Embryological Results of Couples Undergoing ICSI-ET Treatments with Males Carrying the Single Nucleotide Polymorphism rs175080 of the MLH3 Gene
Abstract
:1. Introduction
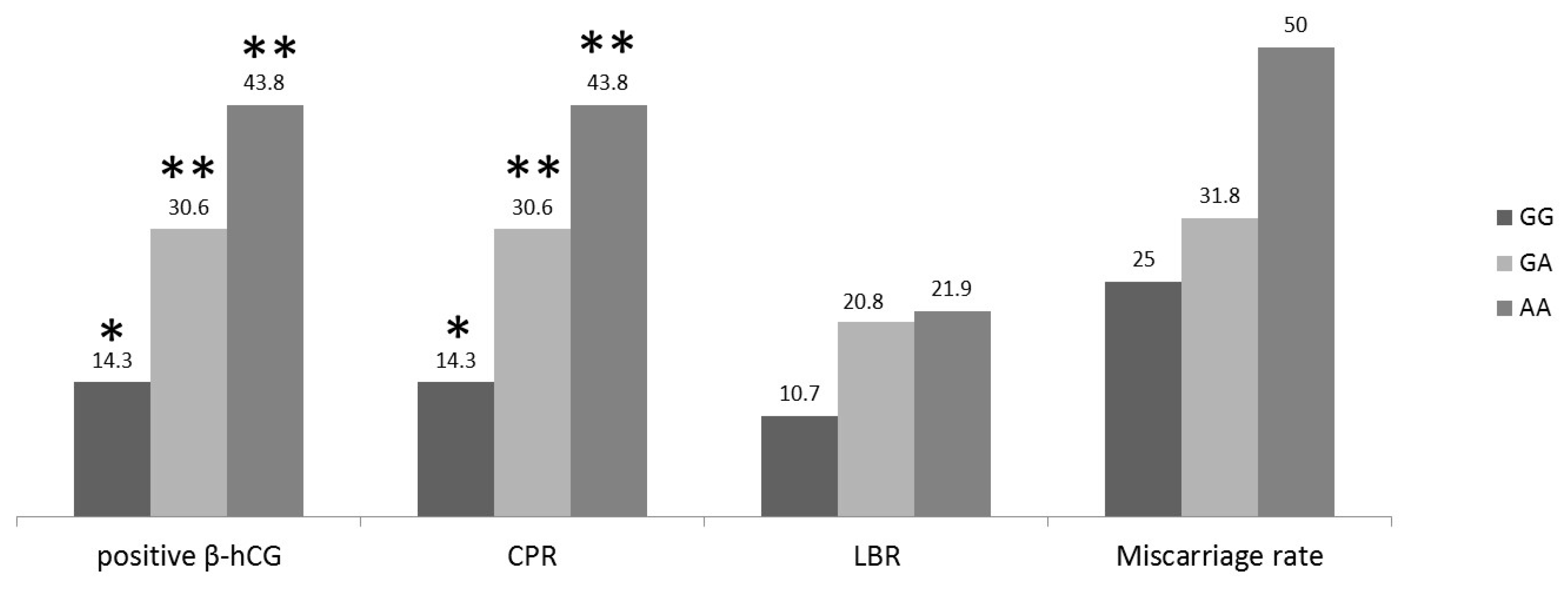
2. Results
3. Discussion
4. Materials and Methods
4.1. DNA Extraction
4.2. Genotyping the Single Nucleotide Polymorphism (SNP)
4.3. Fertilization, Cleavage, Pregnancy Rates and Assessment of Embryo Quality
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Hu, M.; Liu, S.; Wang, N.; Wu, Y.; Jin, F. Impact of DNA mismatch repair system alterations on human fertility and related treatments. J. Zhejiang Univ. Sci. B 2016, 17, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Terribas, E.; Bonache, S.; García-Arévalo, M.; Sánchez, J.; Franco, E.; Bassas, L.; Larriba, S. Changes in the expression profile of the meiosis-involved mismatch repair genes in impaired human spermatogenesis. J. Androl. 2010, 31, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Pashaiefar, H.; Sheikhha, M.H.; Kalantar, S.M.; Jahaninejad, T.; Zaimy, M.A.; Ghasemi, N. Analysis of MLH3 C2531T polymorphism in Iranian women with unexplained infertility. Iran J. Reprod. Med. 2013, 11, 19–24. [Google Scholar] [PubMed]
- Schmidt, M.H.; Pearson, C.E. Disease-associated repeat instability and mismatch repair. DNA Repair 2016, 38, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Muro, Y.; Sugiura, K.; Mimori, T.; Akiyama, M. DNA mismatch repair enzymes: Genetic defects and autoimmunity. Clin. Chim. Acta 2015, 442, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Her, C.; Zhao, N.; Wu, X.; Tompkins, J.D. MutS homologues hMSH4 and hMSH5: Diverse functional implications in humans. Front. Biosci. 2007, 12, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, S.M.; Moens, P.B.; Wang, V.; Lenzi, M.; Shanmugarajah, D.; Gilgeous, A.; Thomas, J.; Cheng, J.; Touchman, J.W.; Green, E.D.; et al. Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat. Genet. 2002, 31, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Kan, R.; Sun, X.; Kolas, N.K.; Avdievich, E.; Kneitz, B.; Edelmann, W.; Cohen, P.E. Comparative analysis of meiotic progression in female mice bearing mutations in genes of the DNA mismatch repair pathway. Biol. Reprod. 2008, 78, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, S.M.; Wang, V.; Jacoby, R.; Banerjee-Basu, S.; Baxevanis, A.D.; Lynch, H.T.; Elliott, R.M.; Collins, F.S. MLH3: A DNA mismatch repair gene associated with mammalian microsatellite instability. Nat. Genet. 2000, 24, 27–35. [Google Scholar] [PubMed]
- Cannavo, E.; Marra, G.; Sabates-Bellver, J.; Menigatti, M.; Lipkin, S.M.; Fischer, F.; Cejka, P.; Jiricny, J. Expression of the MutL homologue hMLH3 in human cells and its role in DNA mismatch repair. Cancer Res. 2005, 65, 10759–10766. [Google Scholar] [CrossRef] [PubMed]
- Santucci-Darmanin, S.; Neyton, S.; Lespinasse, F.; Saunieres, A.; Gaudray, P.; Paquis-Flucklinger, V. The DNA mismatch-repair MLH3 protein interacts with MSH4 in meiotic cells, supporting a role for this MutL homolog in mammalian meiotic recombination. Hum. Mol. Genet. 2002, 11, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Markandona, O.; Dafopoulos, K.; Anifandis, G.; Messini, C.I.; Dimitraki, M.; Tsezou, A.; Georgoulias, P.; Messinis, I.E. Single-nucleotide polymorphism rs 175080 in the MLH3 gene and its relation to male infertility. J. Assist. Reprod. Genet. 2015, 32, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Pashaiefar, H.; Sheikhha, M.H.; Kalantar, S.M.; Jahaninejad, T.; Zaimy, M.A. Analysis of MLH3 C2531T polymorphism in Iranian men with Idiopathic azoospermia or severe oligozoospermia. J. Shahid Sadoughi Univ. Med. Sci. 2013, 21, 62–69. [Google Scholar]
- Xu, K.; Lu, T.; Zhou, H.; Bai, L.; Xiang, Y. The role of MSH5 C85T and MLH3 C2531T polymorphisms in the risk of male infertility with azoospermia or severe oligozoospermia. Clin. Chim. Acta 2010, 411, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Nishant, K.T.; Plys, A.J.; Alani, E. A mutation in the putative MLH3 endonuclease domain confers a defect in both mismatch repair and meiosis in Saccharomyces cerevisiae. Genetics 2008, 179, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, L.; Anand, R.; Cejka, P. The Saccharomyces cerevisiae MLH1-MLH3 heterodimer is an endonuclease that preferentially binds to Holliday junctions. J. Biol. Chem. 2014, 289, 5674–5686. [Google Scholar] [CrossRef] [PubMed]
- Ferrás, C.; Zhou, X.L.; Sousa, M.; Lindblom, A.; Barros, A. DNA mismatch repair gene hMLH3 variants in meiotic arrest. Fertil. Steril. 2005, 88, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Nogués, C.; Fernández, C.; Rajmil, O.; Templado, C. Baseline expression profile of meiotic-specific genes in healthy fertile males. Fertil. Steril. 2009, 92, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Yan, L.; Liu, W.; Huang, C.; Gu, A.; Wang, X. Polymorphisms in double-strand breaks repair genes are associated with impaired fertility in Chinese population. Reproduction 2013, 145, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, M.; Ding, X.; Li, T.; Chen, H. Six polymorphisms in genes involved in DNA double-strand break repair and chromosome synapsis: Association with male infertility. Syst. Biol. Reprod. Med. 2015, 61, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Bounartzi, T.; Messini, C.I.; Dafopoulos, K.; Markandona, R.; Sotiriou, S.; Tzavella, A.; Messinis, I.E. Sperm DNA fragmentation measured by halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia 2015, 47, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Messini, C.I.; Dafopoulos, K.; Messinis, I.E. Genes and conditions controlling mammalian pre- and post-implantation embryo development. Curr. Genomics 2015, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Anifandis, G.; Dafopoulos, K.; Messini, C.I.; Chalvatzas, N.; Messinis, I.E. Effect of the position of the polar body during ICSI on fertilization rate and embryo development. Reprod. Sci. 2010, 17, 849–853. [Google Scholar] [CrossRef] [PubMed]
| Variables | Wild Type (GG) | Heterozygotic Type (GA) | Mutant Type (AA) | p Value |
|---|---|---|---|---|
| Number of cycles | 28 | 72 | 32 | |
| Number of ETs | 22 | 65 | 30 | |
| Age of men (y) | 39.14 ± 1.2 | 38.5 ± 0.7 | 37.87 ± 0.9 | NS |
| Age of women (y) | 35.89 ± 0.7 | 34.71 ± 0.5 | 33.75 ± 0.8 | NS |
| BMI of men (kg/m2) | 27.76 ± 0.8 | 27.58 ± 0.4 | 30.06 ± 0.7 | <0.05 |
| Sperm Concentration (106/mL) | 41.03 ± 6.8 | 38.3 ± 5.4 | 14.57 ± 4.2 | <0.05 |
| FSH (IU/L) | 7.85 ± 1.4 | 6.4 ± 0.5 | 8.3 ± 1 | NS |
| LH (IU/L) | 4.73 ± 0.5 | 4.65 ± 0.2 | 4.96 ± 0.3 | NS |
| E2 (pg/mL) | 45.43 ± 2.8 | 47.51 ± 1.6 | 49.23 ± 2.9 | NS |
| PRL (ng/mL) | 6.83 ± 0.7 | 7.39 ± 0.4 | 8.72 ± 0.9 | NS |
| Testosterone (ng/mL) | 4.81 ± 0.3 | 4.87 ± 0.2 | 4.53 ± 0.3 | NS |
| Variables | Wild Type (GG) | Heterozygotic Type (GA) | Mutant Type (AA) | p Value |
|---|---|---|---|---|
| Number of cycles | 28 | 72 | 32 | |
| Number of ETs | 22 | 65 | 30 | |
| PRM (%) | 39.5 ± 4.1 | 42.8 ± 2.7 | 21.06 ± 3.2 | <0.05 |
| NPM (%) | 12.42 ± 1.9 | 16.71±1.3 | 15.75 ± 2.4 | NS |
| IM (%) | 43.78 ± 4.4 | 37.79 ± 2.6 | 53.81 ± 4.9 | <0.05 |
| CoCs | 4.7 ± 0.7 | 5.6 ± 0.4 | 5.7 ± 0.5 | NS |
| FR (%) | 57.64 ± 7.2 | 61.31 ± 3.3 | 51.29 ± 4.9 | NS |
| CR (%) | 64.89 ± 7.8 | 82.07 ± 3.7 | 89.21 ± 4.6 | <0.05 |
| CES | 17.71 ± 3.3 | 26.5 ± 2.3 | 23.43 ± 2.8 | NS |
| MSEQ | 5.82 ± 0.7 | 7.36 ± 0.4 | 8.12 ± 0.5 | <0.05 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anifandis, G.; Markandona, O.; Dafopoulos, K.; Messini, C.; Tsezou, A.; Dimitraki, M.; Georgoulias, P.; Daponte, A.; Messinis, I. Embryological Results of Couples Undergoing ICSI-ET Treatments with Males Carrying the Single Nucleotide Polymorphism rs175080 of the MLH3 Gene. Int. J. Mol. Sci. 2017, 18, 314. https://doi.org/10.3390/ijms18020314
Anifandis G, Markandona O, Dafopoulos K, Messini C, Tsezou A, Dimitraki M, Georgoulias P, Daponte A, Messinis I. Embryological Results of Couples Undergoing ICSI-ET Treatments with Males Carrying the Single Nucleotide Polymorphism rs175080 of the MLH3 Gene. International Journal of Molecular Sciences. 2017; 18(2):314. https://doi.org/10.3390/ijms18020314
Chicago/Turabian StyleAnifandis, George, Ourania Markandona, Konstantinos Dafopoulos, Christina Messini, Aspasia Tsezou, Marina Dimitraki, Panagiotis Georgoulias, Alexandros Daponte, and Ioannis Messinis. 2017. "Embryological Results of Couples Undergoing ICSI-ET Treatments with Males Carrying the Single Nucleotide Polymorphism rs175080 of the MLH3 Gene" International Journal of Molecular Sciences 18, no. 2: 314. https://doi.org/10.3390/ijms18020314




