Exogenous PTHrP Repairs the Damaged Fracture Healing of PTHrP+/− Mice and Accelerates Fracture Healing of Wild Mice
Abstract
:1. Introduction
2. Results
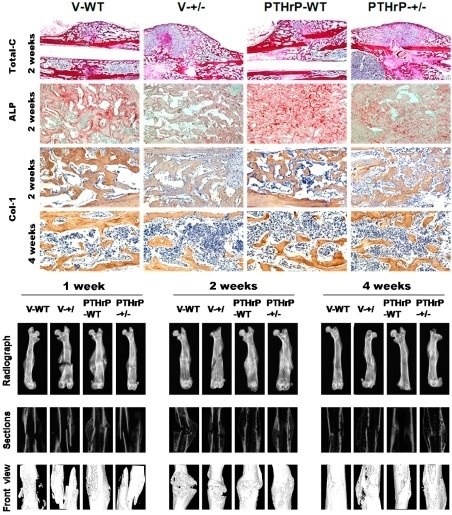
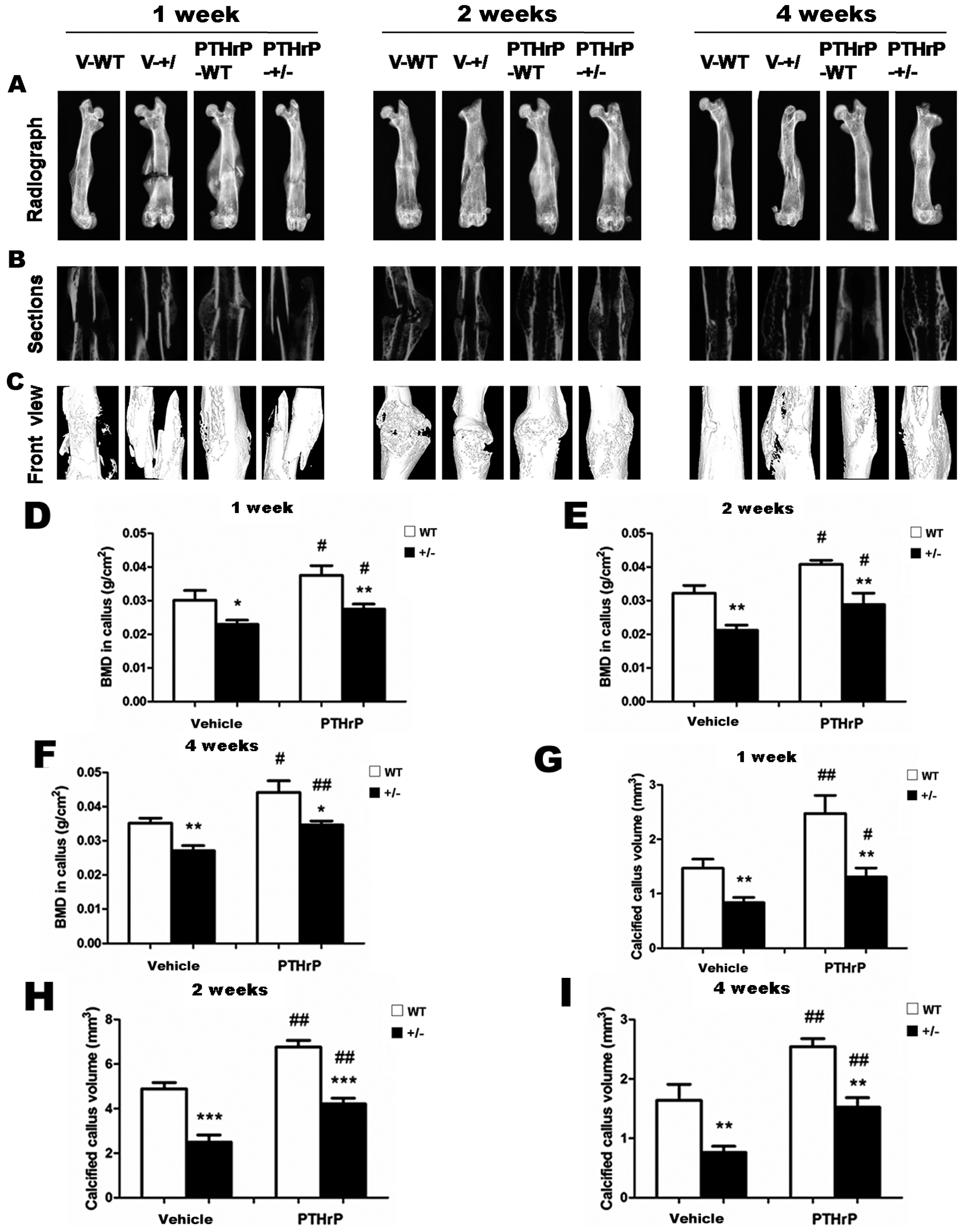
2.1. Exogenous PTHrP Redressed Effect of Endogenous PTHrP-Deficient Delayed Bone Fracture Healing
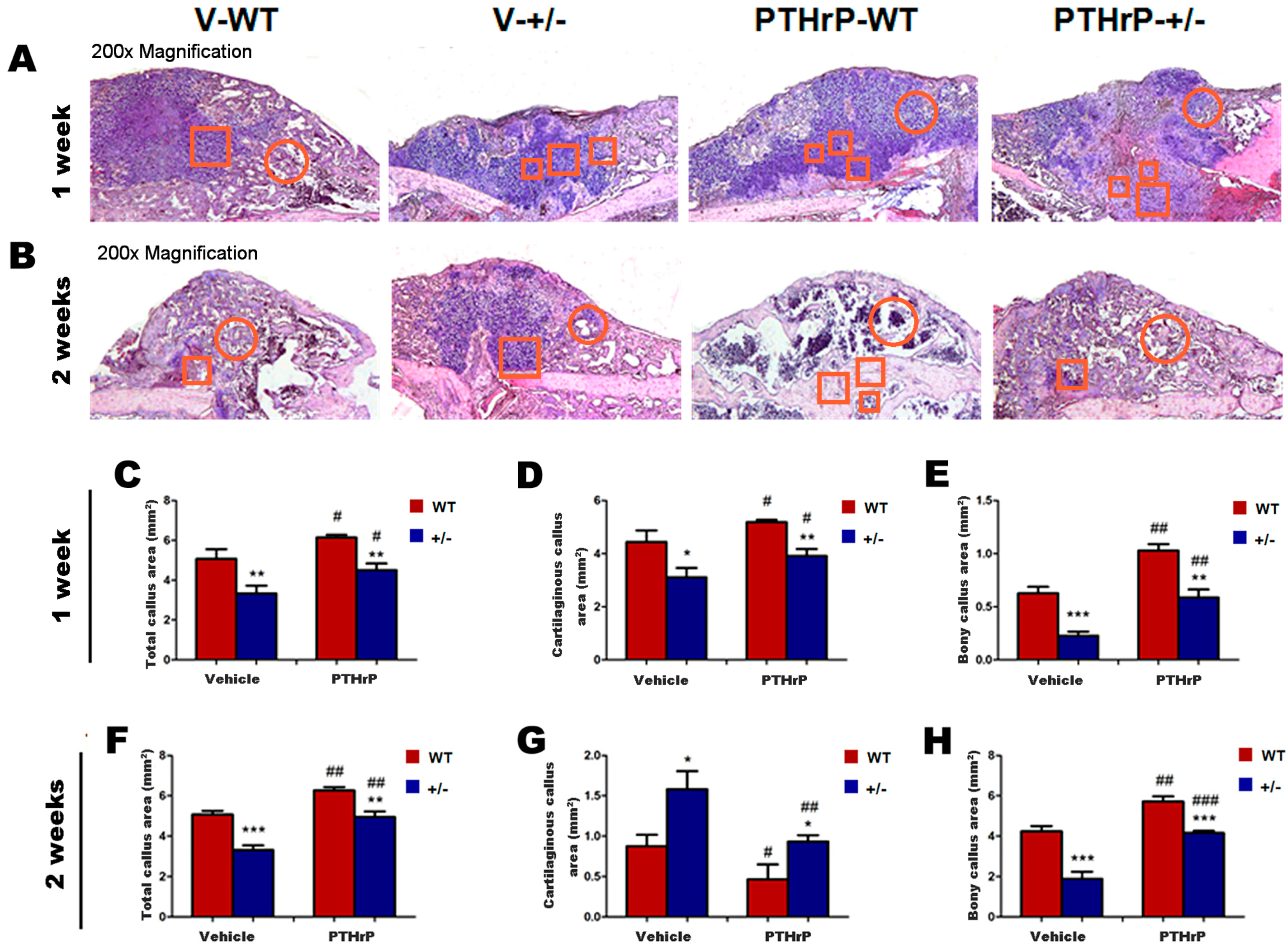
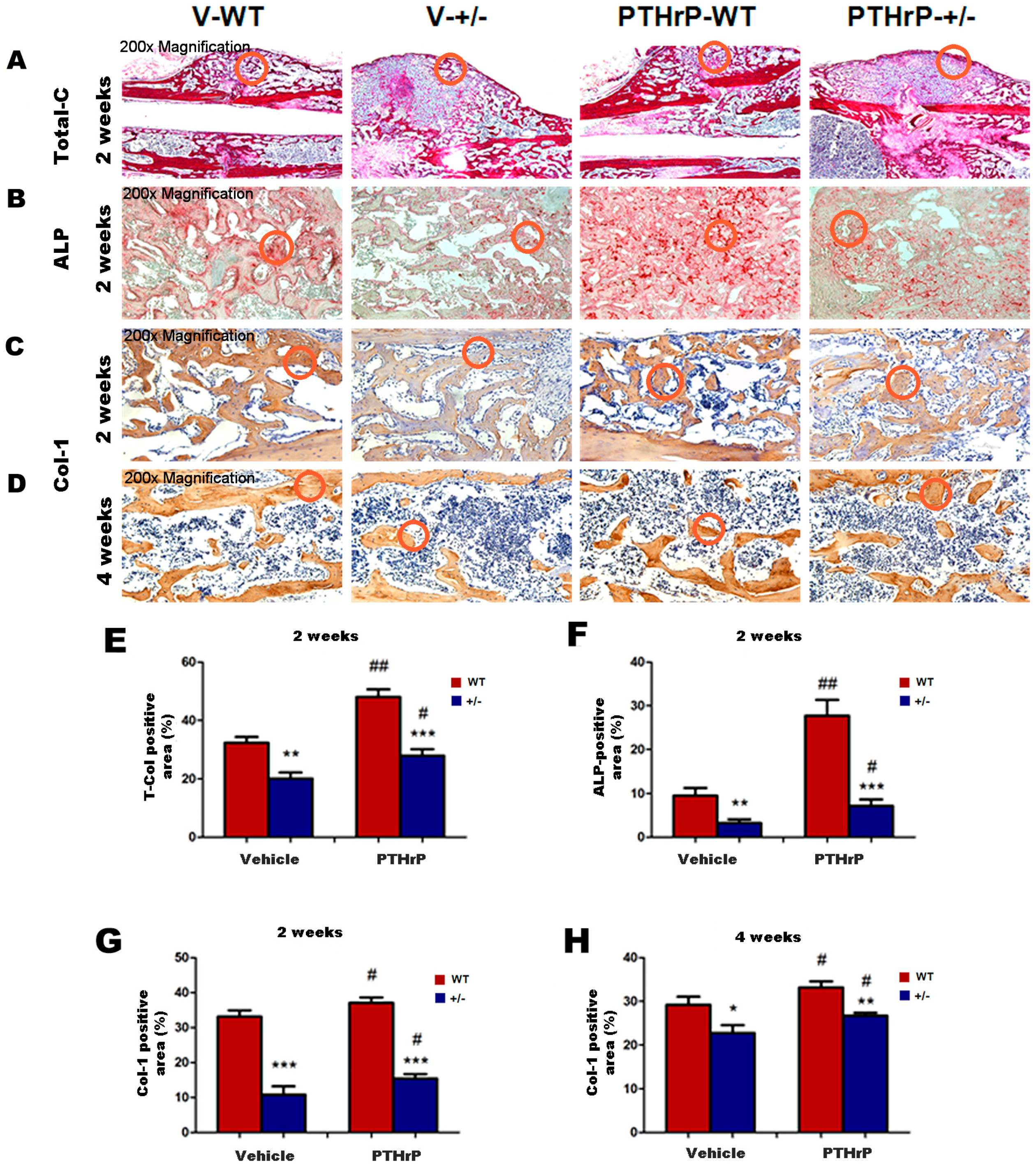
2.2. Endogenous PTHrP Deficiency Inhibited Cartilage Differentiation and Exogenous PTHrP Promoted Cartilaginous Callus Formation and Transformation into Bony Callus
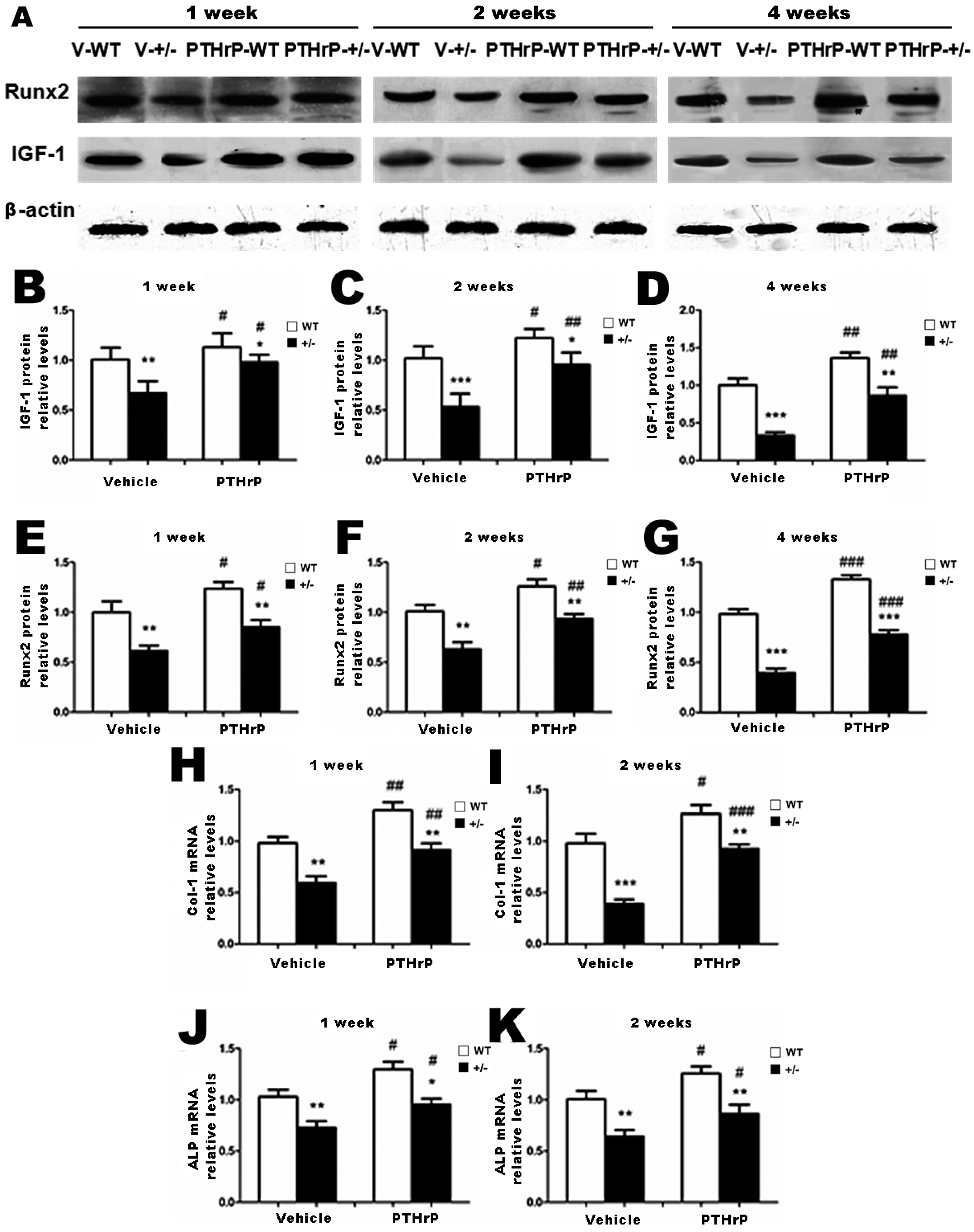
2.3. Effects of Endogenous PTHrP Deficiency and Exogenous PTHrP on the Expression of Osteoblastic Bone Formation-Related Genes and Proteins in Callus Tissues
2.4. Effect of Endogenous PTHrP Deficiency and Exogenous PTHrP on Osteoblastic Bone Formation in Calluses
2.5. Effects of Endogenous PTHrP Deficiency and Exogenous PTHrP on Osteoclastic Bone Resorption in Calluses at Two and Four Weeks PF
2.6. Effects of Endogenous PTHrP Deficiency on Mechanical Properties of Femur Fracture Callus at Four Weeks PF
3. Discussion
4. Materials and Methods
4.1. Sources of Mice
4.2. Animal Fracture Models and Bone Tissue Preparation
4.3. Skeletal Radiography
4.4. Micro Computed Tomography
4.5. RNA Preparation and Quantitative Real-Time Reverse Transcriptase–Polymerase Chain Reaction
4.6. Western Blot Analysis
4.7. Histology
4.8. Immunohistochemical Staining
4.9. Biomechanical Testing
4.10. Computer-Assisted Image Analysis
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.S.; Lee, M.A.; Reddi, A.H. Nonunions and the potential of stem cells in fracture-healing. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. 1), 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Myeroff, C.; Archdeacon, M. Autogenous bone graft: Donor sites and techniques. J. Bone Jt. Surg. Am. 2011, 93, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Laurencin, C.T.; Lyons, K. An aaos-nih symposium. Fracture repair: Challenges, opportunities, and directions for future research. J. Bone Jt. Surg. Am. 2008, 90, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Mazza, E.; Colombo, M.; Ripamonti, C.; Tagliabue, L. Treatment of long bone non-unions with polytherapy: Indications and clinical results. Injury 2011, 42, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Sarahrudi, K.; Thomas, A.; Braunsteiner, T.; Wolf, H.; Vecsei, V.; Aharinejad, S. VEGF serum concentrations in patients with long bone fractures: A comparison between impaired and normal fracture healing. J. Orthop. Res. 2009, 27, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Sarahrudi, K.; Mousavi, M.; Thomas, A.; Eipeldauer, S.; Vecsei, V.; Pietschmann, P.; Aharinejad, S. Elevated levels of macrophage colony-stimulating factor in human fracture healing. J. Orthop. Res. 2010, 28, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Sarahrudi, K.; Thomas, A.; Mousavi, M.; Kaiser, G.; Kottstorfer, J.; Kecht, M.; Hajdu, S.; Aharinejad, S. Elevated transforming growth factor-β 1 (TGF-β1) levels in human fracture healing. Injury 2011, 42, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Suva, L.J.; Winslow, G.A.; Wettenhall, R.E.; Hammonds, R.G.; Moseley, J.M.; Diefenbach-Jagger, H.; Rodda, C.P.; Kemp, B.E.; Rodriguez, H.; Chen, E.Y.; et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: Cloning and expression. Science 1987, 237, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi-Taesch, N.; Sicari, B.; Ubriani, K.; Cozar-Castellano, I.; Takane, K.K.; Stewart, A.F. Mutant parathyroid hormone-related protein, devoid of the nuclear localization signal, markedly inhibits arterial smooth muscle cell cycle and neointima formation by coordinate up-regulation of p15ink4b and p27kip1. Endocrinology 2009, 150, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Karaplis, A.C.; Luz, A.; Glowacki, J.; Bronson, R.T.; Tybulewicz, V.L.; Kronenberg, H.M.; Mulligan, R.C. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994, 8, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Li, J.; Xue, Y.; Su, H.; Karaplis, A.C.; Goltzman, D. Parathyroid hormone-related peptide is required for increased trabecular bone volume in parathyroid hormone-null mice. Endocrinology 2004, 145, 3554–3562. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; Fernandez-de-Castro, L.; Portal-Nunez, S.; Lopez-Herradon, A.; Dapia, S.; Gomez-Barrena, E.; Esbrit, P. The C-terminal fragment of parathyroid hormone-related peptide promotes bone formation in diabetic mice with low-turnover osteopaenia. Br. J. Pharmacol. 2011, 162, 1424–1438. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, B.E., 3rd; Werbeck, J.L.; Thudi, N.K.; Deng, X.; Rosol, T.J.; Toribio, R.E. PTHrP 1–141 and 1–86 increase in vitro bone formation. J. Surg. Res. 2010, 162, e9–e17. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.J.; Tedesco, M.B.; Garcia-Ocana, A.; Sereika, S.M.; Prebehala, L.; Bisello, A.; Hollis, B.W.; Gundberg, C.M.; Stewart, A.F. Parathyroid hormone-related protein for the treatment of postmenopausal osteoporosis: Defining the maximal tolerable dose. J. Clin. Endocrinol. Metab. 2010, 95, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Waterloo, S.; Ahmed, L.A.; Center, J.R.; Eisman, J.A.; Morseth, B.; Nguyen, N.D.; Nguyen, T.; Sogaard, A.J.; Emaus, N. Prevalence of vertebral fractures in women and men in the population-based TromsØ study. BMC Musculoskelet. Disord. 2012, 13, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.H.; Qiu, Y.; Han, X.D.; Xiong, J.; Chen, Y.X.; Shi, H.F.; Karaplis, A. Haploinsufficiency of endogenous parathyroid hormone-related peptide impairs bone fracture healing. Clin. Exp. Pharmacol. Physiol. 2013, 40, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, B.; Feng, Y.; Shu, L.; Cao, X.; Karaplis, A.; Goltzman, D.; Miao, D. Endogenous PTH deficiency impairs fracture healing and impedes the fracture-healing efficacy of exogenous pth(1–34). PLoS ONE 2011, 6, e23060. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C.; Thompson, Z.; Miclau, T.; Werb, Z.; Helms, J.A. Altered fracture repair in the absence of MMP9. Development 2003, 130, 4123–4133. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Jingushi, S.; Ikenoue, T.; Urabe, K.; Sakai, H.; Iwamoto, Y. Expression of parathyroid hormone-related peptide and insulin-like growth factor I during rat fracture healing. J. Orthop. Res. 2003, 21, 511–520. [Google Scholar] [CrossRef]
- Miao, D.; He, B.; Jiang, Y.; Kobayashi, T.; Sorocéanu, M.A.; Zhao, J.; Su, H.; Tong, X.; Amizuka, N.; Gupta, A. Osteoblast-derived pthrp is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1–34. J. Clin. Investig. 2005, 115, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Nasiri, A.R.; Broadus, A.E.; Tommasini, S.M. Periosteal PTHrP regulates cortical bone remodeling during fracture healing. Bone 2015, 81, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Vӓlimӓki, V.V.; Vӓlimӓki, M.J.; Loyttyniemi, E.; Richard, M.; Bukka, P.L.; Goltzman, D.; Karaplis, A.C. Variable number of tandem repeats polymorphism in parathyroid hormone-related protein as predictor of peak bone mass in young healthy finnish males. Eur. J. Endocrinol. 2008, 158, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Karaplis, A.C.; Hendy, G.N.; Goltzman, D.; Miao, D. Genetic models show that parathyroid hormone and 1,25-dihydroxyvitamin D3 play distinct and synergistic roles in postnatal mineral ion homeostasis and skeletal development. Hum. Mol. Genet. 2005, 14, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Lipton, A.; Roodman, G.D.; Guise, T.A.; Boyce, B.F.; Brufsky, A.M.; Clezardin, P.; Croucher, P.I.; Gralow, J.R.; Hadji, P.; et al. Metastasis and bone loss: Advancing treatment and prevention. Cancer Treat. Rev. 2010, 36, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Li, Y.; Wang, Y.; Liu, L.; Shi, H.; Qiu, Y. Exogenous parathyroid hormone-related peptide promotes fracture healing in lepr(−/−) mice. Calcif. Tissue Int. 2015, 97, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhou, X.; Zhu, M.; Wang, Q.; Goltzman, D.; Karaplis, A.; Miao, D. Endogenous parathyroid hormone-related protein compensates for the absence of parathyroid hormone in promoting bone accrual in vivo in a model of bone marrow ablation. J. Bone Miner. Res. 2013, 28, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Le, A.X.; Miclau, T.; Hu, D.; Helms, J.A. Molecular aspects of healing in stabilized and non-stabilized fractures. J. Orthop. Res. 2001, 19, 78–84. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Zhou, X.; Xiao, Y.; Karaplis, A.; Pollak, M.R.; Brown, E.; Goltzman, D.; Miao, D. Alterations in phosphorus, calcium and PTHrP contribute to defects in dental and dental alveolar bone formation in calcium-sensing receptor-deficient mice. Development 2010, 137, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, J.; Wang, L.; Chen, N.; Karaplis, A.; Goltzman, D.; Miao, D. Distinctive anabolic roles of 1,25-dihydroxyvitamin D(3) and parathyroid hormone in teeth and mandible versus long bones. J. Endocrinol. 2009, 203, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Salehi, Z.; Mashayekhi, F.; Naji, M.; Pandamooz, S. Insulin-like growth factor-1 and insulin-like growth factor binding proteins in cerebrospinal fluid during the development of mouse embryos. J. Clin. Neurosci. 2009, 16, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Bai, X.; Panda, D.; McKee, M.D.; Karaplis, A.C.; Goltzman, D. Osteomalacia in hyp mice is associated with abnormal Phex expression and with altered bone matrix protein expression and deposition 1. Endocrinology 2001, 142, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A. Technical aspects of immunohistochemistry. Vet. Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Duvall, C.L.; Taylor, W.R.; Weiss, D.; Wojtowicz, A.M.; Guldberg, R.E. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J. Bone Miner. Res. 2007, 22, 286–297. [Google Scholar] [CrossRef] [PubMed]


| Gene | Nucleotide Sequence |
|---|---|
| ALP | sense: 5′–3′CTTGCTGGTGGAAGGAGGCAGG |
| anti-sense: 5′–3′GGAGCACAGGAAGTTGGGAC | |
| Runx-2 | sense: 5′–3′CTTCATTCGCCTCACAAACA |
| anti-sense: 5′–3′TTGATGCCATAGTCCCTCCT | |
| Col-I | sense: 5′–3′TCTCCACTCTTCTAGTTCCT |
| anti-sense: 5′–3′TTGGGTCATTTCCACATGC | |
| GAPDH | sense: 5′–3′GGTCGGTGTGAACGGATTTG |
| anti-sense: 5′–3′ATGAGCCCTTCCACAATG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Fang, X.; Wang, C.; Ding, C.; Lin, H.; Liu, A.; Wang, L.; Cao, Y. Exogenous PTHrP Repairs the Damaged Fracture Healing of PTHrP+/− Mice and Accelerates Fracture Healing of Wild Mice. Int. J. Mol. Sci. 2017, 18, 337. https://doi.org/10.3390/ijms18020337
Wang Y, Fang X, Wang C, Ding C, Lin H, Liu A, Wang L, Cao Y. Exogenous PTHrP Repairs the Damaged Fracture Healing of PTHrP+/− Mice and Accelerates Fracture Healing of Wild Mice. International Journal of Molecular Sciences. 2017; 18(2):337. https://doi.org/10.3390/ijms18020337
Chicago/Turabian StyleWang, Yinhe, Xin Fang, Chun Wang, Congzhu Ding, Hua Lin, Anlong Liu, Lei Wang, and Yang Cao. 2017. "Exogenous PTHrP Repairs the Damaged Fracture Healing of PTHrP+/− Mice and Accelerates Fracture Healing of Wild Mice" International Journal of Molecular Sciences 18, no. 2: 337. https://doi.org/10.3390/ijms18020337