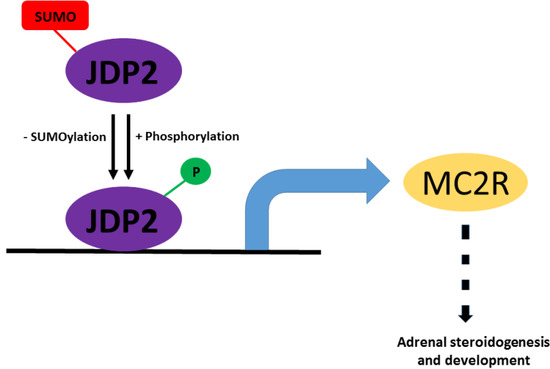
Jun Dimerization Protein 2 Activates Mc2r Transcriptional Activity: Role of Phosphorylation and SUMOylation
Abstract
:1. Introduction
2. Results
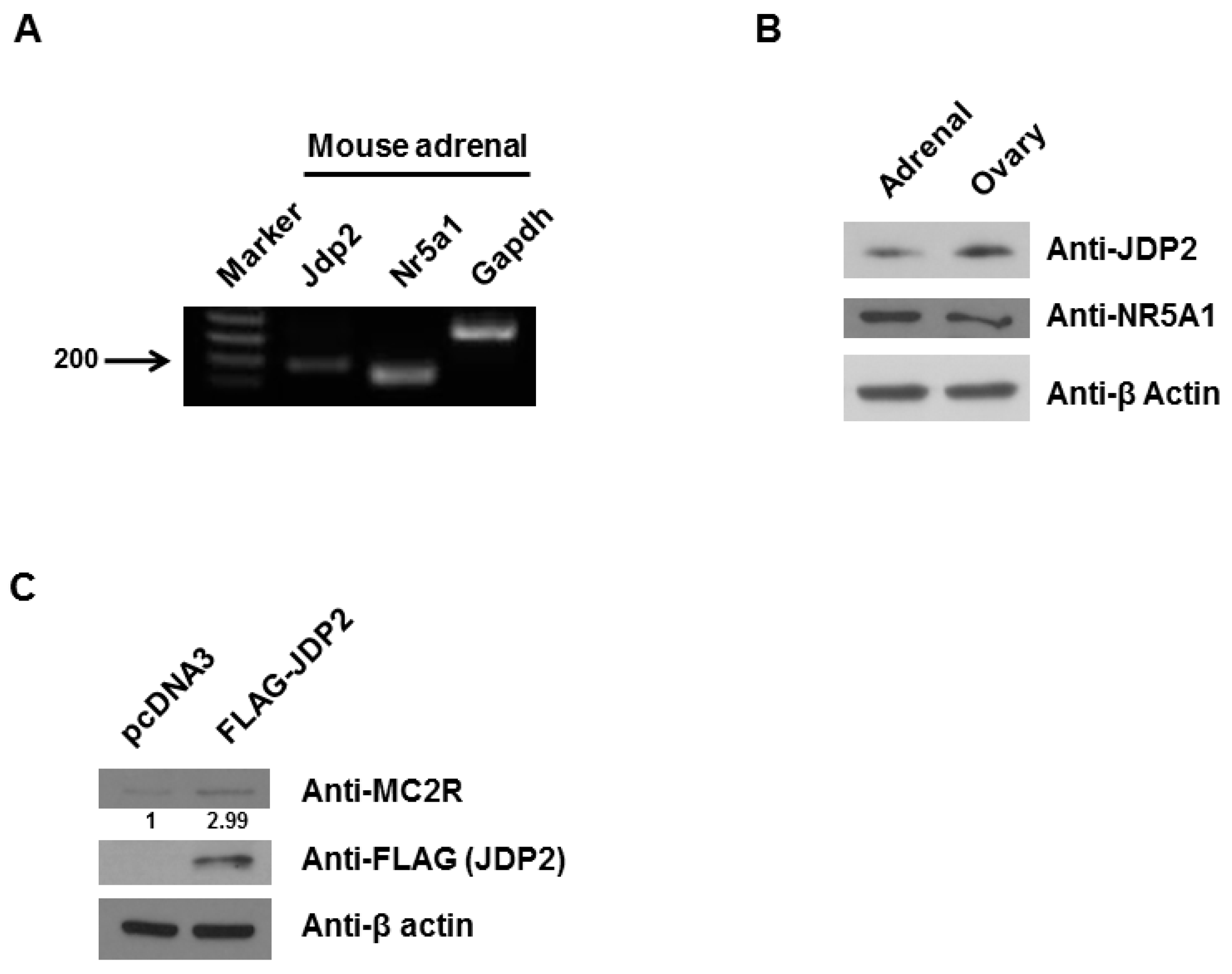
2.1. JDP2 Is Expressed in Adrenal Glands and Increases MC2R Level
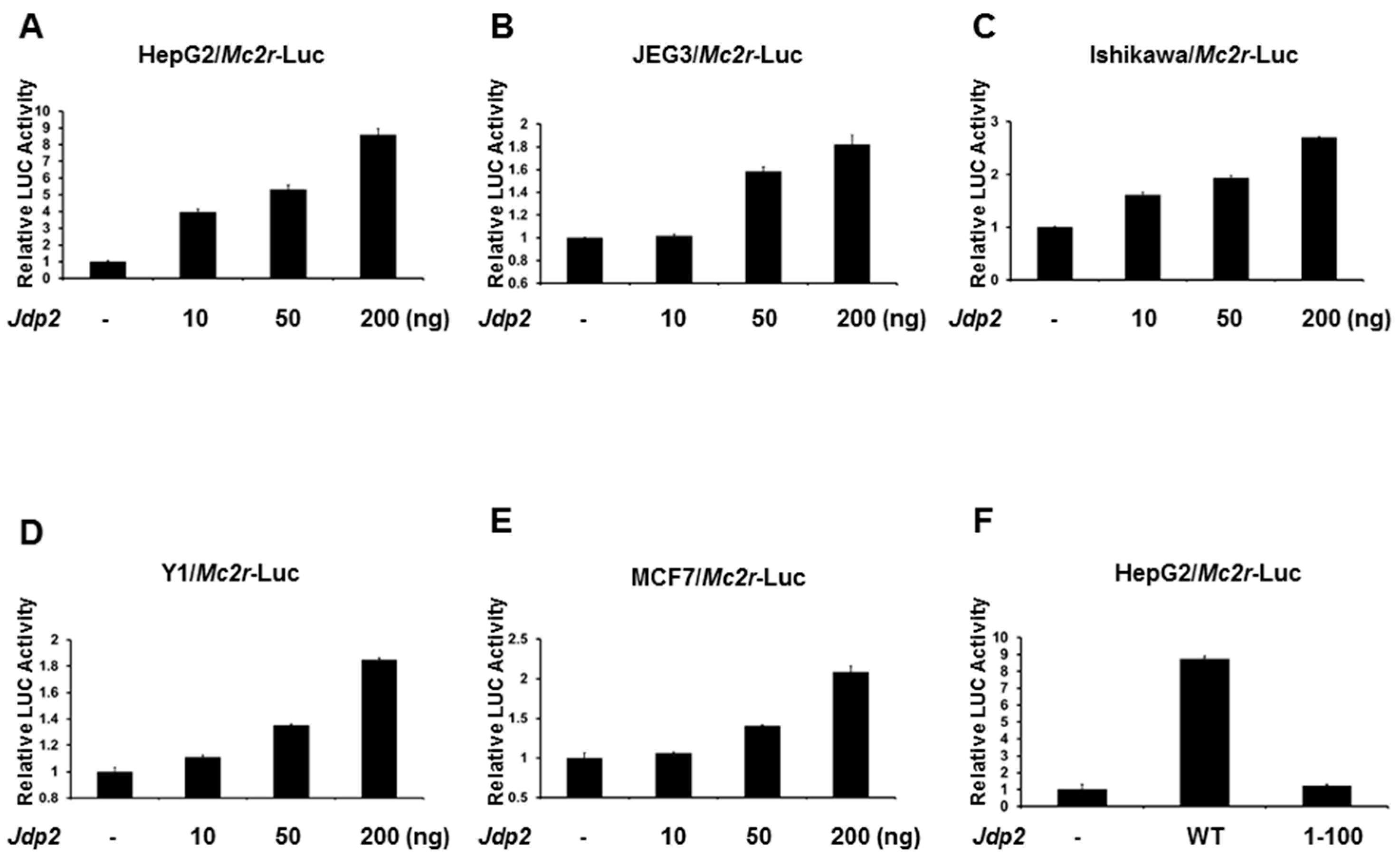
2.2. JDP2 Is an Activator of the Mc2r Promoter
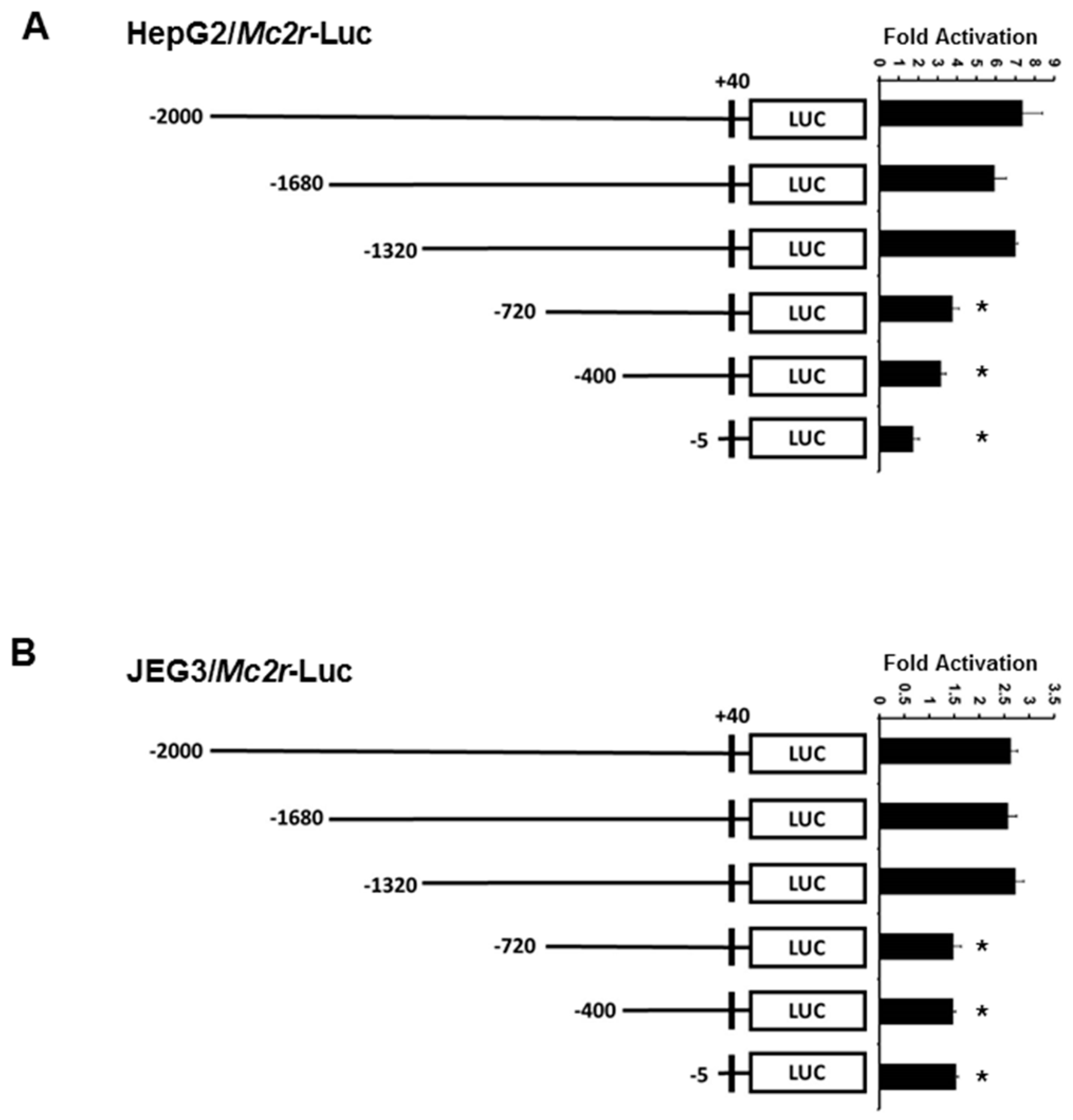
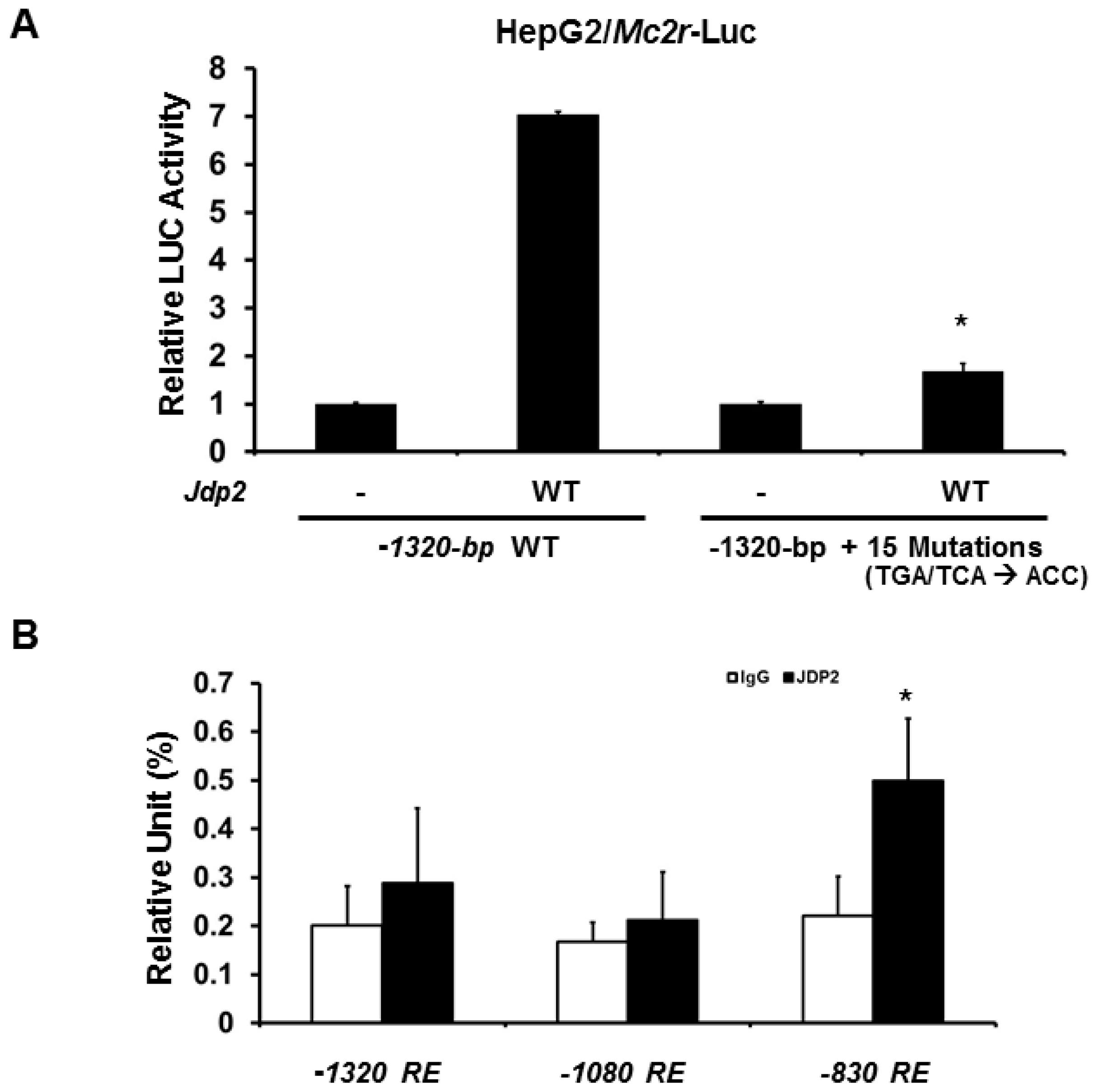
2.3. Minimal Mc2r Promoter Region Responsive to JDP2 Activation
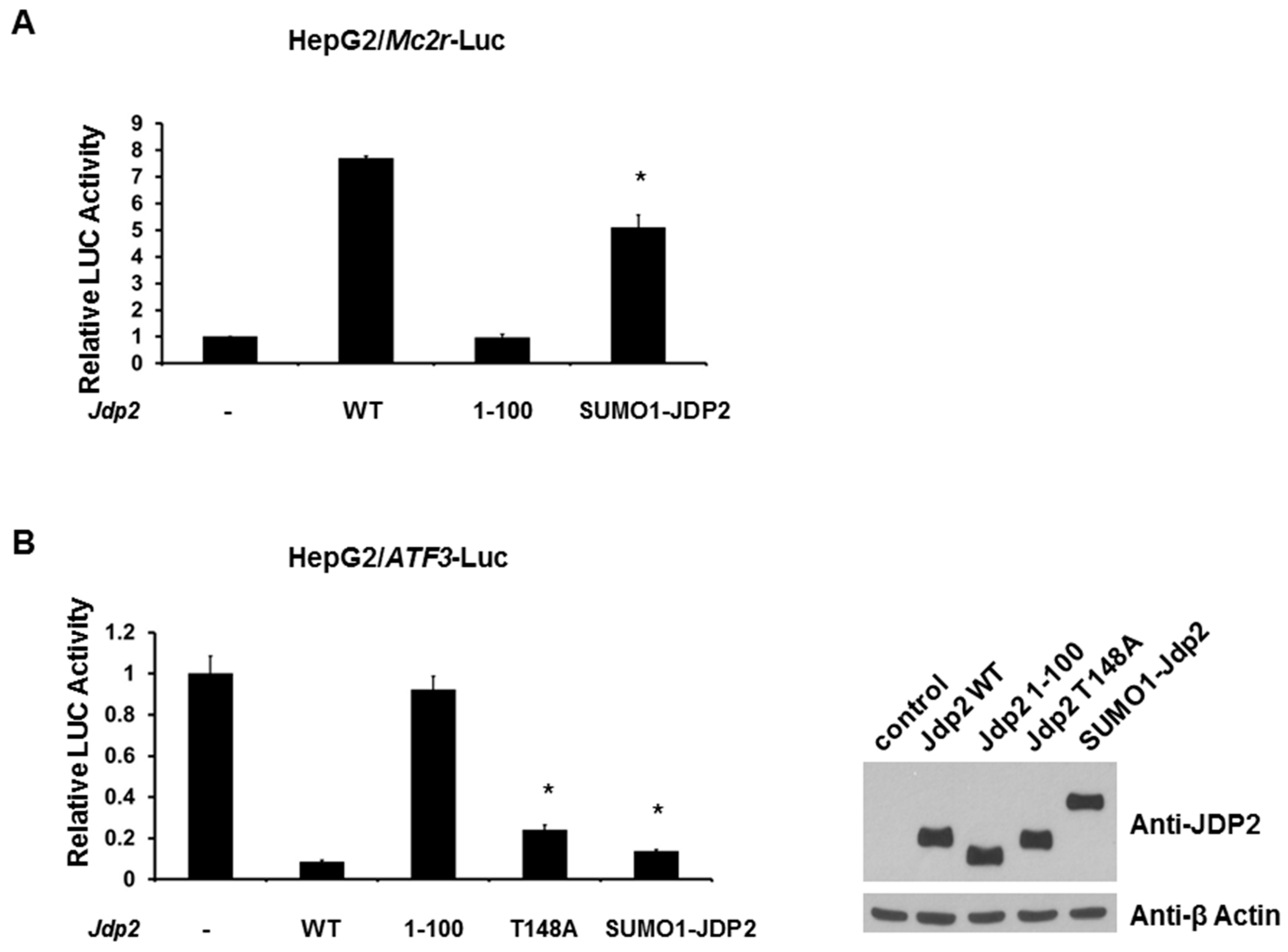
2.4. Phosphorylation of JDP2 at T148 Is Required for Full JDP2-Mediated Mc2r Transcriptional Activity
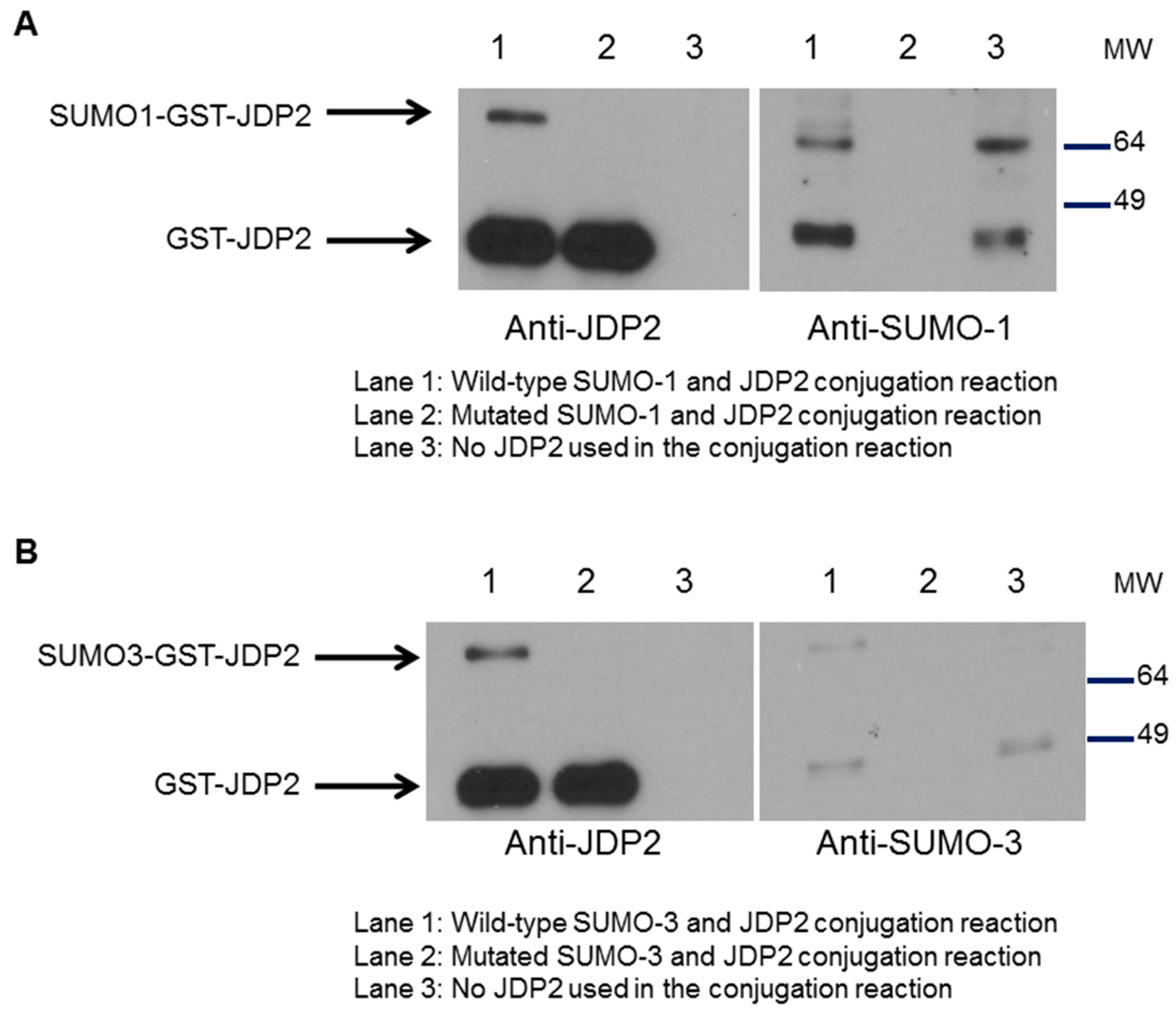
2.5. SUMOylation of JDP2 Affects Its Transcriptional Activities
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. DNA Constructs
4.3. Cell Culture and Transfection
4.4. RT-PCR and Real-Time ChIP
4.5. Immunoblotting
4.6. Cell-Free SUMOylation Assays
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| JDP2 | Jun dimerization protein 2 |
| MC2R | Melanocortin 2 receptor |
| SUMO | Small ubiquitin-like modifier |
References
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Li, H.; Murata, T.; Sun, K.; Horikoshi, M.; Chiu, R.; Yokoyama, K.K. JDP2, a repressor of AP-1, recruits a histone deacetylase 3 complex to inhibit the retinoic acid-induced differentiation of F9 cells. Mol. Cell. Biol. 2002, 22, 4815–4826. [Google Scholar] [CrossRef] [PubMed]
- Darlyuk-Saadon, I.; Weidenfeld-Baranboim, K.; Yokoyama, K.K.; Hai, T.; Aronheim, A. The bZIP repressor proteins, c-Jun dimerization protein 2 and activating transcription factor 3, recruit multiple HDAC members to the ATF3 promoter. Biochim. Biophys. Acta 2012, 1819, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Blazek, E.; Wasmer, S.; Kruse, U.; Aronheim, A.; Aoki, M.; Vogt, P.K. Partial oncogenic transformation of chicken embryo fibroblasts by Jun dimerization protein 2, a negative regulator of TRE- and CRE-dependent transcription. Oncogene 2003, 22, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Jin, C.; Murata, T.; Yokoyama, K.K. Sequence specific transcription factor, JDP2 interacts with histone and inhibits p300-mediated histone acetylation. Nucleic Acids Symp. Ser. 2003, 3, 305–306. [Google Scholar] [CrossRef]
- Jin, C.; Kato, K.; Chimura, T.; Yamasaki, T.; Nakade, K.; Murata, T.; Li, H.; Pan, J.; Zhao, M.; Sun, K.; et al. Regulation of histone acetylation and nucleosome assembly by transcription factor JDP2. Nat. Struct. Mol. Biol. 2006, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Nakade, K.; Pan, J.; Yoshiki, A.; Ugai, H.; Kimura, M.; Liu, B.; Li, H.; Obata, Y.; Iwama, M.; Itohara, S.; et al. JDP2 suppresses adipocyte differentiation by regulating histone acetylation. Cell. Death Differ. 2007, 14, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Nakade, K.; Pan, J.; Yamasaki, T.; Murata, T.; Wasylyk, B.; Yokoyama, K.K. JDP2 (Jun Dimerization Protein 2)-deficient mouse embryonic fibroblasts are resistant to replicative senescence. J. Biol. Chem. 2009, 284, 10808–10817. [Google Scholar] [CrossRef] [PubMed]
- Ostrovsky, O.; Bengal, E.; Aronheim, A. Induction of terminal differentiation by the c-Jun dimerization protein JDP2 in C2 myoblasts and rhabdomyosarcoma cells. J. Biol. Chem. 2002, 277, 40043–40054. [Google Scholar] [CrossRef] [PubMed]
- Kawaida, R.; Ohtsuka, T.; Okutsu, J.; Takahashi, T.; Kadono, Y.; Oda, H.; Hikita, A.; Nakamura, K.; Tanaka, S.; Furukawa, H. Jun dimerization protein 2 (JDP2), a member of the AP-1 family of transcription factor, mediates osteoclast differentiation induced by RANKL. J. Exp. Med. 2003, 197, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Heinrich, R.; Ben-Izhak, O.; Miyazaki, H.; Gutkind, J.S.; Aronheim, A. Inhibition of basic leucine zipper transcription is a major mediator of atrial dilatation. Cardiovasc. Res. 2006, 70, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Wardell, S.E.; Boonyaratanakornkit, V.; Adelman, J.S.; Aronheim, A.; Edwards, D.P. Jun dimerization protein 2 functions as a progesterone receptor N-terminal domain coactivator. Mol. Cell. Biol. 2002, 22, 5451–5466. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.K.; Roemer, S.C.; Jones, D.N.; Churchill, M.E.; Edwards, D.P. A progesterone receptor co-activator (JDP2) mediates activity through interaction with residues in the carboxyl-terminal extension of the DNA binding domain. J. Biol. Chem. 2009, 284, 24415–24424. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Noda, C.; Saito, S.; Kawashima, D.; Sugimoto, A.; Isomura, H.; Kanda, T.; Yokoyama, K.K.; Tsurumi, T. Involvement of Jun dimerization protein 2 (JDP2) in the maintenance of Epstein-Barr virus latency. J. Biol. Chem. 2011, 286, 22007–22016. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Fukasaka, M.; Vandenbon, A.; Saitoh, T.; Kawasaki, T.; Kondo, T.; Yokoyama, K.K.; Kidoya, H.; Takakura, N.; Standley, D.; et al. The transcription factor Jdp2 controls bone homeostasis and antibacterial immunity by regulating osteoclast and neutrophil differentiation. Immunity 2012, 37, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Barbarov, Y.; Timaner, M.; Alishekevitz, D.; Hai, T.; Yokoyama, K.K.; Shaked, Y.; Aronheim, A. Host JDP2 expression in the bone marrow contributes to metastatic spread. Oncotarget 2015, 6, 37737–37749. [Google Scholar] [PubMed]
- Pan, J.; Nakade, K.; Huang, Y.C.; Zhu, Z.W.; Masuzaki, S.; Hasegawa, H.; Murata, T.; Yoshiki, A.; Yamaguchi, N.; Lee, C.H.; et al. Suppression of cell-cycle progression by Jun dimerization protein-2 (JDP2) involves downregulation of cyclin-A2. Oncogene 2011, 29, 6245–6256. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, R.; Livne, E.; Ben-Izhak, O.; Aronheim, A. The c-Jun dimerization protein 2 inhibits cell transformation and acts as a tumor suppressor gene. J. Biol. Chem. 2004, 279, 5708–5715. [Google Scholar] [CrossRef] [PubMed]
- Yuanhong, X.; Feng, X.; Qingchang, L.; Jianpeng, F.; Zhe, L.; Kejian, G. Downregulation of AP-1 repressor JDP2 is associated with tumor metastasis and poor prognosis in patients with pancreatic carcinoma. Int. J. Biol. Mark. 2010, 25, 136–140. [Google Scholar] [PubMed]
- Järvinen, A.K.; Autio, R.; Kilpinen, S.; Saarela, M.; Leivo, I.; Grénman, R.; Mäkitie, A.A.; Monni, O. High-resolution copy number and gene expression microarray analyses of head and neck squamous cell carcinoma cell lines of tongue and larynx. Genes Chromosomes Cancer 2008, 47, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Bitton-Worms, K.; Pikarsky, E.; Aronheim, A. The AP-1 repressor protein, JDP2, potentiates hepatocellular carcinoma in mice. Mol. Cancer 2010, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Kilianova, Z.; Basora, N.; Kilian, P.; Payet, M.D.; Gallo-Payet, N. Human melanocortin receptor 2 expression and functionality: effects of protein kinase A and protein kinase C on desensitization and internalization. Endocrinology 2006, 147, 2325–2337. [Google Scholar] [CrossRef] [PubMed]
- Weidenfeld-Baranboim, K.; Koren, L.; Aronheim, A. Phosphorylation of JDP2 on threonine-148 by the c-Jun N-terminal kinase targets it for proteosomal degradation. Biochem. J. 2011, 436, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Ji, W.K.; Hu, X.H.; Hu, W.F.; Tang, X.C.; Huang, Z.X.; Li, L.; Liu, M.; Xiang, S.H.; Wu, E.; et al. Sumoylation differentially regulates Sp1 to control cell differentiation. Proc. Natl. Acad. Sci. USA. 2014, 111, 5574–5579. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Jang, H.; Lee, J.H.; Huh, J.Y.; Choi, S.; Chung, J.; Kim, J.B. PIASy-Mediated Sumoylation of SREBP1c Regulates Hepatic Lipid Metabolism upon Fasting Signaling. Mol. Cell. Biol. 2014, 34, 1720. [Google Scholar] [CrossRef]
- Wang, C.M.; Brennan, V.C.; Gutierrez, N.M.; Wang, X.; Wang, L.; Yang, W.H. SUMOylation of ATF3 alters its transcriptional activity on regulation of TP53 gene. J. Cell. Biochem. 2013, 114, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Nepveu-Traversy, M.É.; Berthoux, L. The conserved sumoylation consensus site in TRIM5α modulates its immune activation functions. Virus Res. 2014, 184, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Taylor-Jaffe, K.M.; Nordin, K.M.; Prasad, M.S.; Lander, R.M.; LaBonne, C. SUMOylated SoxE factors recruit Grg4 and function as transcriptional repressors in the neural crest. J. Cell. Biol. 2012, 198, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bao, H.; Pan, Y.; Yin, M.; Liu, Y.; Wu, S.; Li, H. SUMOylation alterations are associated with multidrug resistance in hepatocellular carcinoma. Mol. Med. Rep. 2014, 9, 877–881. [Google Scholar] [PubMed]
- Wang, C.M.; Liu, R.; Wang, L.; Nascimento, L.; Brennan, V.C.; Yang, W.H. SUMOylation of FOXM1B alters its transcriptional activity on regulation of miR-200 family and JNK1 in MCF7 human breast cancer cells. Int. J. Mol. Sci. 2014, 15, 10233–10251. [Google Scholar] [CrossRef] [PubMed]
- Meredith, L.J.; Wang, C.M.; Nascimento, L.; Liu, R.; Wang, L.; Yang, W.H. The Key Regulator for Language and Speech Development, FOXP2, is a Novel Substrate for SUMOylation. J. Cell. Biochem. 2016, 117, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Weidenfeld-Baranboim, K.; Hasin, T.; Darlyuk, I.; Heinrich, R.; Elhanani, O.; Pan, J.; Yokoyama, K.K.; Aronheim, A. The ubiquitously expressed bZIP inhibitor, JDP2, suppresses the transcription of its homologue immediate early gene counterpart, ATF3. Nucleic Acids Res. 2009, 37, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Frigeri, C.; Tsao, J.; Czerwinski, W.; Schimmer, B.P. Impaired steroidogenic factor 1 (NR5A1) activity in mutant Y1 mouse adrenocortical tumor cells. Mol. Endocrinol. 2000, 14, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Gutierrez, N.M.; Wang, L.; Ellsworth, B.S.; Wang, C.M. Synergistic activation of the Mc2r promoter by FOXL2 and NR5A1 in mice. Biol. Reprod. 2010, 83, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Yang, W.H. Loss of SUMOylation on ATF3 inhibits proliferation of prostate cancer cells by modulating CCND1/2 activity. Int. J. Mol. Sci. 2013, 14, 8367–8380. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-M.; Wang, R.X.; Liu, R.; Yang, W.-H. Jun Dimerization Protein 2 Activates Mc2r Transcriptional Activity: Role of Phosphorylation and SUMOylation. Int. J. Mol. Sci. 2017, 18, 304. https://doi.org/10.3390/ijms18020304
Wang C-M, Wang RX, Liu R, Yang W-H. Jun Dimerization Protein 2 Activates Mc2r Transcriptional Activity: Role of Phosphorylation and SUMOylation. International Journal of Molecular Sciences. 2017; 18(2):304. https://doi.org/10.3390/ijms18020304
Chicago/Turabian StyleWang, Chiung-Min, Raymond X. Wang, Runhua Liu, and Wei-Hsiung Yang. 2017. "Jun Dimerization Protein 2 Activates Mc2r Transcriptional Activity: Role of Phosphorylation and SUMOylation" International Journal of Molecular Sciences 18, no. 2: 304. https://doi.org/10.3390/ijms18020304