Oral Administration of Surface-Deacetylated Chitin Nanofibers and Chitosan Inhibit 5-Fluorouracil-Induced Intestinal Mucositis in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. SDACNFs Suppress the Effects of 5-FU-Induced Intestinal Mucositis
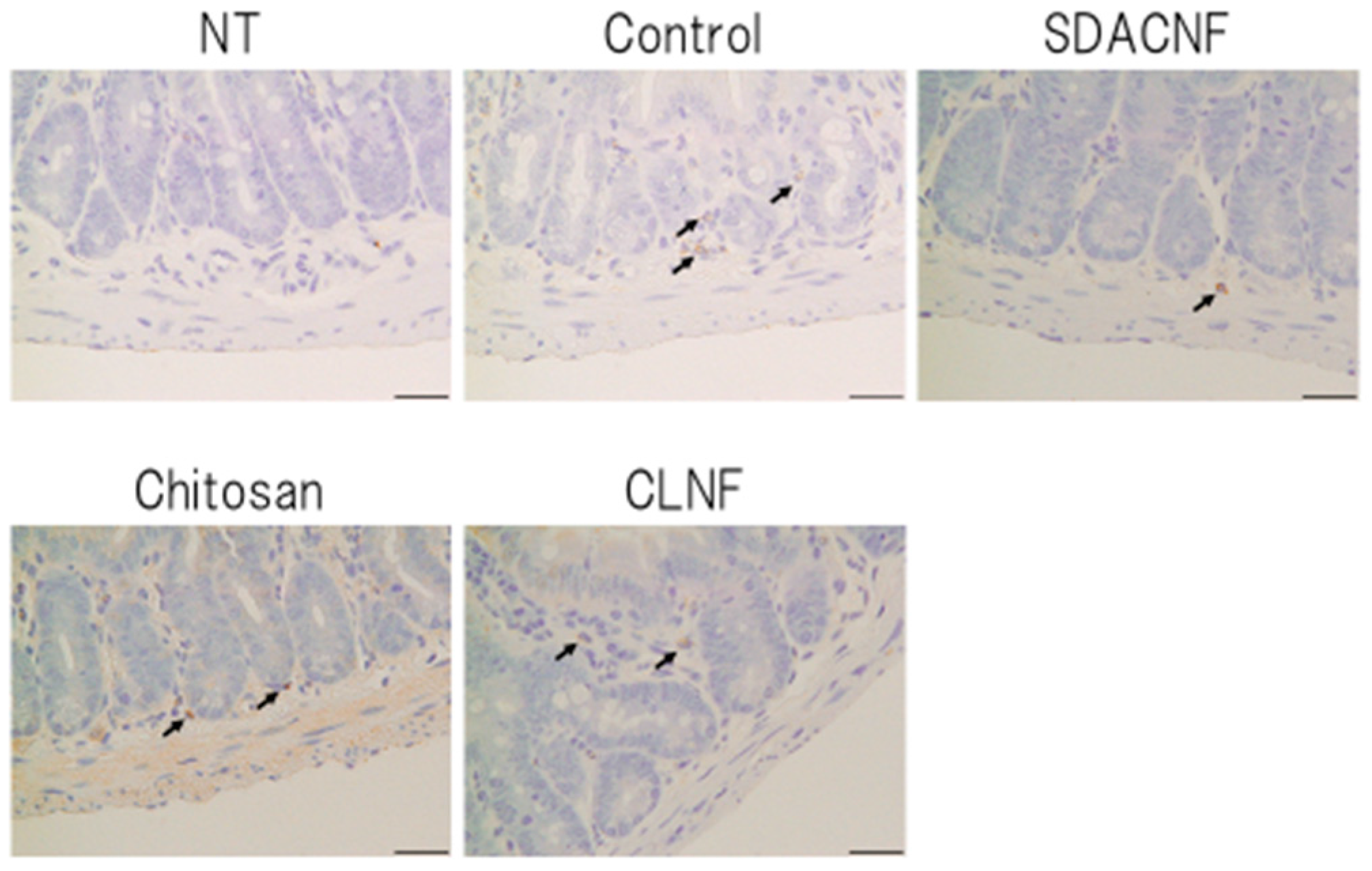
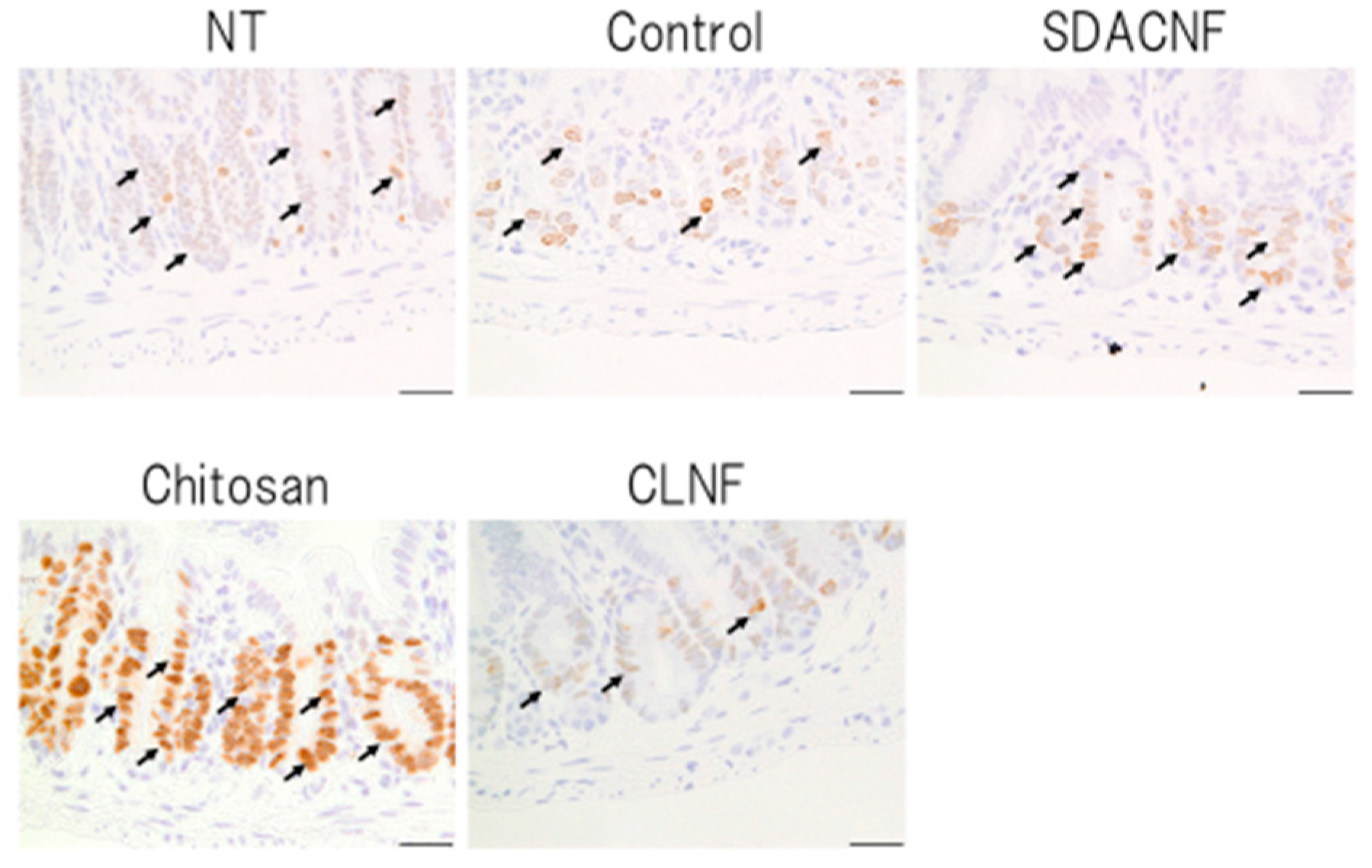
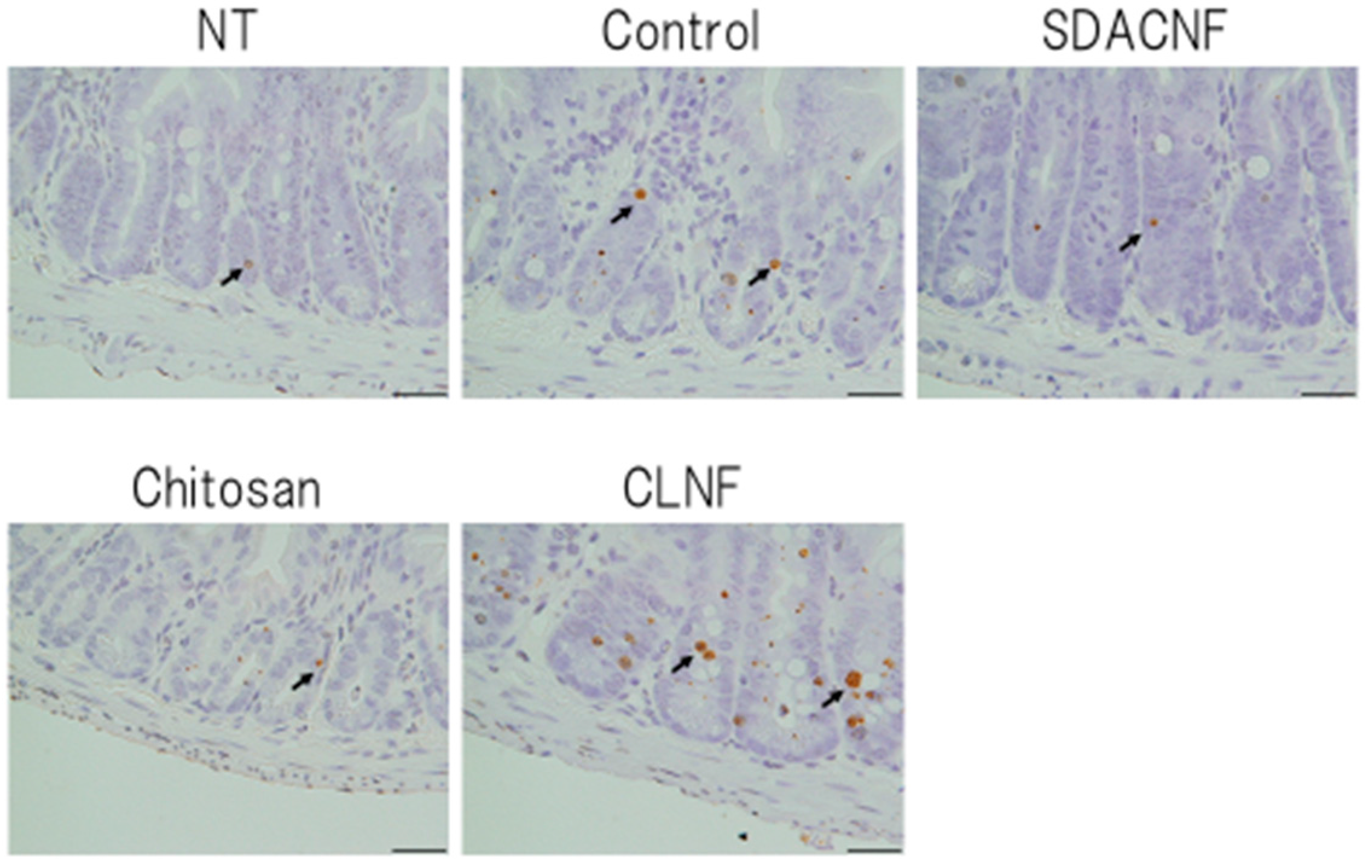
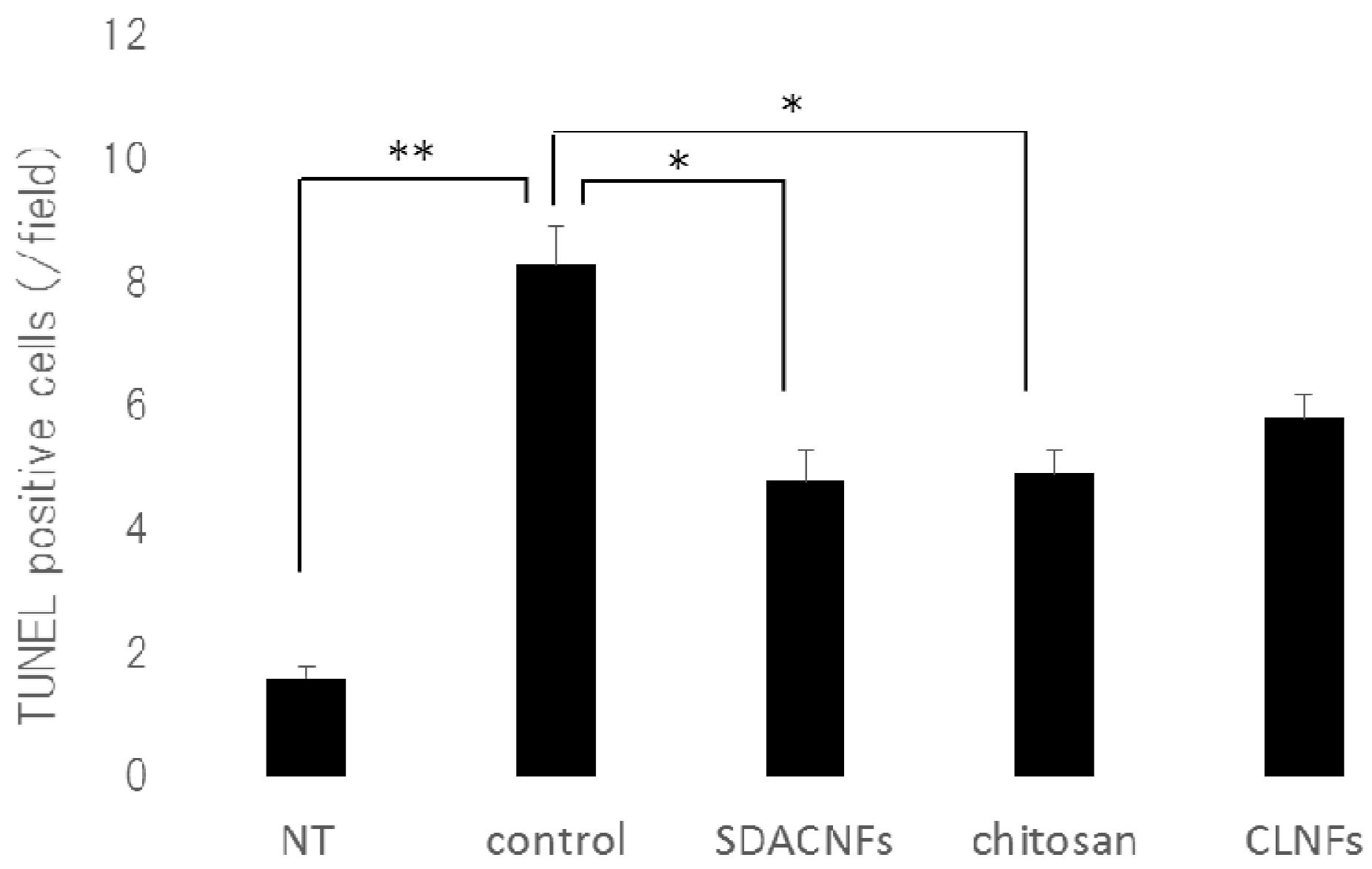
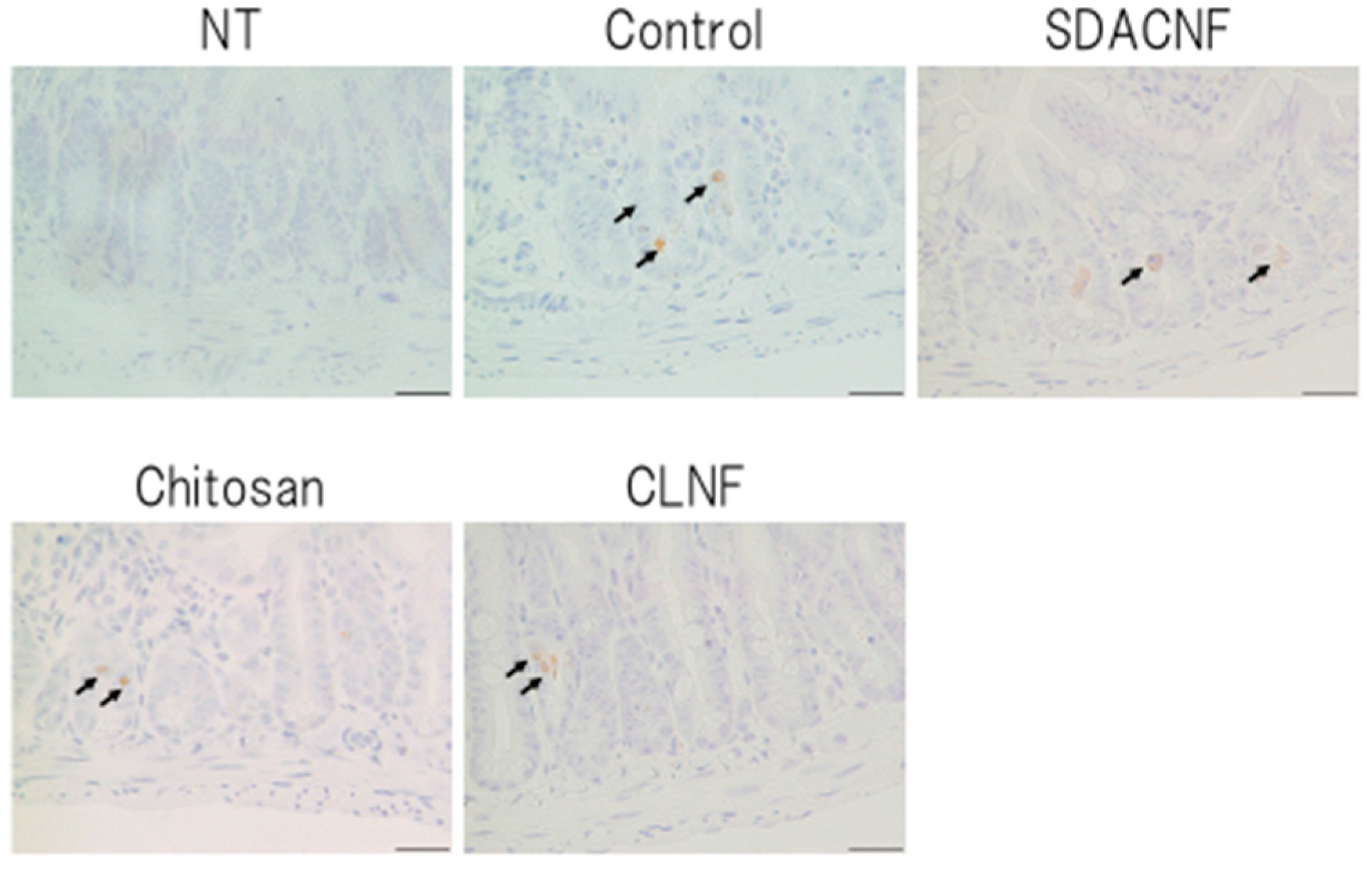
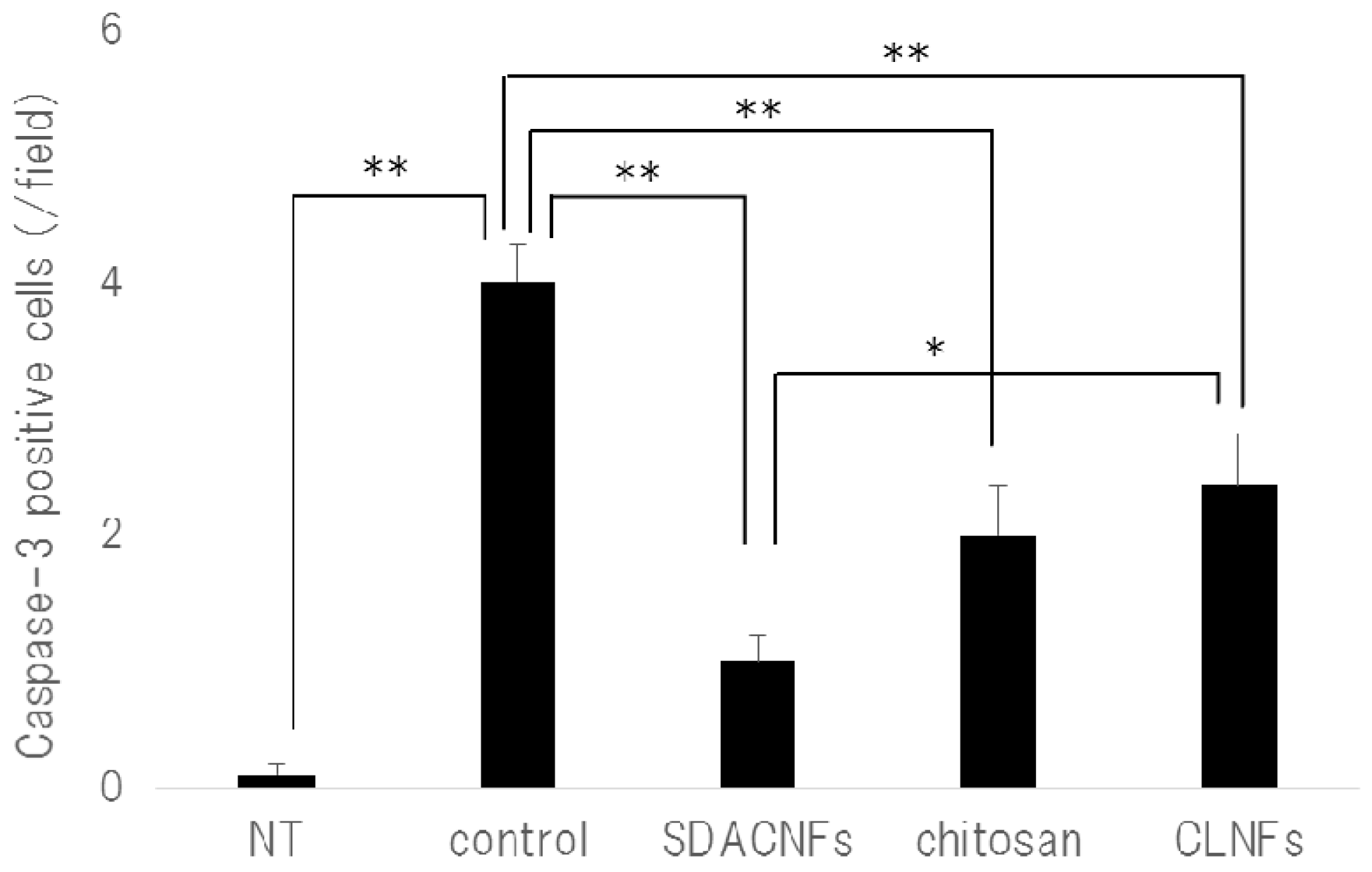
2.2. SDACNF Prevents Apoptosis of Intestinal Crypt Cells Induced by 5-FU
3. Materials and Methods
3.1. Animals and Regents
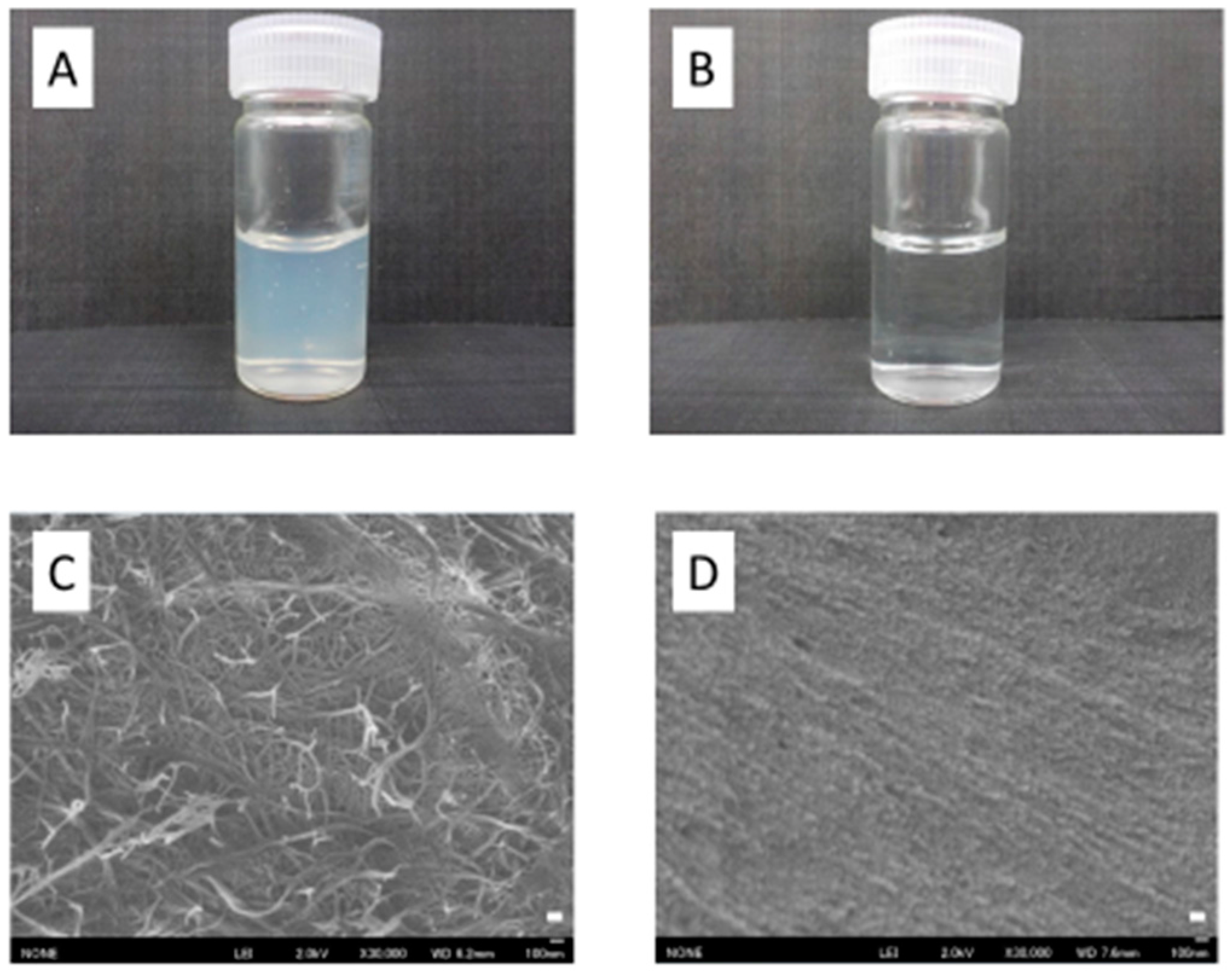
3.2. Preparation of SDACNFs
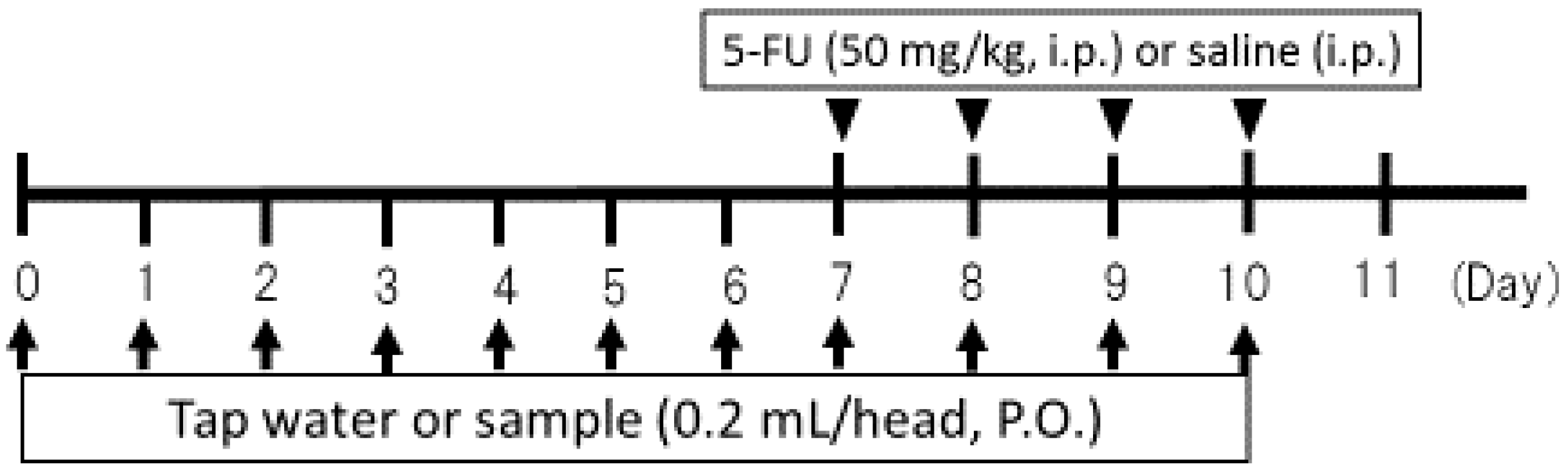
3.3. Study Design
3.4. Histological Analysis
3.5. Ki-67 Immunohistochemistry
3.6. Caspase-3 Immunohistochemistry
3.7. TUNEL Assay
3.8. MPO Staining
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ribeiro, R.A.; Wanderley, C.W.; Wong, D.V.; Mota, J.M.; Leite, C.A.; Souza, M.H.; Cunha, F.Q.; Lima-Júnior, R.C. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: Insights into pathogenesis and therapeutic perspectives. Cancer Chemother. Pharmacol. 2016, 78, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B., III; Ajani, J.A.; Catalano, R.B.; Engelking, C.; Kornblau, S.M.; Martenson, J.A., Jr.; McCallum, R.; Mitchell, E.P.; O’Dorisio, T.M.; Vokes, E.E.; et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol. 2004, 22, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Symonds, R.P. Treatment-induced mucositis: An old problem with new remedies. Br. J. Cancer 1998, 77, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Wadler, S.; Benson, A.B., III; Engelking, C.; Catalano, R.; Field, M.; Kornblau, S.M.; Mitchell, E.; Rubin, J.; Trotta, P.; Vokes, E. Recommended guidelines for the treatment of chemotherapy-induced diarrhea. J. Clin. Oncol. 1998, 16, 3169–3178. [Google Scholar] [PubMed]
- Deepthi, S.; Venkatesan, J.; Kim, S.K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okuda, H. Prevention by chitosan of myelotoxicity, gastrointestinal toxicity and immunocompetent organic toxicity induced by 5-fluorouracil without loss of antitumor activity in mice. Jpn. J. Cancer Res. 1999, 90, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sawai, N.; Okuda, H. Antitumour activity and adverse reactions of combined treatment with chitosan and doxorubicin in tumour-bearing mice. J. Pharm. Pharmacol. 2001, 53, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S. Chitin and chitosan nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Nagae, T.; Nagai, T.; Izawa, H.; Morimoto, M.; Murahata, Y.; Osaki, T.; Tsuka, T.; Imagawa, T.; Ito, N.; et al. Effects of surface-deacetylated chitin nanofibers in an experimental model of hypercholesterolemia. Int. J. Mol. Sci. 2015, 16, 17445–17455. [Google Scholar] [CrossRef] [PubMed]
- Izumi, R.; Komada, S.; Ochi, K.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, R.; Azuma, K.; Izumi, R.; Tanou, T.; Okamoto, Y.; Nagae, T.; Iohara, D.; Uekama, K.; Otagiri, M.; Hirayama, F.; et al. Biomaterials based on freeze dried surface-deacetylated chitin nanofibers reinforced with sulfobutyl ether β-cyclodextrin gel in wound dressing applications. Int. J. Pharm 2016, 511, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.M.; Mota, J.M.; Gomes, A.S.; Oliveira, R.B.; Assreuy, A.M.; Brito, G.A.; Santos, A.A.; Ribeiro, R.A.; Souza, M.H. Gastrointestinal dysmotility in 5-fluorouracil-induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemother. Pharmacol. 2008, 63, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Daniele, B.; Secondulfo, M.; de Vivo, R.; Pignata, S.; de Magistris, L.; Delrio, P.; Palaia, R.; Barletta, E.; Tambaro, R.; Carratù, R. Effect of chemotherapy with 5-fluorouracil on intestinal permeability and absorption in patients with advanced colorectal cancer. J. Clin. Gastroenterol. 2001, 32, 228–320. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.; Grant, G. Oral and intestinal mucositis—Causes and possible treatments. Aliment. Pharmacol. Ther. 2003, 18, 853–874. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Kato, S.; Yamanaka, N.; Iimori, M.; Utsumi, D.; Kitahara, Y.; Iwata, K.; Matsuno, K.; Amagase, K.; Yabe-Nishimura, C.; et al. Potential role of the NADPH oxidase NOX1 in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1133–G1142. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C.; Gatter, K.C. Ki67 protein: The immaculate deception? Histopathology 2002, 40, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, T.V.; Sarraf, C.E.; Hunt, T.; Alison, M.R. The nature of cytotoxic drug-induced cell death in murine intestinal crypts. Br. J. Cancer 1992, 65, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Inomata, A.; Horii, I.; Suzuki, K. 5-Fluorouracil-induced intestinal toxicity: What determines the severity of damage to murine intestinal crypt epithelia? Toxicol. Lett. 2002, 133, 231–240. [Google Scholar] [CrossRef]
- Keefe, D.M.K.; Brealey, J.; Goland, G.J.; Cummins, A.G. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 2000, 47, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.M.; Potten, C.S.; Hickman, J.A. The relationships between p53-dependent apoptosis, inhibition of proliferation, and 5-fluorouracil-induced histopathology in murine intestinal epithelia. Cancer Res. 1998, 58, 5453–5465. [Google Scholar] [PubMed]
- Kumar, S. Caspase function in programmed cell death. Cell Death Differ. 2007, 14, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.M.; Gibson, R.J.; Keefe, D.M.; Cummins, A.G. Cytotoxic chemotherapy upregulates pro-apoptotic BAX and BAK in the small intestine of rats and humans. Pathology 2005, 37, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ito, K.; Sumi, S.; Fuwa, T.; Horie, T. Protective effect of aged garlic extract (AGE) on the apoptosis of intestinal epithelial cells caused by methotrexate. Cancer Chemother. Pharmacol. 2009, 63, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Q.; Han, X.D.; Wang, Y.; Yuan, K.L.; Jin, Z.M.; Di, J.Z.; Yan, J.; Pan, Y.; Zhang, P.; Huang, X.Y.; et al. Interleukin-1 receptor antagonist reduced apoptosis and attenuated intestinal mucositis in a 5-fluorouracil chemotherapy model in mice. Cancer Chemother. Pharmacol. 2011, 68, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Gong, M.; He, Q.T.; Zhou, P.H. Controlled release of interleukin-1 receptor antagonist from hyaluronic acid-chitosan microspheres attenuates interleukin-1β-induced inflammation and apoptosis in chondrocytes. Biomed. Res. Int. 2016, 2016, 6290957. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Marciello, M.; Bonferoni, M.C.; Ferrari, F.; Sandri, G.; Dacarro, C.; Grisoli, P.; Caramella, C. Thermally sensitive gels based on chitosan derivatives for the treatment of oral mucositis. Eur. J. Pharm. Biopharm. 2010, 74, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nitta, M.; Azuma, K.; Hata, K.; Takahashi, S.; Ogiwara, K.; Tsuka, T.; Imagawa, T.; Yokoe, I.; Osaki, T.; Minami, S.; et al. Systemic and local injections of lupeol inhibit tumor growth in a melanoma-bearing mouse model. Biomed. Rep. 2013, 1, 641–645. [Google Scholar] [PubMed]
- Takagi, H.; Azuma, K.; Tsuka, T.; Imagawa, T.; Osaki, T.; Okamoto, Y. Antitumor effects of high-temperature hyperthermia on a glioma-bearing rat model according to temperature. Oncol. Lett. 2014, 7, 1007–1010. [Google Scholar] [PubMed]
- Azuma, K.; Osaki, T.; Ifuku, S.; Saimoto, H.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. α-Chitin nanofibrils improve inflammatory and fibrosis responses in inflammatory bowel disease mice model. Carbohydr. Polym. 2012, 90, 197–200. [Google Scholar] [CrossRef] [PubMed]



| NT | Control | SDACNF | Chitosan | CLNF | |
|---|---|---|---|---|---|
| MA | 0.0 ± 0.0 | 1.8 ± 0.6 | 0.8 ± 0.6 *,† | 1.1 ± 0.6 | 1.8 ± 0.8 |
| MU | 0.0 ± 0.0 | 1.8 ± 0.6 | 0.8 ± 0.6 *,† | 1.2 ± 0.6 | 1.8 ± 0.6 |
| MI | 0.0 ± 0.0 | 1.9 ± 0.5 | 0.7 ± 0.5 *,† | 0.9 ± 0.5 * | 1.5 ± 0.5 |
| VI | 0.0 ± 0.0 | 1.6 ± 0.7 | 0.8 ± 0.5 *,† | 1.1 ± 0.7 | 1.8 ± 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koizumi, R.; Azuma, K.; Izawa, H.; Morimoto, M.; Ochi, K.; Tsuka, T.; Imagawa, T.; Osaki, T.; Ito, N.; Okamoto, Y.; et al. Oral Administration of Surface-Deacetylated Chitin Nanofibers and Chitosan Inhibit 5-Fluorouracil-Induced Intestinal Mucositis in Mice. Int. J. Mol. Sci. 2017, 18, 279. https://doi.org/10.3390/ijms18020279
Koizumi R, Azuma K, Izawa H, Morimoto M, Ochi K, Tsuka T, Imagawa T, Osaki T, Ito N, Okamoto Y, et al. Oral Administration of Surface-Deacetylated Chitin Nanofibers and Chitosan Inhibit 5-Fluorouracil-Induced Intestinal Mucositis in Mice. International Journal of Molecular Sciences. 2017; 18(2):279. https://doi.org/10.3390/ijms18020279
Chicago/Turabian StyleKoizumi, Ryo, Kazuo Azuma, Hironori Izawa, Minoru Morimoto, Kosuke Ochi, Takeshi Tsuka, Tomohiro Imagawa, Tomohiro Osaki, Norihiko Ito, Yoshiharu Okamoto, and et al. 2017. "Oral Administration of Surface-Deacetylated Chitin Nanofibers and Chitosan Inhibit 5-Fluorouracil-Induced Intestinal Mucositis in Mice" International Journal of Molecular Sciences 18, no. 2: 279. https://doi.org/10.3390/ijms18020279






