Behavior of Gingival Fibroblasts on Titanium Implant Surfaces in Combination with either Injectable-PRF or PRP
Abstract
:1. Introduction
2. Results
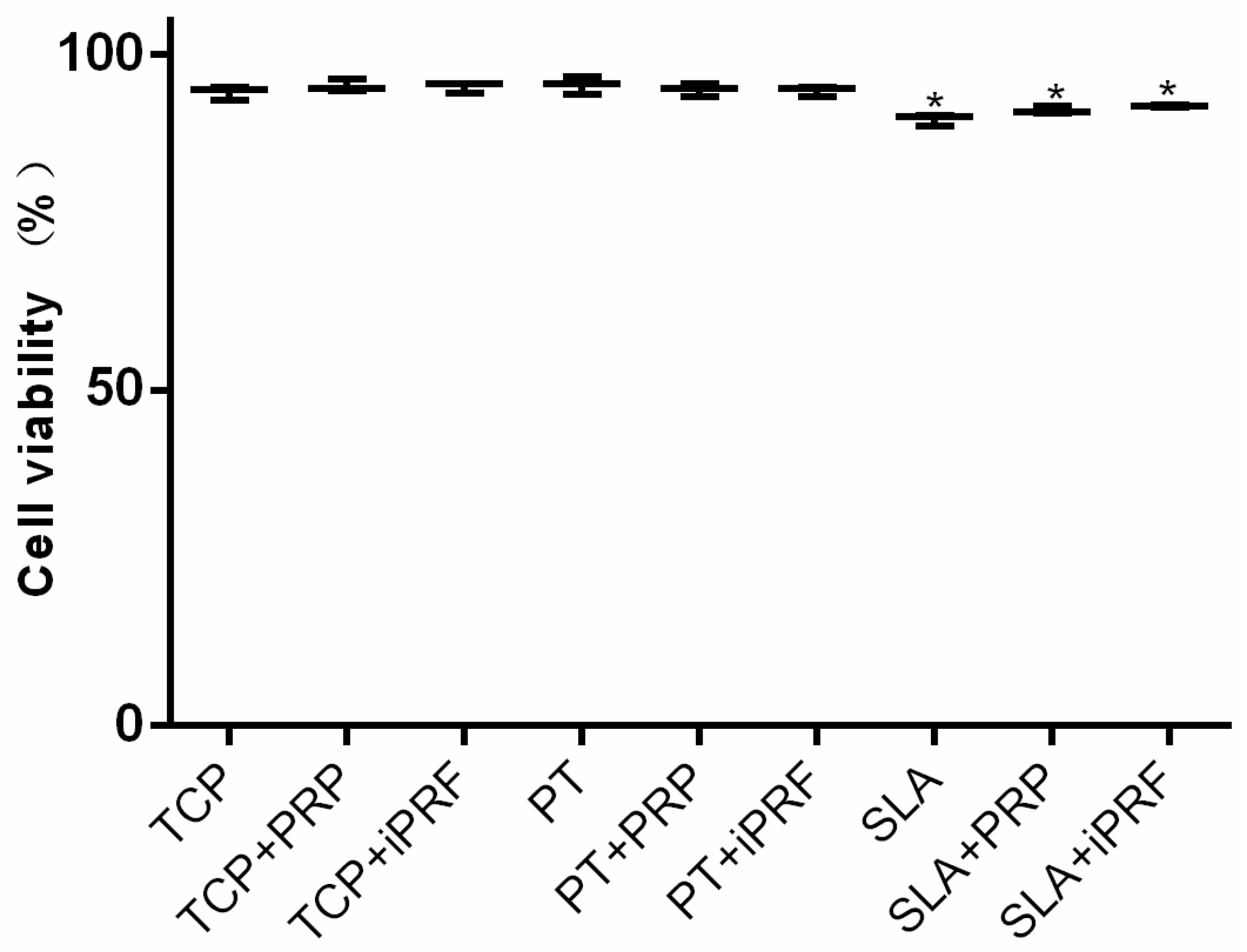
2.1. Cell Viability
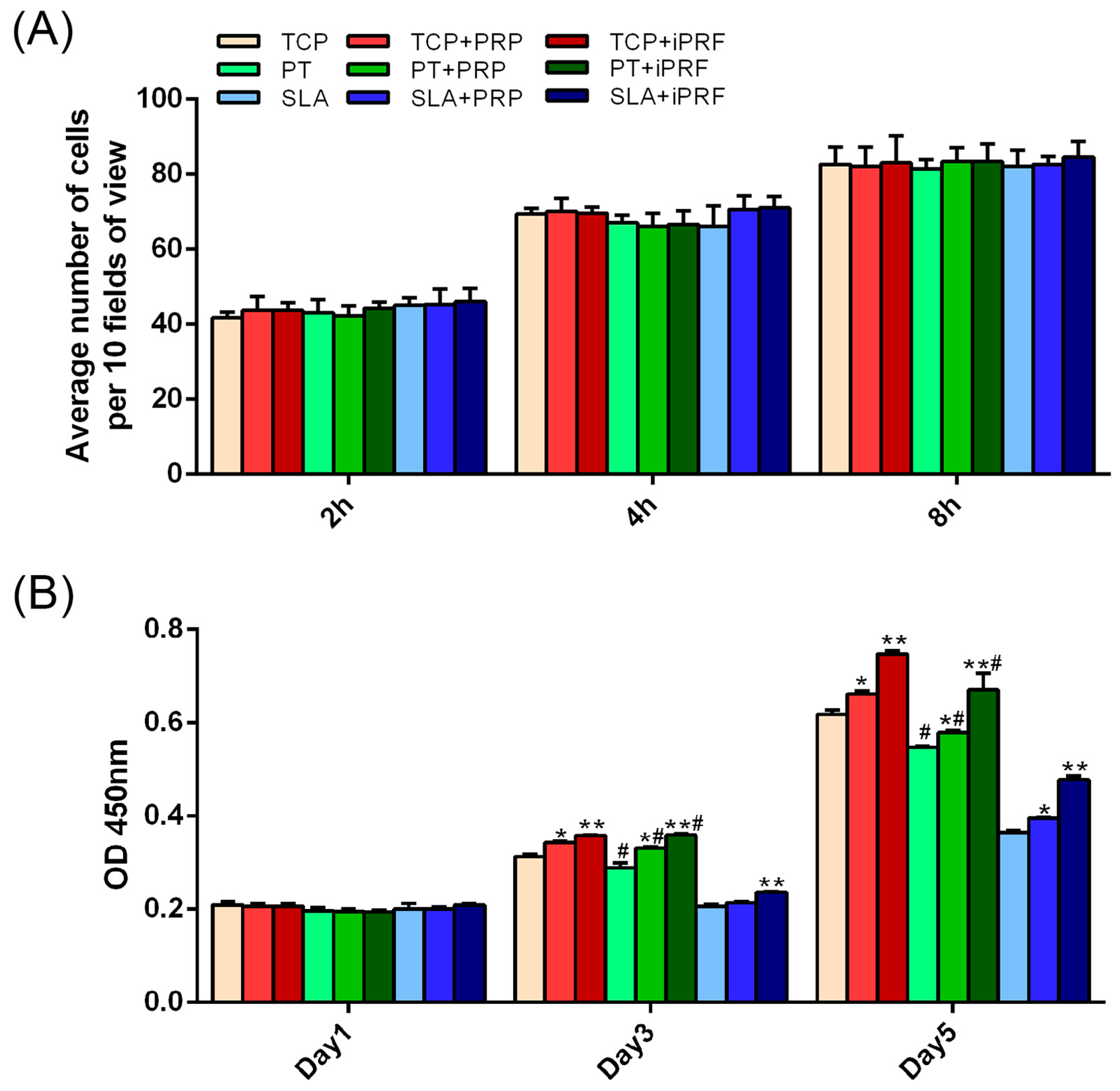
2.2. Cell Migration
2.3. Cell Adhesion, Proliferation and Morphology
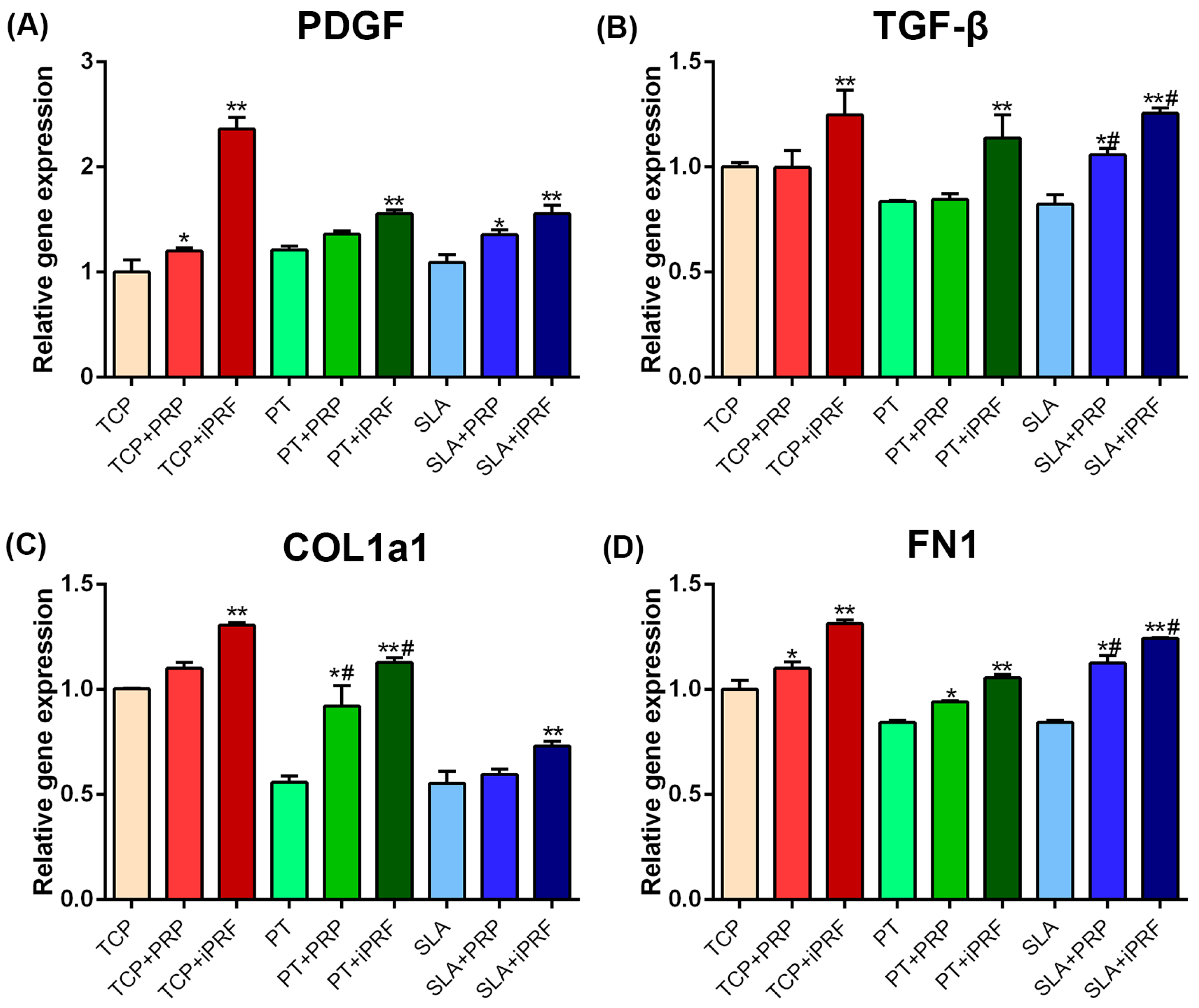
2.4. Human Gingival Fibroblast Expression of Regeneration-Related and Extracellular Matrix (ECM)-Related Genes
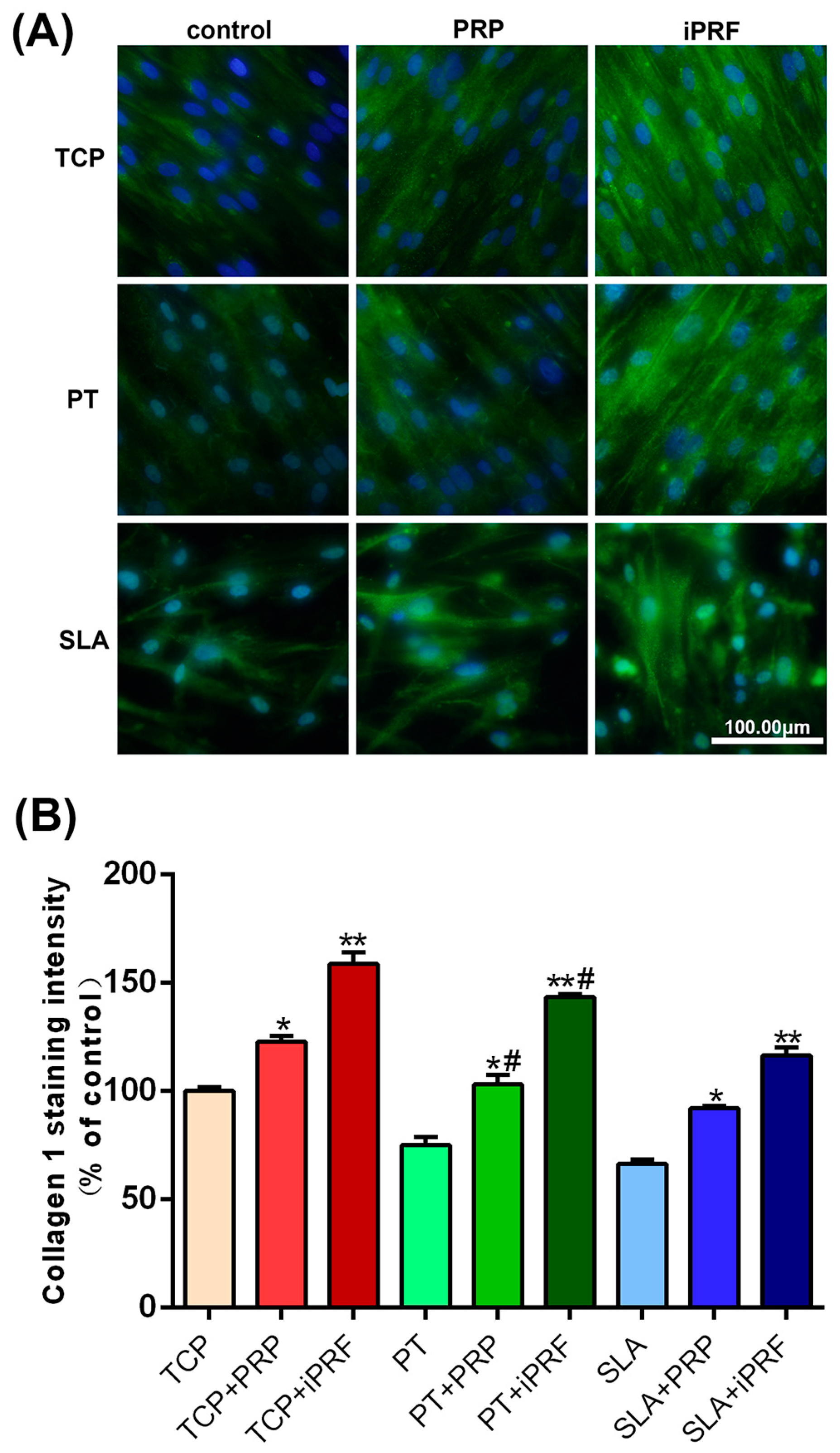
2.5. Collagen Type I Staining
3. Discussion
4. Materials and Methods
4.1. PT and SLA Titanium Discs
4.2. Preparation of PRP and i-PRF
4.3. Isolation of Human Gingival Fibroblasts
4.4. Cell Culture
4.5. Cell Viability
4.6. Cell Migration Assay
4.7. Cell Adhesion and Proliferation Assays
4.8. Cell Morphology
4.9. Real-Time PCR Analysis
4.10. Collagen Type I Staining
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| i-PRF | Injectable Platelet-Rich Fibrin |
| PRP | Platelet-Rich Plasma |
| PT | Pickled Implant Surfaces |
| SLA | Sand-Blasted by Large Grit followed by Acid Etching Implant Surfaces |
| PDGF | Platelet-Derived Growth Factor |
| TGF-β | Transforming Growth Factor-β |
| VEGF | Vascular Endothelial Growth Factor |
| HCl | Hydrochloric Acid |
| H2SO4 | Sulfuric Acid |
| EDTA | Ethylenediaminetetraacetic Acid |
| PPP | Platelet-Poor Plasma |
| RBC | Red Blood Cells |
| DMEM | Dulbecco’s Modified Eagle Medium |
| PBS | Phosphate Buffered Solution |
| FBS | Fetal Bovine Serum |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| HGF | Human Gingival Fibroblast |
| FITC | Fluorescein Isothiocyanate |
| COL1a1 | Collagen type I α1 |
| FN1 | Fibronectin |
| GAPDH | Glyceraldehyde 3-Phosphate Dehydrogenase |
| RT | Room Temperature |
References
- Mendonça, G.; Mendonça, D.B.; Aragao, F.J.; Cooper, L.F. Advancing dental implant surface technology—From micron-to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhang, W.; Shi, B.; Cheng, X.; Zhang, Y. Enhanced bone regeneration around dental implant with bone morphogenetic protein 2 gene and vascular endothelial growth factor protein delivery. Clin. Oral Implant. Res. 2012, 23, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.; Lojudice, F.; Halcsik, E.; Navarro, R.; Sogayar, M.; Granjeiro, J. Bone morphogenetic proteins facts, challenges, and future perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Schenk, R.; Buser, D.; Wozney, J.M.; Jones, A.A. Recombinant human bone morphogenetic protein-2 stimulation of bone formation around endosseous dental implants. J. Periodontol. 1999, 70, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Weibrich, G.; Hansen, T.; Kleis, W.; Buch, R.; Hitzler, W. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004, 34, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Zechner, W.; Tangl, S.; Tepper, G.; Fürst, G.; Bernhart, T.; Haas, R.; Watzek, G. Influence of platelet-rich plasma on osseous healing of dental implants: A histologic and histomorphometric study in minipigs. Int. J. Oral Maxillofac. Implant. 2003, 18, 15–22. [Google Scholar]
- Wang, Y.; Zhang, Y.; Miron, R.J. Health, Maintenance, and Recovery of Soft Tissues around Implants. Clin. Implant Dent. Relat. Res. 2016, 18, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef] [PubMed]
- Peerbooms, J.C.; van Laar, W.; Faber, F.; Schuller, H.M.; van der Hoeven, H.; Gosens, T. Use of platelet rich plasma to treat plantar fasciitis: Design of a multi centre randomized controlled trial. BMC Musculoskelet. Disord. 2010, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet Rich Fibrin With the Low Speed Concept: Growth Factor Release, Biocompatibility and Cellular Response. J. Periodontol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Zhang, C.; Lin, X.J. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: A meta-analysis. J. Shoulder Elbow Surg. 2015, 24, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Goldberg, L.J. Autologous Platelet-Rich Plasma for the Treatment of Pattern Hair Loss. Am. J. Clin. Dermatol. 2016, 17, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, G.; Trovati, M.; Mularoni, E.; Massucco, P.; Calcamuggi, G.; Emanuelli, G. Influence of propranolol on platelet aggregation and thromboxane B2 production from platelet-rich plasma and whole blood. Prostaglandins Leukot. Essent. Fatty Acids 1989, 36, 1–7. [Google Scholar] [CrossRef]
- Fijnheer, R.; Pietersz, R.N.; de Korte, D.; Gouwerok, C.W.; Dekker, W.J.; Reesink, H.W.; Roos, D. Platelet activation during preparation of platelet concentrates: A comparison of the platelet-rich plasma and the buffy coat methods. Transfusion 1990, 30, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2001, 42, e62. (In French) [Google Scholar]
- Toffler, M. Guided bone regeneration (GBR) using cortical bone pins in combination with leukocyte-and platelet-rich fibrin (L-PRF). Compend. Contin. Educ. Dent. 2014, 35, 192–198. [Google Scholar] [PubMed]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.; Camargo, P. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal Res. 2012, 47, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, V.Y.; Johns, D.A.; Vidyanath, S.; Sam, G. Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory periapical lesion. J. Conserv. Dent. 2013, 16, 261. [Google Scholar] [CrossRef] [PubMed]
- Medina-Porqueres, I.; Alvarez-Juarez, P. The Efficacy of Platelet-Rich Plasma Injection in the Management of Hip Osteoarthritis: A Systematic Review Protocol. Musculoskelet. Care 2015. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Veronesi, F.; Maglio, M.; Della Bella, E. New and emerging strategies in platelet-rich plasma application in musculoskeletal regenerative procedures: General overview on still open questions and outlook. BioMed Res. Int. 2015, 2015, 846045. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Licata, M.E.; Polizzi, B.; Campisi, G. Platelet-rich plasma (PRP) in dental and oral surgery: From the wound healing to bone regeneration. Immun. Ageing 2013, 10, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.; Doraiswamy, J.; Malaiappan, S.; Varghese, S.S.; del Fabbro, M. Additive effect of autologous platelet concentrates in treatment of intrabony defects: A systematic review and meta-analysis. J. Investig. Clin. Dent. 2016, 7, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Tatullo, M. Influence of PRF in the healing of bone and gingival tissues. Clinical and histological evaluations. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1958–1962. [Google Scholar] [PubMed]
- Moraschini, V.; Barboza Edos, S. Use of Platelet-Rich Fibrin Membrane in the Treatment of Gingival Recession: A Systematic Review and Meta-Analysis. J. Periodontol. 2016, 87, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Marrelli, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Odontogenic differentiation of human dental pulp stem cells on hydrogel scaffolds derived from decellularized bone extracellular matrix and collagen type I. PLoS ONE 2016, 11, e0148225. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Fluckiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implant. 1999, 14, 529–535. [Google Scholar]
- Anitua, E.; Prado, R.; Troya, M.; Zalduendo, M.; de la Fuente, M.; Pino, A.; Muruzabal, F.; Orive, G. Implementation of a more physiological plasma rich in growth factor (PRGF) protocol: Anticoagulant removal and reduction in activator concentration. Platelets 2016, 27, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, T.; Dohan Ehrenfest, D.M.; Everts, P.A.; Wiczkowski, A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: New perspectives. Curr. Pharm. Biotechnol. 2012, 13, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Barrick, B.; Campbell, E.J.; Owen, C.A. Leukocyte proteinases in wound healing: Roles in physiologic and pathologic processes. Wound Repair Regen. 1999, 7, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Oates, C.J.; Molenberg, A.; Dard, M.; Hamilton, D.W. The effect of enamel matrix proteins on the spreading, proliferation and differentiation of osteoblasts cultured on titanium surfaces. Biomaterials 2010, 31, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Bossert, J.; Jandt, K.D. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf. B Biointerfaces 2006, 49, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Deligianni, D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y. Effect of surface roughness of the titanium alloy Ti–6Al–4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Falisi, G.; Rastelli, C.; Palmieri, F.; Gargari, M.; Zavan, B.; Paduano, F.; Benagiano, V. Mechanical influence of tissue culture plates and extracellular matrix on mesenchymal stem cell behavior: A topical review. Int. J. Immunopathol. Pharmacol. 2015, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Falisi, G.; Apicella, A.; Apicella, D.; Amantea, M.; Cielo, A.; Bonanome, L.; Palmieri, F.; Santacroce, L.; Giannini, S. Behaviour of dental pulp stem cells on different types of innovative mesoporous and nanoporous silicon scaffolds with different functionalizations of the surfaces. J. Biol. Regul. Homeost. Agents 2014, 29, 991–997. [Google Scholar]
- Weibrich, G.; Kleis, W.K.; Hafner, G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: Curasan-type PRP kit versus PCCS PRP system. Int. J. Oral Maxillofac. Implant. 2002, 17, 184–190. [Google Scholar]
- Wang, Y.; Zhang, Y.; Jing, D.; Shuang, Y.; Miron, R.J. Enamel matrix derivative improves gingival fibroblast cell behavior cultured on titanium surfaces. Clin. Oral Investig. 2016, 20, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Hedbom, E.; Ruggiero, S.; Bosshardt, D.D.; Zhang, Y.; Mauth, C.; Gemperli, A.C.; Iizuka, T.; Buser, D.; Sculean, A. Premature osteoblast clustering by enamel matrix proteins induces osteoblast differentiation through up-regulation of connexin 43 and N-cadherin. PLoS ONE 2011, 6, e23375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wei, L.; Chang, J.; Miron, R.J.; Shi, B.; Yi, S.; Wu, C. Strontium-incorporated mesoporous bioactive glass scaffolds stimulating in vitro proliferation and differentiation of bone marrow stromal cells and in vivo regeneration of osteoporotic bone defects. J. Mater. Chem. B 2013, 1, 5711–5722. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Miron, R.J.; Shi, B.; Bian, Z. Anabolic Bone Formation Via a Site Specific Bone Targeting Delivery System by Interfering with Semaphorin 4D Expression. J. Bone Miner. Res. 2015, 30, 286–296. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequence (5′ to 3′) |
|---|---|
| hPDGF F | cacacctcctcgctgtagtattta |
| hPDGF R | gttatcggtgtaaatgtcatccaa |
| hTGF-β F | actactacgccaaggaggtcac |
| hTGF-β R | tgcttgaacttgtcatagatttcg |
| hCOL1a1 F | cccagccaagaactggtatagg |
| hCOL1a1 R | ggctgccagcattgatagtttc |
| hFN1 F | acctacggatgactcgtgctttga |
| hFN1 R | caaagcctaagcactggcacaaca |
| hGAPDH F | agccacatcgctcagacac |
| hGAPDH R | gcccaatacgaccaaatcc |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Behavior of Gingival Fibroblasts on Titanium Implant Surfaces in Combination with either Injectable-PRF or PRP. Int. J. Mol. Sci. 2017, 18, 331. https://doi.org/10.3390/ijms18020331
Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ. Behavior of Gingival Fibroblasts on Titanium Implant Surfaces in Combination with either Injectable-PRF or PRP. International Journal of Molecular Sciences. 2017; 18(2):331. https://doi.org/10.3390/ijms18020331
Chicago/Turabian StyleWang, Xuzhu, Yufeng Zhang, Joseph Choukroun, Shahram Ghanaati, and Richard J. Miron. 2017. "Behavior of Gingival Fibroblasts on Titanium Implant Surfaces in Combination with either Injectable-PRF or PRP" International Journal of Molecular Sciences 18, no. 2: 331. https://doi.org/10.3390/ijms18020331






