Antioxidant and Cytoprotective Activities of Fucus spiralis Seaweed on a Human Cell in Vitro Model
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Activity
2.2. Protective Effect of Seaweed Fractions on MCF-7 Cells Exposed to H2O2
2.3. Study of the Cellular Mechanisms Involved in the Cytotoxicity Induced by H2O2 on MCF-7 Cells in the Presence or Absence of Fucus spiralis Fractions
2.3.1. Real-Time Quantification of H2O2 Production
2.3.2. Mitochondrial Membrane Potential (ΔΨm)
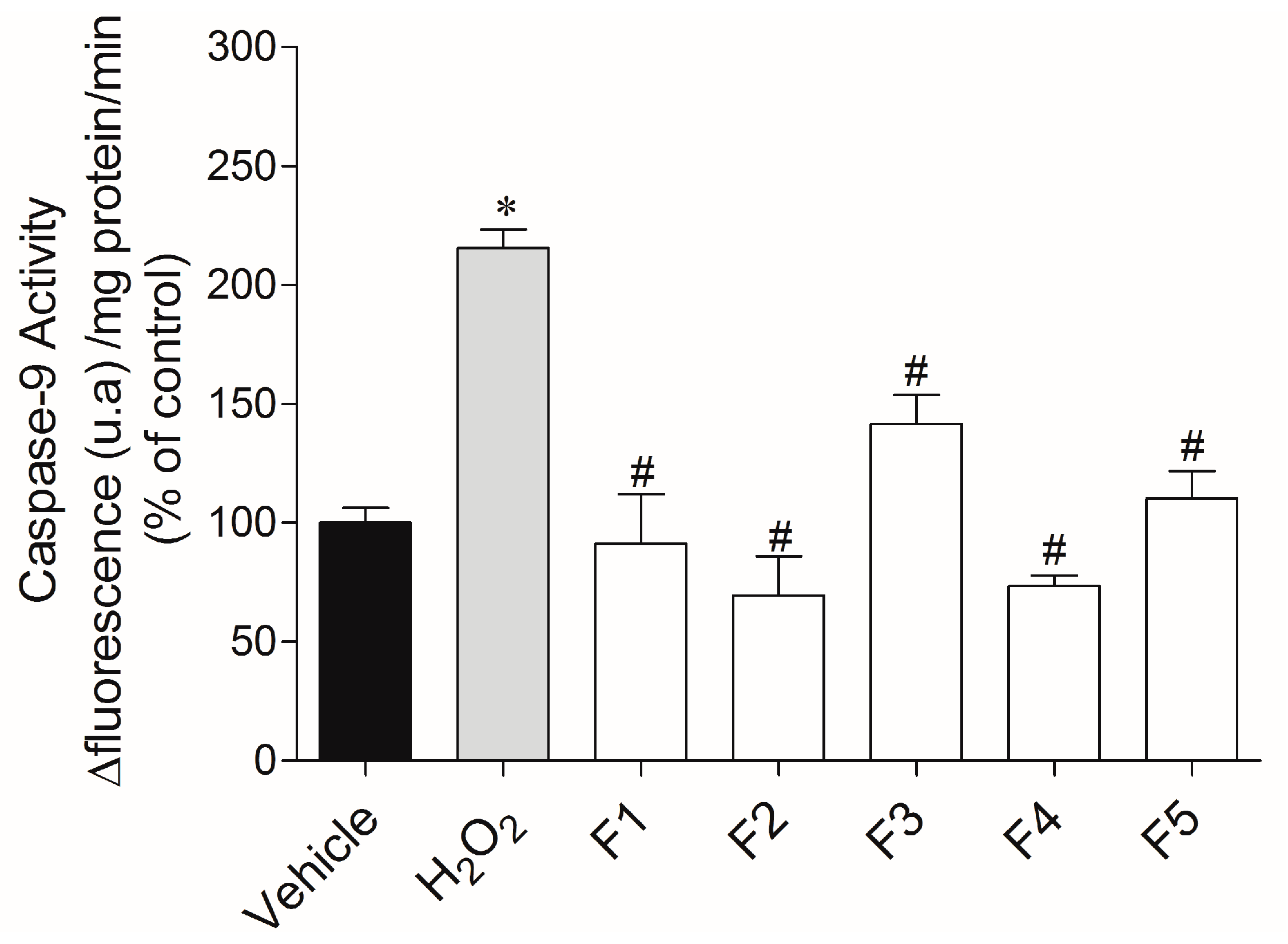
2.3.3. Caspase-9 Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection, Preparation and Extraction of Fucus spiralis
4.3. Fractionation of Fucus spiralis Methanolic Extract by Vacuum Liquid Chromatography (VLC)
4.4. Analysis of Total Phenolic Content (TPC)
4.5. Evaluation of Antioxidant Activities
4.5.1. DPPH (1,1-Diphenyl-2-picrylhydrazyl) Radical Scavenging Activity
4.5.2. Oxygen Radical Absorbance Capacity (ORAC)
4.5.3. Hydroxyl Radical Scavenging Activity (•OH)
4.6. In Vitro Assay of Oxidative Stress Prevention
4.6.1. Cell Maintenance Culture Conditions
4.6.2. Fucus spiralis Fractions Cytotoxicity Evaluation
4.6.3. Evaluation of the Protective Effect of Seaweed Fractions in an Oxidative Stress Condition Induced by H2O2 on MCF-7 Cells
4.6.4. Real-Time Quantification of H2O2 Production
4.6.5. Mitochondrial Membrane Potential (ΔΨm)
4.6.6. Caspase-9 Activity
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hasan, M.R.; Chakrabarti, R. Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture—A Review; FAO: Rome, Italy, 2009; Volume 531, p. 123. [Google Scholar]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Burtin, P. Nutritional value of seaweeds. Electron. J. Environ. Agric. Food Chem. 2003, 2, 498–503. [Google Scholar]
- Skibola, C.F. The effect of Fucus vesiculosus, an edible brown seaweed, upon menstrual cycle length and hormonal status in three pre-menopausal women: A case report. BMC Complement. Altern. Med. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Gutierrez, C.M.; Guerra-Rivas, G.; Soria-Mercado, I.E.; Ayala-Sánchez, N.E. Chapter 3—Marine edible algae as disease preventers. Adv. Food Nutr. Res. 2011, 64, 29–39. [Google Scholar] [PubMed]
- Julia, K.; Sarah, E.L.; William, F.; Mark, E.H. Ambiguous role of phlorotannins as chemical defenses in the brown alga Fucus vesiculosus. Mar. Ecol. Prog.Ser. 2004, 277, 79–93. [Google Scholar]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Cornish, M.L.; Garbary, D.J. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae 2010, 25, 155–171. [Google Scholar] [CrossRef]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: In vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.M.; Moane, S.; Collins, C.; Beletskaya, T.; Thomas, O.P.; Duarte, A.W.F.; Nobre, F.S.; Owoyemi, I.O.; Pagnocca, F.C.; Sette, L.D.; et al. Sustainable production of biologically active molecules of marine based origin. New Biotechnol. 2013, 30, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-Y.; Choi, H. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: Their molecular targets and action mechanisms. Arch. Pharm. Res. 2015, 38, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Pintéus, S.; Azevedo, S.; Alves, C.; Mouga, T.; Cruz, A.; Afonso, C.; Sampaio, M.; Rodrigues, A.; Pedrosa, R. High antioxidant potential of Fucus spiralis extracts collected from Peniche coast (Portugal). New Biotechnol. 2009, 25, S296. [Google Scholar] [CrossRef]
- Cerantola, S.; Breton, F.; Ar Gall, E.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar] [CrossRef]
- Tierney, M.; Soler-Vila, A.; Croft, A.; Hayes, M. Antioxidant activity of the brown macroalga Fucus spiralis Linnaeus harvested from the West Coast of Ireland. Curr. Res. J. Biol. Sci. 2013, 5, 81–90. [Google Scholar]
- Dasgupta, A.; Klein, K. Chapter 10—Role of Oxidative Stress in Neurodegenerative Diseases and Other Diseases Related to Aging. In Antioxidants in Food, Vitamins and Supplements; Klein, A.D., Ed.; Elsevier: San Diego, CA, USA, 2014; pp. 167–184. [Google Scholar]
- Kim, Y.R.; Lee, J.S.; Lee, K.R.; Kim, Y.E.; Baek, N.I.; Hong, E.K. Effects of mulberry ethanol extracts on hydrogen peroxide-induced oxidative stress in pancreatic β-cells. Int. J. Mol. Med. 2014, 33, 128–134. [Google Scholar] [PubMed]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Inês Rodrigues, A.; Mendes, S.; Pedrosa, R. Cytoprotective effect of seaweeds with high antioxidant activity from the Peniche coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Chang, T.-S.; Jeong, W.; Kang, D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, T.; Diao, X.; Kong, B. Protective effects of black currant (Ribes nigrum L.) extract on hydrogen peroxide-induced damage in lung fibroblast MRC-5 cells in relation to the antioxidant activity. J. Funct. Foods 2014, 11, 142–151. [Google Scholar]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Lee, Y.; Kim, S.Y.; Kim, H.S.; Joo, H.G.; Park, J.W. Eckol isolated from Ecklonia cava attenuates oxidative stress induced cell damage in lung fibroblast cells. FEBS Lett. 2005, 579, 6295–6304. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Cha, S.-H.; Ko, J.-Y.; Kang, M.-C.; Kim, D.; Heo, S.-J.; Kim, J.-S.; Heu, M.S.; Kim, Y.-T.; Jung, W.-K. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharm. 2012, 34, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jeon, Y.-J. Evaluation of diphlorethohydroxycarmalol isolated from Ishige okamurae for radical scavenging activity and its protective effect against H2O2-induced cell damage. Process Biochem. 2009, 44, 412–418. [Google Scholar] [CrossRef]
- Kim, E.-K.; Tang, Y.; Kim, Y.-S.; Hwang, J.-W.; Choi, E.-J.; Lee, J.-H.; Lee, S.-H.; Jeon, Y.-J.; Park, P.-J. First evidence that Ecklonia cava-derived dieckol attenuates MCF-7 human breast carcinoma cell migration. Mar. Drugs 2015, 13, 1785. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Enokido, Y.; Aoshima, H.; Uchiyama, Y.; Hatanaka, H. Changes in mitochondrial membrane potential during oxidative stress—Induced apoptosis in PC12 cells. J. Neurosci. Res. 1997, 50, 413–420. [Google Scholar] [CrossRef]
- Cook, S.A.; Sugden, P.H.; Clerk, A. Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes association with changes in mitochondrial membrane potential. Circ. Res. 1999, 85, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lee, I.K.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Kim, B.J.; Lee, N.H.; Choi, J.-Y.; Choi, J.; Hyun, J.W. Cytoprotective Effects of triphlorethol-A against formaldehyde-induced oxidative damage and apoptosis: Role of mitochondria-mediated caspase-dependent pathway. J. Toxicol. Environ. Health Part A 2010, 73, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Kim, J.-A.; Yoon, N.-Y.; Kim, S.-K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.; Kanthimathi, M.; Khoo, K.; Rajarajeswaran, J.; Cheng, H.; Yap, W. Antioxidant and cytotoxic activities of three species of tropical seaweeds. BMC Complement. Altern. Med. 2015, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Shin, T.; Utsuki, T.; Choi, J.-S.; Byun, D.-S.; Kim, H.-R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and hepatoprotective properties in tacrine-treated HepG2 cells. J. Agric. Food Chem. 2012, 60, 5340–5349. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vit. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1980, 165, 215–219. [Google Scholar] [CrossRef]
- Kunchandy, E.; Rao, M. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Walsh, N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006, 44, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.; Jaffe, J.S.; Schulman, E.S.; Raible, D.G. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods 1997, 202, 133–141. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Ter Braak, C.; Smilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows—Software for Canonical Community Ordination; Version 4; Microcomputer Power: New York, NY, USA, 1998. [Google Scholar]
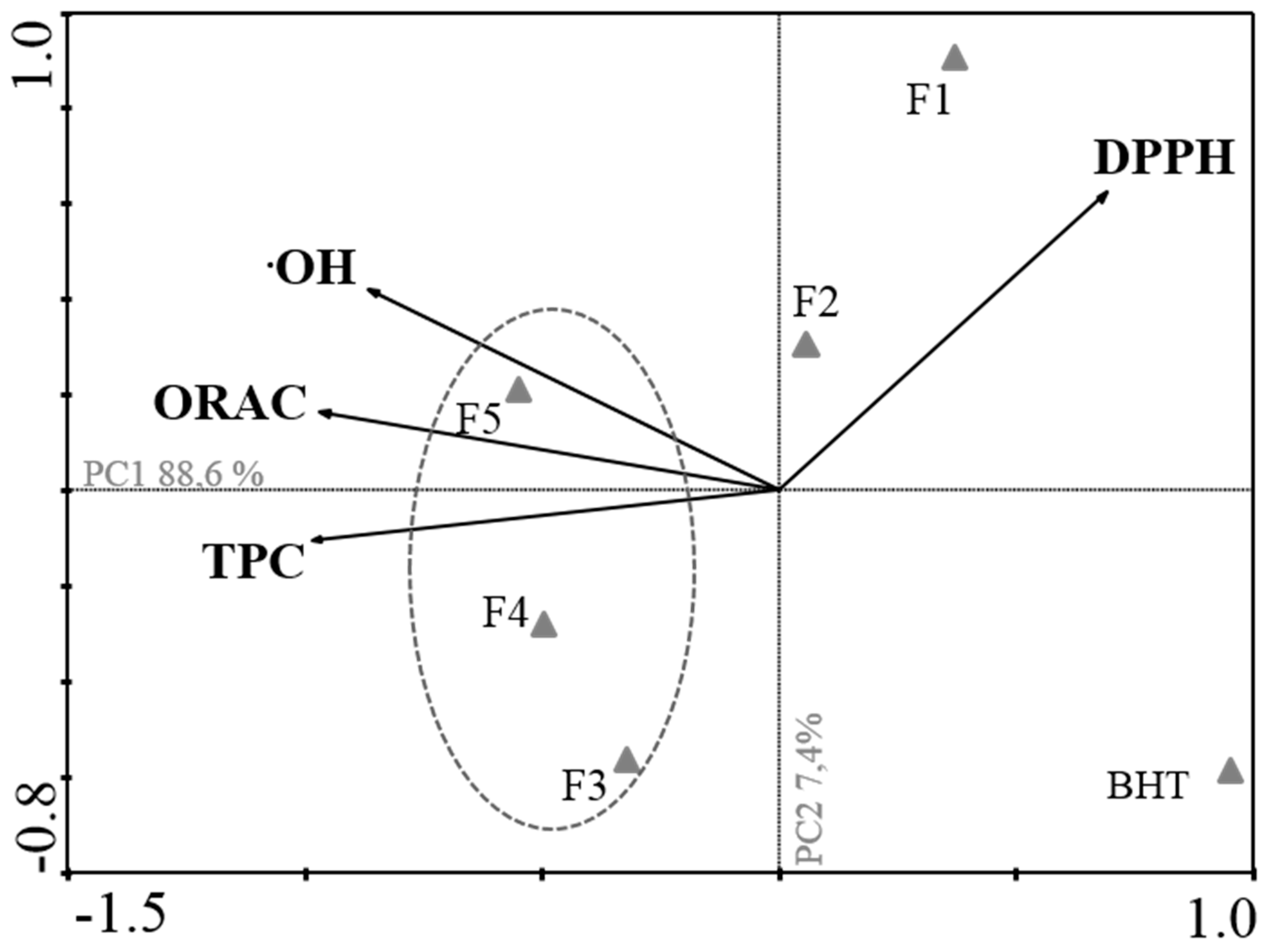
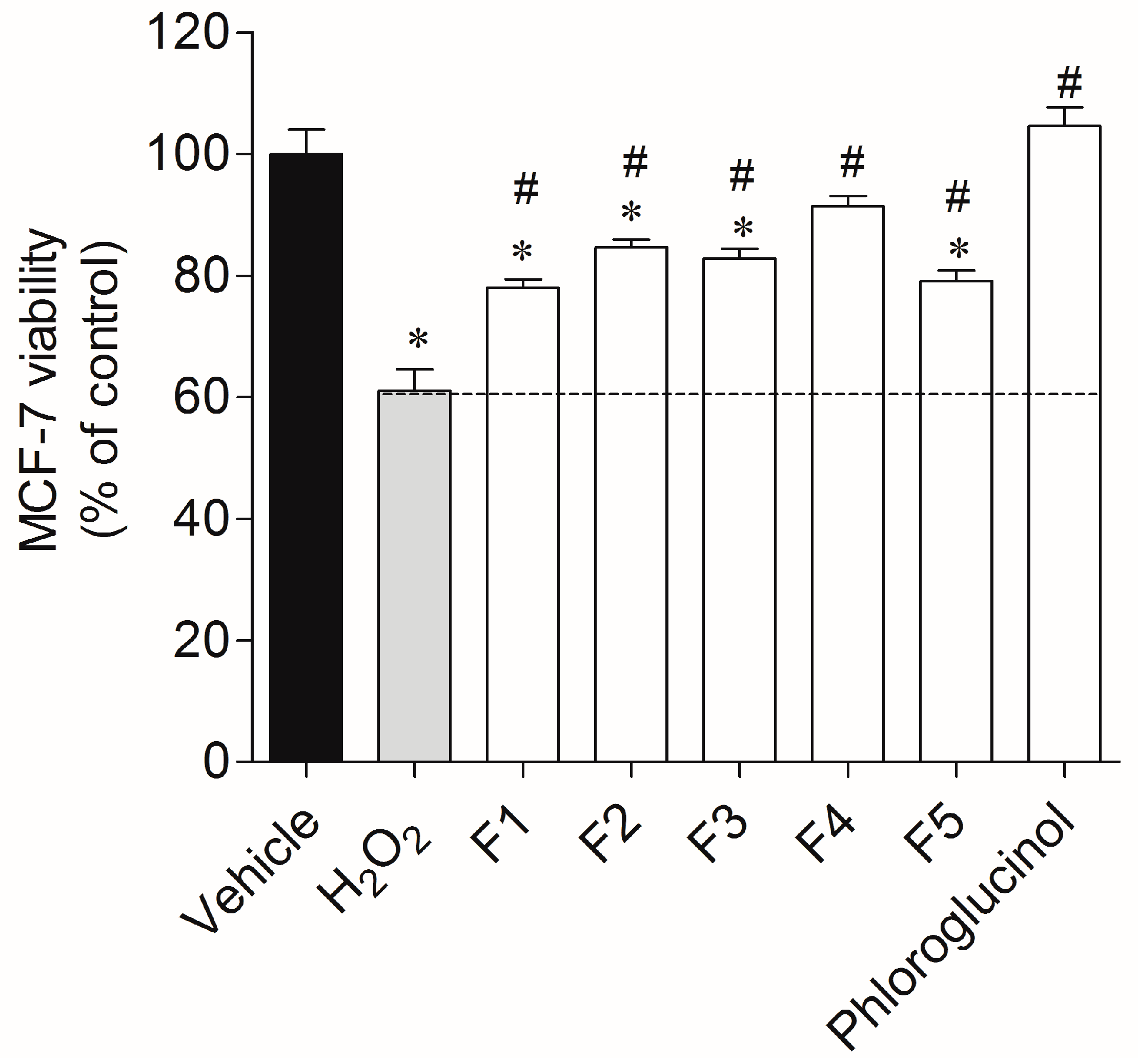
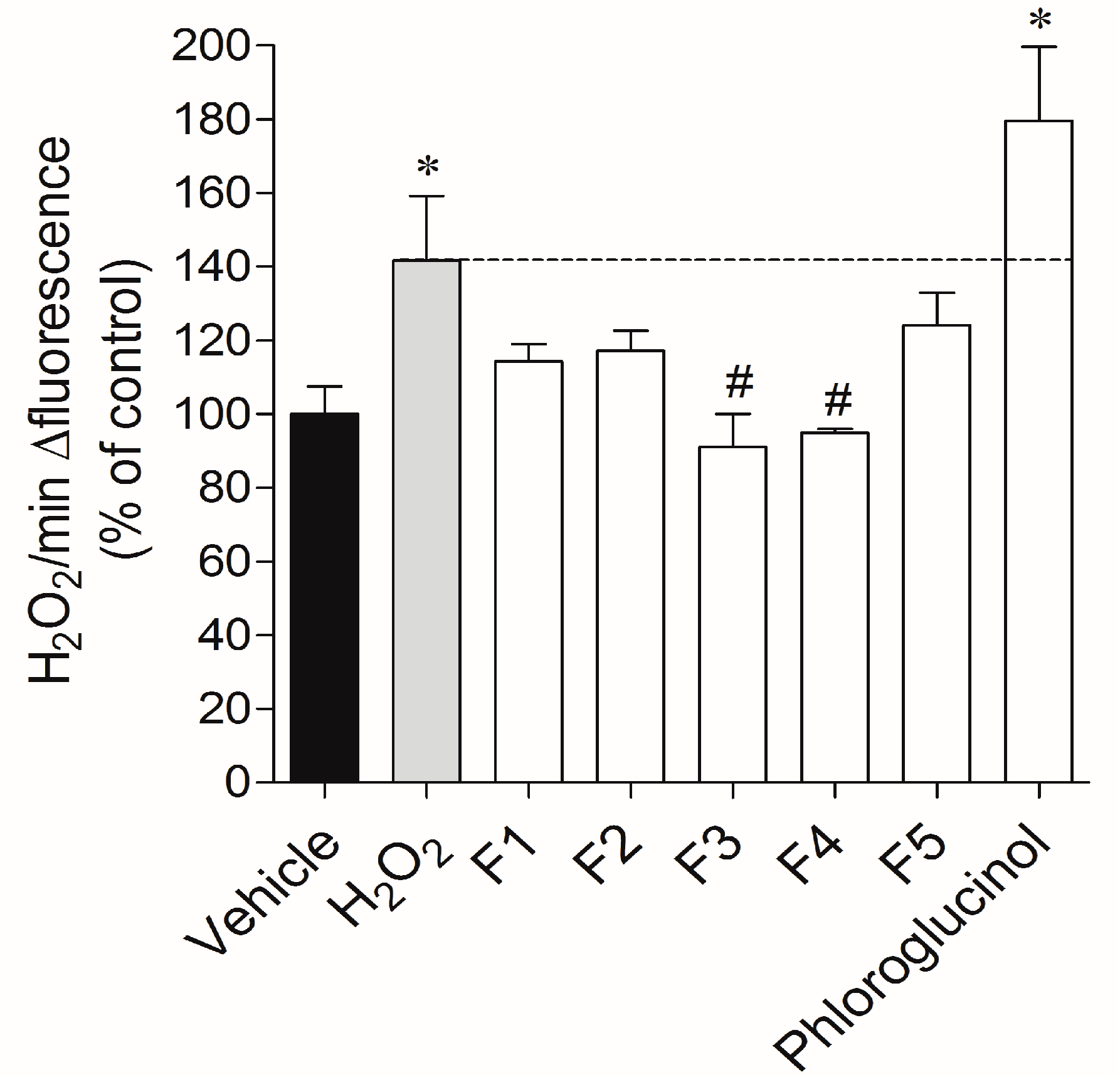
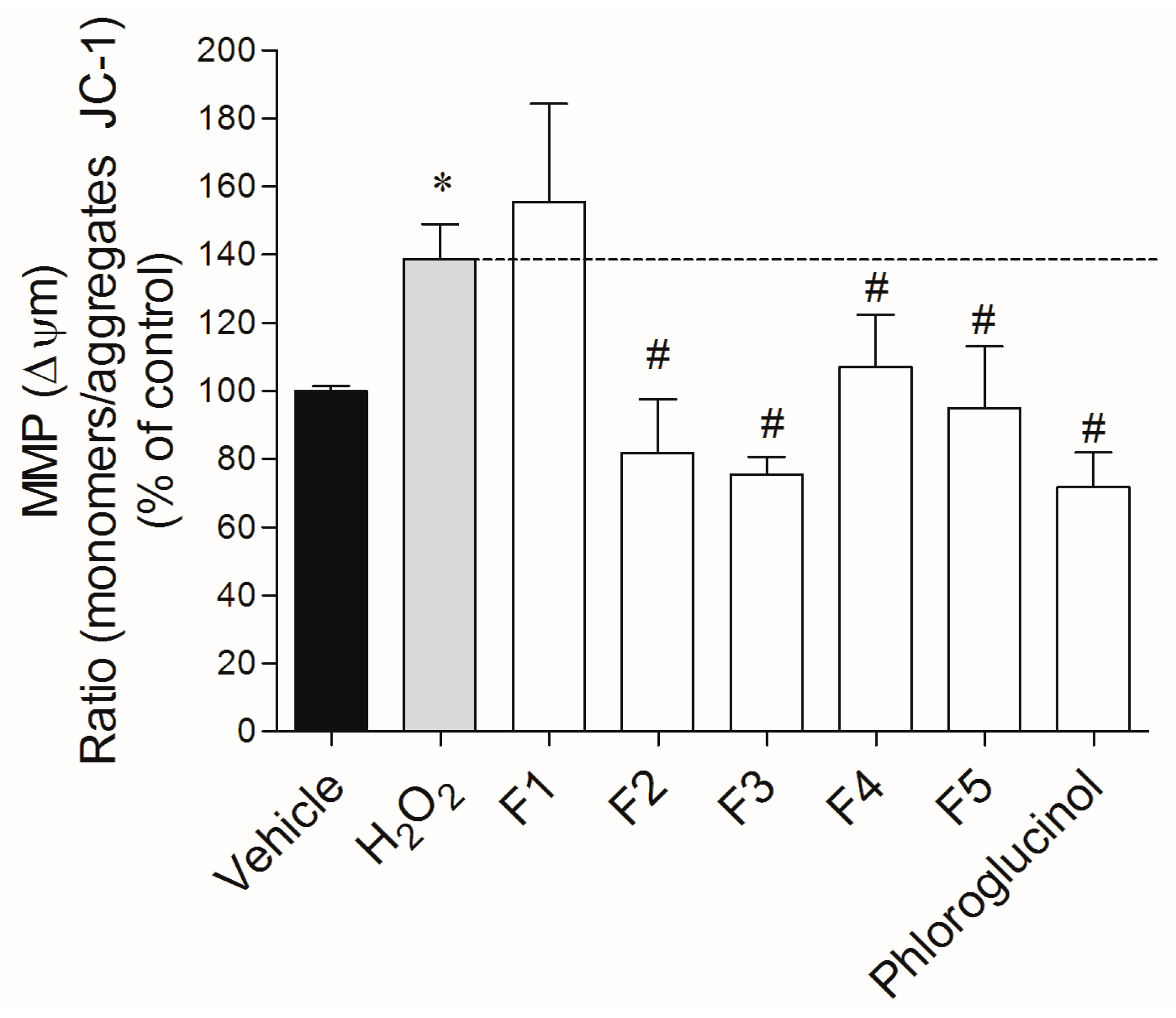
| Fractions | TPC a | DPPH b | •OH c | ORAC d |
|---|---|---|---|---|
| 1 | 8.00 ± 1.10 | 182.90 (124.30–269.00) | 7.90 (5.60–11.60) | 2,988.00 ± 107.77 |
| 2 | 33.00 ± 18.00 | 44.40 (38.30–51.47) | 11.52 (8.03–16.52) | 5,411.00 ± 91.54 |
| 3 | 379.00 ± 34.0 | 15.58 (13.31–18.22) | 9.73 (6.51–14.55) | 6,877.00 ± 92.56 |
| 4 | 419.00 ± 3.00 | 13.94 (11.13–17.46) | 10.86 (5.90–19.95) | 34,893.68 ± 945.20 |
| 5 | 285.00 ± 12.00 | 9.74 (8.14–11.66) | 58.61 (41.32–83.14) | 30,691.00 ± 1172.90 |
| BHT | - | 40.55 (25.74–63.87) | >1000 | 320.49 ± 32.31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Thomas, O.P.; Pedrosa, R. Antioxidant and Cytoprotective Activities of Fucus spiralis Seaweed on a Human Cell in Vitro Model. Int. J. Mol. Sci. 2017, 18, 292. https://doi.org/10.3390/ijms18020292
Pinteus S, Silva J, Alves C, Horta A, Thomas OP, Pedrosa R. Antioxidant and Cytoprotective Activities of Fucus spiralis Seaweed on a Human Cell in Vitro Model. International Journal of Molecular Sciences. 2017; 18(2):292. https://doi.org/10.3390/ijms18020292
Chicago/Turabian StylePinteus, Susete, Joana Silva, Celso Alves, André Horta, Olivier P. Thomas, and Rui Pedrosa. 2017. "Antioxidant and Cytoprotective Activities of Fucus spiralis Seaweed on a Human Cell in Vitro Model" International Journal of Molecular Sciences 18, no. 2: 292. https://doi.org/10.3390/ijms18020292






