Neurol. 2003, 62, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Mazzon, E.; Esposito, E.; Di Paola, R.; Murthy, K.; Neville, L.; Bramanti, P.; Cuzzocrea, S. Effects of a metalloporphyrinic peroxynitrite decomposition catalyst, ww-85, in a mouse model of spinal cord injury. Free Radic. Res. 2009, 43, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Fleming, J.C.; Golshani, R.; Pearse, D.D.; Kasabov, L.; Brown, A.; Weaver, L.C. A selective phosphodiesterase-4 inhibitor reduces leukocyte infiltration, oxidative processes, and tissue damage after spinal cord injury. J. Neurotrauma 2011, 28, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, J.T.; Morton, P.D.; Jayakumar, A.R.; Johnstone, A.L.; Gao, H.; Bracchi-Ricard, V.; Pearse, D.D.; Norenberg, M.D.; Bethea, J.R. Inhibition of NADPH oxidase activation in oligodendrocytes reduces cytotoxicity following trauma. PLoS ONE 2013, 8, e80975. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Aguirre, V.; Wai, K.; Felfly, H.; Dietrich, W.D.; Pearse, D.D. The interplay between cyclic AMP, MAPK, and NF-κB pathways in response to proinflammatory signals in microglia. BioMed Res. Int. 2015, 2015, 308461. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.P. Role of nitric oxide in the regulation of monoaminergic neurotransmission. Brain Res. Bull. 2000, 52, 459–466. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Zhang, F.; Casey, R.; Nagayama, M.; Ross, M.E. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997, 17, 9157–9164. [Google Scholar] [PubMed]
- Chen, K.; Northington, F.J.; Martin, L.J. Inducible nitric oxide synthase is present in motor neuron mitochondria and Schwann cells and contributes to disease mechanisms in ALS mice. Brain Struct. Funct. 2010, 214, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Hall, E.D.; Detloff, M.R.; Johnson, K.; Kupina, N.C. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma 2004, 21, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Wang, J.A.; Miller, D.M. Relationship of nitric oxide synthase induction to peroxynitrite-mediated oxidative damage during the first week after experimental traumatic brain injury. Exp. Neurol. 2012, 238, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Carrico, K.M.; Vaishnav, R.; Hall, E.D. Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. J. Neurotrauma 2009, 26, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, J.P.; Heales, S.J.; Land, J.M.; Clark, J.B. Effect of peroxynitrite on the mitochondrial respiratory chain: Differential susceptibility of neurones and astrocytes in primary culture. J. Neurochem. 1995, 64, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett. 2003, 140–141, 105–112. [Google Scholar] [CrossRef]
- Maggio, D.M.; Chatzipanteli, K.; Masters, N.; Patel, S.P.; Dietrich, W.D.; Pearse, D.D. Acute molecular perturbation of inducible nitric oxide synthase with an antisense approach enhances neuronal preservation and functional recovery after contusive spinal cord injury. J. Neurotrauma 2012, 29, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Schwentker, A.; Billiar, T.R. Nitric oxide and wound repair. Surg. Clin. N. Am. 2003, 83, 521–530. [Google Scholar] [CrossRef]
- Debats, I.B.; Wolfs, T.G.; Gotoh, T.; Cleutjens, J.P.; Peutz-Kootstra, C.J.; van der Hulst, R.R. Role of arginine in superficial wound healing in man. Nitric Oxide 2009, 21, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Chatzipanteli, K.; Garcia, R.; Marcillo, A.E.; Loor, K.E.; Kraydieh, S.; Dietrich, W.D. Temporal and segmental distribution of constitutive and inducible nitric oxide synthases after traumatic spinal cord injury: Effect of aminoguanidine treatment. J. Neurotrauma 2002, 19, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, J.; Farooque, M.; Olsson, Y. Improved functional outcome after spinal cord injury in iNOS-deficient mice. Spinal Cord 2005, 43, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Kotil, K.; Kuscuoglu, U.; Kirali, M.; Uzun, H.; Akcetin, M.; Bilge, T. Investigation of the dose-dependent neuroprotective effects of agmatine in experimental spinal cord injury: A prospective randomized and placebo-control trial. J. Neurosurg. Spine 2006, 4, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Sinz, E.H.; Kochanek, P.M.; Dixon, C.E.; Clark, R.S.; Carcillo, J.A.; Schiding, J.K.; Chen, M.; Wisniewski, S.R.; Carlos, T.M.; Williams, D.; et al. Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J. Clin. Investig. 1999, 104, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Kubes, P.; Zochodne, D.W. Delayed peripheral nerve degeneration, regeneration, and pain in mice lacking inducible nitric oxide synthase. J. Neuropathol. Exp. Neurol. 2001, 60, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Fenyk-Melody, J.E.; Garrison, A.E.; Brunnert, S.R.; Weidner, J.R.; Shen, F.; Shelton, B.A.; Mudgett, J.S. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J. Immunol. 1998, 160, 2940–2946. [Google Scholar] [PubMed]
- Sahrbacher, U.C.; Lechner, F.; Eugster, H.P.; Frei, K.; Lassmann, H.; Fontana, A. Mice with an inactivation of the inducible nitric oxide synthase gene are susceptible to experimental autoimmune encephalomyelitis. Eur. J. Immunol. 1998, 28, 1332–1338. [Google Scholar] [CrossRef]
- Arnett, H.A.; Hellendall, R.P.; Matsushima, G.K.; Suzuki, K.; Laubach, V.E.; Sherman, P.; Ting, J.P. The protective role of nitric oxide in a neurotoxicant-induced demyelinating model. J. Immunol. 2002, 168, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.K.; Wittmer, S. Nitric-oxide-dependent and independent mechanisms of protection from CNS inflammation during Th1-mediated autoimmunity: Evidence from EAE in iNOS KO mice. J. Neuroimmunol. 2005, 160, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Okuda, Y.; Sakoda, S.; Fujimura, H.; Yanagihara, T. Aminoguanidine, a selective inhibitor of the inducible nitric oxide synthase, has different effects on experimental allergic encephalomyelitis in the induction and progression phase. J. Neuroimmunol. 1998, 81, 201–210. [Google Scholar] [CrossRef]
- Wei, X.Q.; Charles, I.G.; Smith, A.; Ure, J.; Feng, G.J.; Huang, F.P.; Xu, D.; Muller, W.; Moncada, S.; Liew, F.Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 1995, 375, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.P.; Niedbala, W.; Wei, X.Q.; Xu, D.; Feng, G.J.; Robinson, J.H.; Lam, C.; Liew, F.Y. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur. J. Immunol. 1998, 28, 4062–4070. [Google Scholar] [CrossRef]
- Niedbala, W.; Wei, X.Q.; Piedrafita, D.; Xu, D.; Liew, F.Y. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur. J. Immunol. 1999, 29, 2498–2505. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, T. Expression of constitutive endothelial and inducible nitric oxide synthase in the sciatic nerve of Lewis rats with experimental autoimmune neuritis. J. Neuroimmunol. 2002, 126, 78–85. [Google Scholar] [CrossRef]
- Kahl, K.G.; Schmidt, H.H.; Jung, S.; Sherman, P.; Toyka, K.V.; Zielasek, J. Experimental autoimmune encephalomyelitis in mice with a targeted deletion of the inducible nitric oxide synthase gene: Increased T-helper 1 response. Neurosci. Lett. 2004, 358, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Choi, Y.B.; Pan, Z.H.; Lei, S.Z.; Chen, H.S.; Sucher, N.J.; Loscalzo, J.; Singel, D.J.; Stamler, J.S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 1993, 364, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S. Production of nitric oxide by glial cells: Regulation and potential roles in the CNS. Glia 2000, 29, 1–13. [Google Scholar] [CrossRef]
- Shahani, N.; Sawa, A. Protein S-nitrosylation: Role for nitric oxide signaling in neuronal death. Biochim. Biophys. Acta 2012, 1820, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, T.; Jain, R.K.; Mumtaz, N.; Morris, R. Inhibition of neuronal nitric oxide synthase results in neurodegenerative changes in the axotomised dorsal root ganglion neurons: Evidence for a neuroprotective role of nitric oxide in vivo. Neurosci. Res. 2001, 40, 37–44. [Google Scholar] [CrossRef]
- Donnini, S.; Ziche, M. Constitutive and inducible nitric oxide synthase: Role in angiogenesis. Antioxid. Redox Signal. 2002, 4, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Yang, H.I.; Chen, S.D.; Shaw, F.Z.; Yang, D.I. Protective effects of lipopolysaccharide preconditioning against nitric oxide neurotoxicity. J. Neurosci. Res. 2008, 86, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Z.A. Nitric oxide show its survival role by NO-PKC pathway through cGMP-dependent or independent on the culture of cerebella granular neurons. Neurosci. Lett. 2014, 583, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, T.; McKay, J.S.; Morris, R. Bax and caspases are inhibited by endogenous nitric oxide in dorsal root ganglion neurons in vitro. Eur. J. Neurosci. 2001, 14, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Keilhoff, G.; Fansa, H.; Wolf, G. Differences in peripheral nerve degeneration/regeneration between wild-type and neuronal nitric oxide synthase knockout mice. J. Neurosci. Res. 2002, 68, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Keilhoff, G.; Fansa, H.; Wolf, G. Nitric oxide synthase, an essential factor in peripheral nerve regeneration. Cell Mol. Biol. 2003, 49, 885–897. [Google Scholar] [PubMed]
- Keilhoff, G.; Fansa, H.; Wolf, G. Neuronal NOS deficiency promotes apoptotic cell death of spinal cord neurons after peripheral nerve transection. Nitric Oxide 2004, 10, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; Negrete, R.; Leanez, S.; Martin-Campos, J.M.; Pol, O. The spinal cord expression of neuronal and inducible nitric oxide synthases and their contribution in the maintenance of neuropathic pain in mice. PLoS ONE 2010, 5, e14321. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, T.; McKay, J.S.; Morris, R.; Quinn, J.; Wong, L.F.; Murphy, D. Glial-mediated neuroprotection: Evidence for the protective role of the NO-cGMP pathway via neuron-glial communication in the peripheral nervous system. Glia 2005, 49, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Keswani, S.C.; Bosch-Marce, M.; Reed, N.; Fischer, A.; Semenza, G.L.; Hoke, A. Nitric oxide prevents axonal degeneration by inducing HIF-1-dependent expression of erythropoietin. Proc. Natl. Acad. Sci. USA 2011, 108, 4986–4990. [Google Scholar] [CrossRef] [PubMed]
- Colasanti, M.; Persichini, T.; Cavalieri, E.; Fabrizi, C.; Mariotto, S.; Menegazzi, M.; Lauro, G.M.; Suzuki, H. Rapid inactivation of NOS-I by lipopolysaccharide plus interferon-γ-induced tyrosine phosphorylation. J. Biol. Chem. 1999, 274, 9915–9917. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Colasanti, M. Cross-talk between constitutive and inducible nitric oxide synthases. Circulation 2001, 103, E81. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ruiz, A.; Ibarra, A.; Perez-Severiano, F.; Guizar-Sahagun, G.; Grijalva, I.; Rios, C. Constitutive and inducible nitric oxide synthase activities after spinal cord contusion in rats. Neurosci. Lett. 2002, 319, 129–132. [Google Scholar] [CrossRef]
- Conti, A.; Cardali, S.; Genovese, T.; Di Paola, R.; La Rosa, G. Role of inflammation in the secondary injury following experimental spinal cord trauma. J. Neurosurg. Sci. 2003, 47, 89–94. [Google Scholar] [PubMed]
- Conti, A.; Miscusi, M.; Cardali, S.; Germano, A.; Suzuki, H.; Cuzzocrea, S.; Tomasello, F. Nitric oxide in the injured spinal cord: Synthases cross-talk, oxidative stress and inflammation. Brain Res. Rev. 2007, 54, 205–218. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Cardali, S.; Genovese, T.; Conti, A.; di Paola, R.; la Torre, D.; Cacciola, F.; Cuzzocrea, S. Inhibition of the nuclear factor-κB activation with pyrrolidine dithiocarbamate attenuating inflammation and oxidative stress after experimental spinal cord trauma in rats. J. Neurosurg. Spine 2004, 1, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Mazzon, E.; Mariotto, S.; Menegazzi, M.; Cardali, S.; Conti, A.; Suzuki, H.; Bramanti, P.; Cuzzocrea, S. Modulation of nitric oxide homeostasis in a mouse model of spinal cord injury. J. Neurosurg. 2006, 4, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129 Pt 12, 3249–3269. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Caputi, A.P.; Zingarelli, B. Peroxynitrite-mediated DNA strand breakage activates poly (ADP-ribose) synthetase and causes cellular energy depletion in carrageenan-induced pleurisy. Immunology 1998, 93, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; Calabro, G.; Dugo, L.; de Sarro, A.; van De, L.F.; Caputi, A.P. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am. J. Respir. Crit. Care Med. 2000, 162, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ling, X.; Wen, J.; Liu, J. The role of reactive nitrogen species in secondary spinal cord injury: Formation of nitric oxide, peroxynitrite, and nitrated protein. J. Neurochem. 2000, 75, 2144–2154. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kim, G.M.; Chen, S.; Yan, P.; Ahmed, S.H.; Ku, G.; Beckman, J.S.; Xu, X.M.; Hsu, C.Y. Inos and nitrotyrosine expression after spinal cord injury. J. Neurotrauma 2001, 18, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; McGaughy, V.M.; Popovich, P.G. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J. Comp. Neurol. 2006, 494, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Popovich, P.G.; Hickey, W.F. Bone marrow chimeric rats reveal the unique distribution of resident and recruited macrophages in the contused rat spinal cord. J. Neuropathol. Exp. Neurol. 2001, 60, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Popovich, P.G.; Wei, P.; Stokes, B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997, 377, 443–464. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Semple-Rowland, S.L.; Hurley, S.D.; Miller, R.C.; Popovich, P.G.; Stokes, B.T. Cytokine mrna profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp. Neurol. 1998, 152, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Rabchevsky, A.G.; Hall, E.D. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 2007, 100, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Schaal, S.M.; Garg, M.S.; Ghosh, M.; Lovera, L.; Lopez, M.; Patel, M.; Louro, J.; Patel, S.; Tuesta, L.; Chan, W.M.; et al. The therapeutic profile of rolipram, pde target and mechanism of action as a neuroprotectant following spinal cord injury. PLoS ONE 2012, 7, e43634. [Google Scholar] [CrossRef] [PubMed]
- Bareyre, F.M.; Kerschensteiner, M.; Misgeld, T.; Sanes, J.R. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat. Med. 2005, 11, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Field, P.M.; Raisman, G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 1997, 277, 2000–2002. [Google Scholar] [CrossRef] [PubMed]
- Datto, J.P.; Bastidas, J.C.; Miller, N.L.; Shah, A.K.; Arheart, K.L.; Marcillo, A.E.; Dietrich, W.D.; Pearse, D.D. Female Rats Demonstrate Improved Locomotor Recovery and Greater Preservation of White and Gray Matter after Traumatic Spinal Cord Injury Compared to Males. J. Neurotrauma 2015, 32, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Roof, R.L.; Hall, E.D. Gender differences in acute CNS trauma and stroke: Neuroprotective effects of estrogen and progesterone. J. Neurotrauma 2000, 17, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Sribnick, E.A.; Samantaray, S.; Das, A.; Smith, J.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J. Neurosci. Res. 2010, 88, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Sribnick, E.A.; Wingrave, J.M.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann. N. Y. Acad. Sci. 2003, 993, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.G.; Wright, D.W.; Kellermann, A.L. Does progesterone have neuroprotective properties? Ann. Emerg. Med. 2008, 51, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Sribnick, E.A.; Das, A.; Thakore, N.P.; Matzelle, D.; Yu, S.P.; Ray, S.K.; Wei, L.; Banik, N.L. Neuroprotective efficacy of estrogen in experimental spinal cord injury in rats. Ann. N. Y. Acad. Sci. 2010, 1199, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Jakeman, L.B.; Guan, Z.; Wei, P.; Ponnappan, R.; Dzwonczyk, R.; Popovich, P.G.; Stokes, B.T. Traumatic spinal cord injury produced by controlled contusion in mouse. J. Neurotrauma 2000, 17, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Lo, T.P., Jr.; Cho, K.S.; Lynch, M.P.; Garg, M.S.; Marcillo, A.E.; Sanchez, A.R.; Cruz, Y.; Dietrich, W.D. Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J. Neurotrauma 2005, 22, 680–702. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef] [PubMed]
- De Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982, 77, 634–643. [Google Scholar] [CrossRef]
- Pearse, D.D.; Marcillo, A.E.; Oudega, M.; Lynch, M.P.; Wood, P.M.; Bunge, M.B. Transplantation of schwann cells and olfactory ensheathing glia after spinal cord injury: Does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J. Neurotrauma 2004, 21, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, D.M.; Singh, A.; Iorgulescu, J.B.; Bleicher, D.H.; Ghosh, M.; Lopez, M.M.; Tuesta, L.M.; Flora, G.; Dietrich, W.D.; Pearse, D.D. Identifying the Long-Term Role of Inducible Nitric Oxide Synthase after Contusive Spinal Cord Injury Using a Transgenic Mouse Model. Int. J. Mol. Sci. 2017, 18, 245. https://doi.org/10.3390/ijms18020245
Maggio DM, Singh A, Iorgulescu JB, Bleicher DH, Ghosh M, Lopez MM, Tuesta LM, Flora G, Dietrich WD, Pearse DD. Identifying the Long-Term Role of Inducible Nitric Oxide Synthase after Contusive Spinal Cord Injury Using a Transgenic Mouse Model. International Journal of Molecular Sciences. 2017; 18(2):245. https://doi.org/10.3390/ijms18020245
Chicago/Turabian StyleMaggio, Dominic M., Amanpreet Singh, J. Bryan Iorgulescu, Drew H. Bleicher, Mousumi Ghosh, Michael M. Lopez, Luis M. Tuesta, Govinder Flora, W. Dalton Dietrich, and Damien D. Pearse. 2017. "Identifying the Long-Term Role of Inducible Nitric Oxide Synthase after Contusive Spinal Cord Injury Using a Transgenic Mouse Model" International Journal of Molecular Sciences 18, no. 2: 245. https://doi.org/10.3390/ijms18020245




