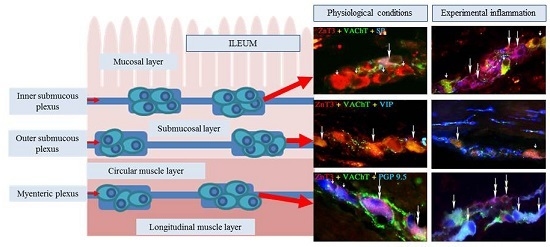
Zinc Transporter 3 (ZnT3) in the Enteric Nervous System of the Porcine Ileum in Physiological Conditions and during Experimental Inflammation
Abstract
:1. Introduction
2. Results
2.1. ZnT3 in the ENS under Physiological Conditions
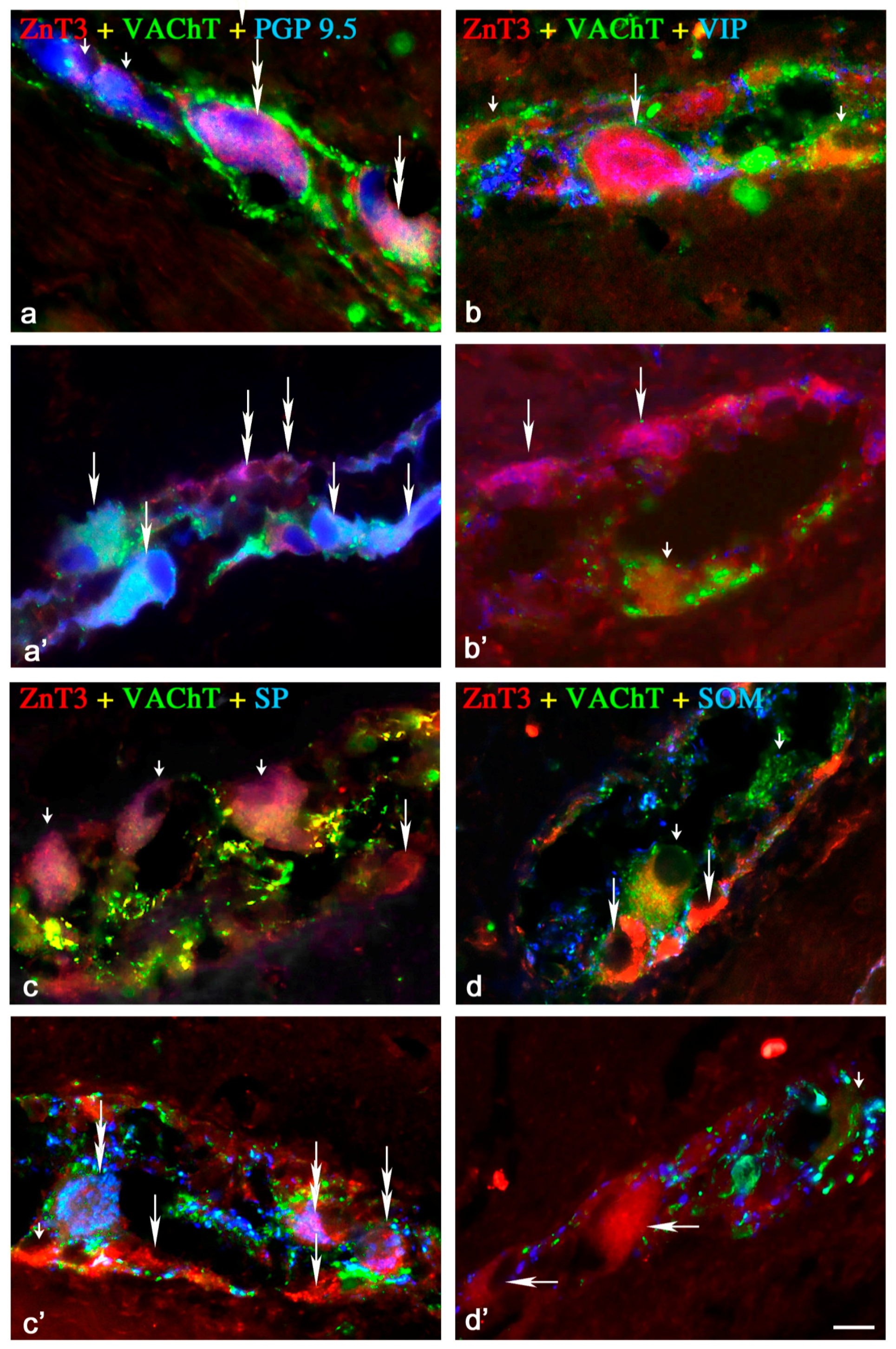
2.1.1. Neurochemical Characterisation of ZnT3+ Cholinergic Enteric Neurons
2.1.2. Neurochemical Characterisation of ZnT3+ Non-Cholinergic Enteric Neurons
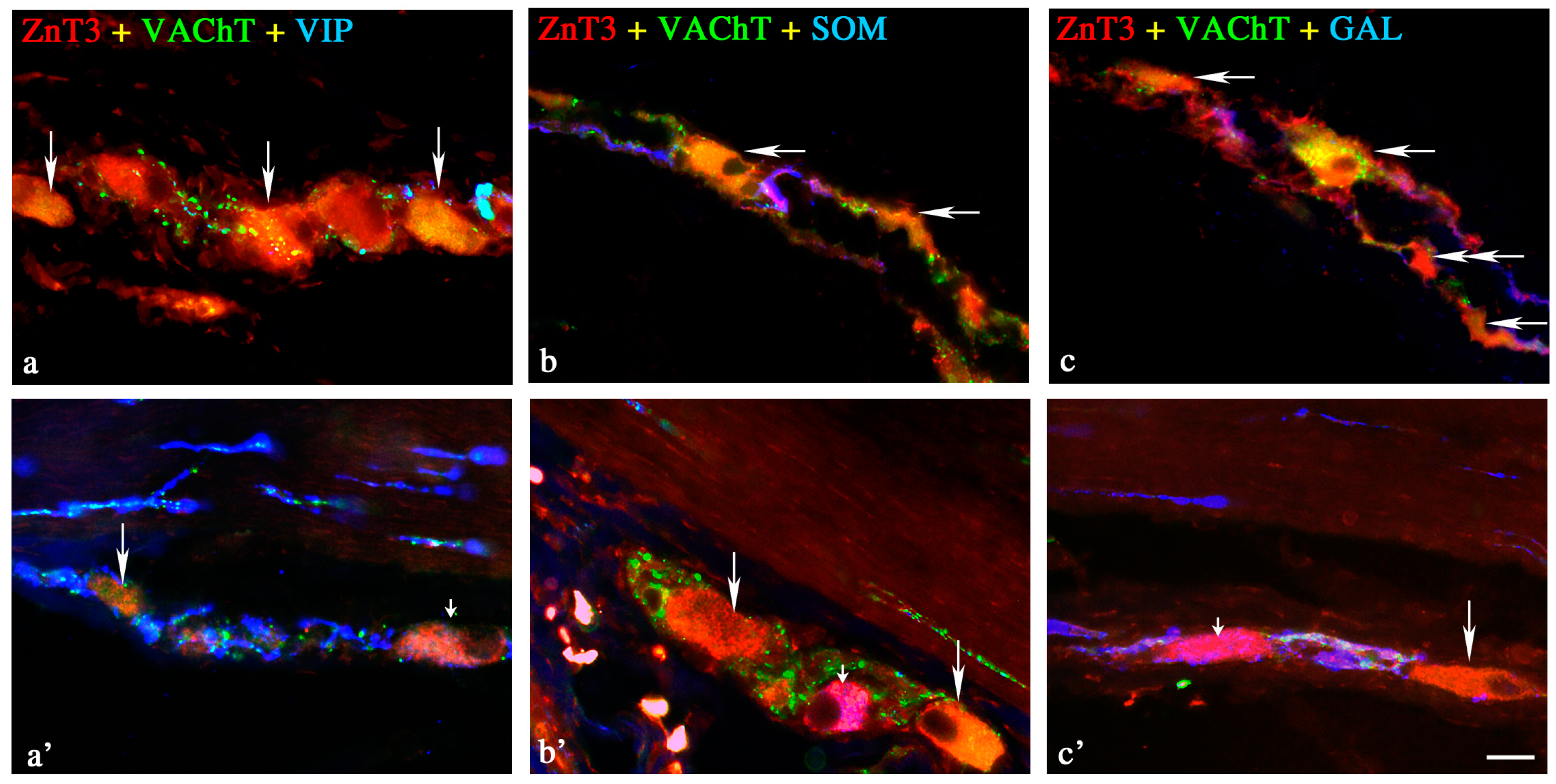
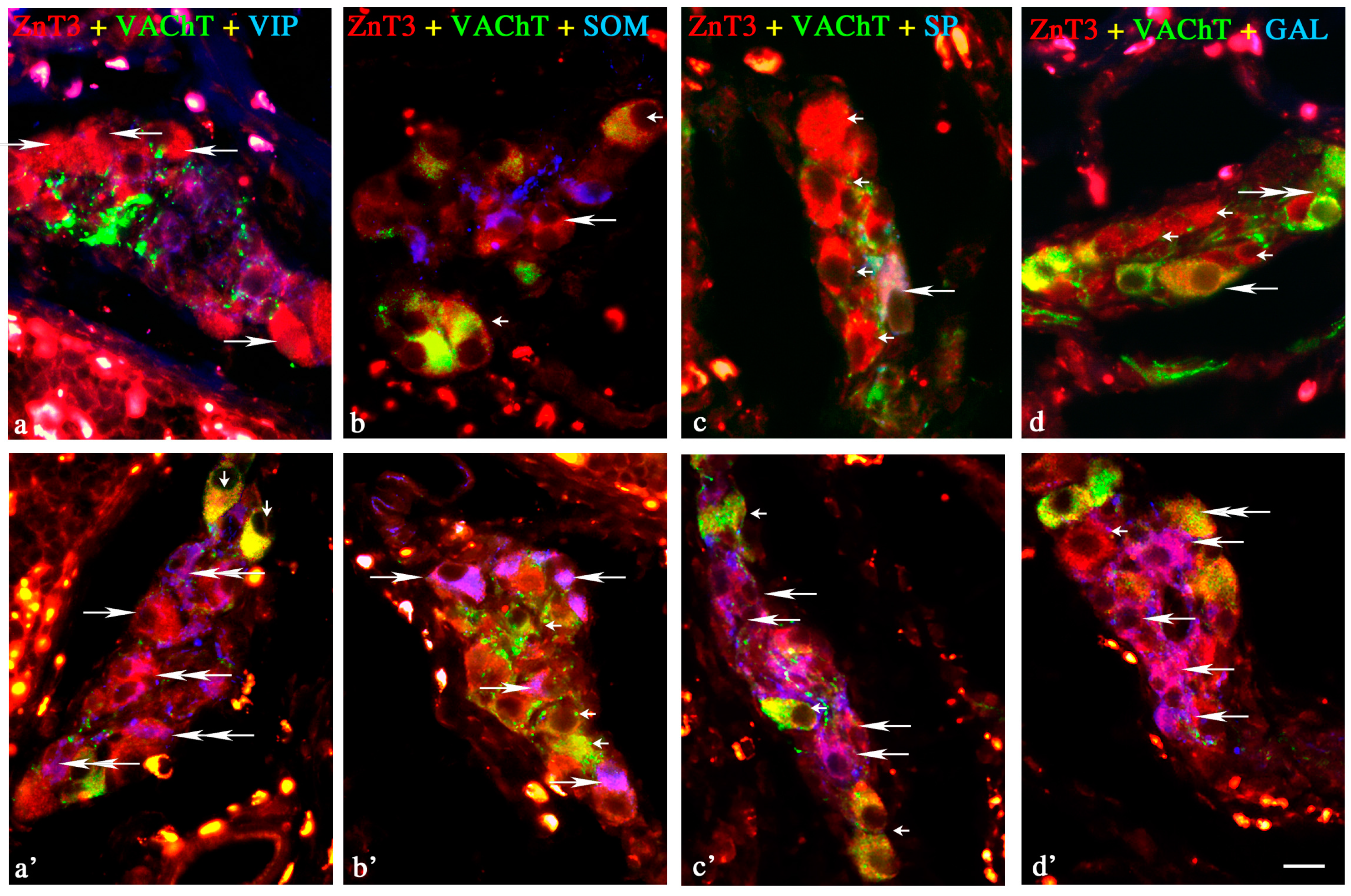
2.2. ZnT3 in the ENS during the Inflammatory Process
Changes in Neurochemical Characterisation of ZnT3+ Enteric Neurons during Inflammation
Myenteric Plexus
Outer Submucous Plexus
Inner Submucous Plexus
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wojtkiewicz, J.; Gonkowski, S.; Bladowski, M.; Majewski, M. Characterization of cocaine- and amphetamine-regulated transcript-like immunoreactive (CART-LI) enteric neurons in the porcine small intestine. Acta Vet. Hung. 2012, 60, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Burlinski, P.; Calka, J. Proliferative enteropathy (PE)-induced changes in galanin—Like immunoreactivity in the enteric nervous system of the porcine distal colon. Acta Vet. Beograd. 2009, 59, 321–330. [Google Scholar]
- Porter, A.J.; Wattchow, D.A.; Brookes, S.J.; Schemann, M.; Costa, M. Choline acetyltransferase immunoreactivity in the human small and large intestine. Gastroenterology 1996, 111, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.J.; Wattchow, D.A.; Brookes, S.J.; Costa, M. Cholinergic and nitrergic interneurones in the myenteric plexus of the human colon. Gut 2002, 51, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kaleczyc, J.; Klimczuk, M.; Franke-Radowiecka, A.; Sienkiewicz, W.; Majewski, M.; Łakomy, M. The distribution and chemical coding of intramural neurons supplying the porcine stomach—The study on normal pigs and on animals suffering from swine dysentery. Anat. Histol. Embryol. 2007, 36, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Pidsudko, Z.; Kaleczyc, J.; Wasowicz, K.; Sienkiewicz, W.; Majewski, M.; Zajac, W.; Lakomy, M. Distribution and chemical coding of intramural neurons in the porcine ileum during proliferative enteropathy. J. Comp. Pathol. 2008, 138, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S. Substance P as a neuronal factor in the enteric nervous system of the porcine descending colon in physiological conditions and during selected pathogenic processes. Biofactors 2013, 39, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Kaminska, B.; Landowski, P.; Skobowiat, C.; Burlinski, P.; Majewski, M.; Calka, J. A population of zinc transporter 3-like immunoreactive neurons is present in the ganglia of human descending colon. Adv. Clin. Exp. Med. 2009, 18, 243–248. [Google Scholar]
- Palmiter, R.D.; Huang, L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflug. Arch. 2004, 447, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, H.J.; Cole, T.B.; Born, D.E.; Schwartzkroin, P.A.; Palmiter, R.D. Ultrastructural localization of zinc transporter-3 (ZnT3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. USA 1997, 94, 12676–12681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Danscher, G.; Dahlstrom, A.; Li, J.Y. Zinc transporter 3 and zinc ions in the rodent superior cervical ganglion neurons. Neuroscience 2003, 120, 605–616. [Google Scholar] [CrossRef]
- Kaneko, M.; Noguchi, T.; Ikegami, S.; Sakurai, T.; Kakita, A.; Toyoshima, Y.; Kambe, T.; Yamada, M.; Inden, M.; Hara, H.; et al. Zinc transporters ZnT3 and ZnT6 are downregulated in the spinal cords of patients with sporadic amyotrophic lateral sclerosis. J. Neurosci. Res. 2015, 93, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.M.; Won, M.H.; Cole, T.B.; Jansen, M.S.; Palmiter, R.D.; Danscher, G. Zinc-enriched (ZEN) terminals in mouse olfactory bulb. Brain Res. 2000, 865, 227–236. [Google Scholar] [CrossRef]
- Takeda, A. Movement of zinc and its functional significance in the brain. Brain Res. Rev. 2000, 34, 137–148. [Google Scholar] [CrossRef]
- Molnar, P.; Nadler, J.V. Lack of effects of mossy fiber—Released zinc on granule cell (GABAA receptors in the pilocarine model of epilepsy. J. Neurophysiol. 2001, 85, 1932–1940. [Google Scholar] [PubMed]
- Wojtkiewicz, J.; Równiak, M.; Crayton, R.; Majewski, M.; Gonkowski, S. Chemical coding of zinc-enriched neurons in the intramural ganglia of the porcine jejunum. Cell Tissue Res. 2012, 350, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Wojtkiewicz, J.; Gonkowski, S.; Równiak, M.; Crayton, R.; Majewski, M.; Jałyński, M. Neurochemical characterization of zinc transporter 3-like immunoreactive (ZnT3+) neurons in the intramural ganglia of the porcine duodenum. J. Mol. Neurosci. 2012, 48, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [PubMed]
- Dineley, K.E.; Votyakova, T.V.; Reynolds, I.J. Zinc inhibition of cellular energy production: Implications for mitochondria and neurodegenaration. J. Neurochem. 2003, 85, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Barañano, D.E.; Ferris, C.D.; Snyder, S.H. Atypical neural messengers. Trends. Neurosci. 2001, 24, 99–106. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Bird, S.M.; Black, R.E.; Brown, K.H.; Gardner, J.M.; Hidayat, A.; Khatun, F.; Martorell, R.; Ninh, N.X.; Penny, M.E.; et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: Pooled analysis of randomized controlled trials. Am. J. Clin. Nutr. 2000, 72, 1516–1522. [Google Scholar] [PubMed]
- Hegarty, S.; Sullivan, A.M.; O’Keeffe, G.W. Zeb2: A multifunctional regulator of nervous system development. Prog. Neurobiol. 2015, 132, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; de Ponti, F.; de Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 126–127, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Calka, J. Changes in the somatostatin (SOM)-like immunoreactivity within nervous structures of the porcine descending colon under various pathological factors. Exp. Mol. Pathol. 2010, 88, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Obremski, K.; Calka, J. The influence of low doses of zearalenone on distribution of selected active substances in nerve fibers within the circular muscle layer of porcine ileum. J. Mol. Neurosci. 2015, 56, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Gonkowski, S.; Zielonka, L.; Dabrowski, M.; Calka, J. T2 toxin-induced changes in cocaine- and amphetamine-regulated transcript (CART)—Like immunoreactivity in the enteric nervous system within selected fragments of the porcine digestive tract. Neurotox. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Timmermans, J.P. Lessons from the porcine enteric nervous system. Neurogastroenterol. Motil. 2004, 16, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Litten-Brown, J.C.; Corson, A.M.; Clarke, L. Porcine models for the metabolic syndrome, digestive and bone disorders: A general overview. Animal 2010, 4, 899–920. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Smidt, K.; Larsen, A.; Brønden, A.; Sørensen, K.S.; Nielsen, J.V.; Praetorius, J.; Martensen, P.M.; Rungby, J. The zinc transporter ZNT3 co-localizes with insulin in INS-1E pancreatic β cells and influences cell survival, insulin secretion capacity, and ZNT8 expression. Biometals 2016, 29, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Bobo, J.A.; Lichten, L.A.; Samuelson, D.A.; Cousins, R.J. Responsive transporter genes within the murine intestinal—Pancreatic axis form a basis of zinc homeostasis. Proc. Natl. Acad. Sci. USA 2004, 101, 14355–14360. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Kelleher, S.L.; Lonnerdal, B. Zinc defficiency is associated with increased brain zinc import and LIV-1 expression and decreased ZnT-1 expression in neonatal rats. J. Nutr. 2005, 135, 1002–1007. [Google Scholar] [PubMed]
- Pfaffl, M.W.; Windisch, W. Influence of zinc deficiency on the mRNA expression of zinc transporters in adult rats. J. Trace Elem. Med. Biol. 2003, 17, 97–106. [Google Scholar] [CrossRef]
- Danscher, G.; Jo, S.M.; Varea, E.; Wang, Z.; Cole, T.B.; Schrøder, H.D. Inhibitory zinc-enriched terminals in mouse spinal cord. Neuroscience 2001, 105, 941–947. [Google Scholar] [CrossRef]
- Danscher, G.; Wang, Z.; Kim, Y.K.; Kim, S.J.; Sun, Y.; Jo, S.M. Immunocytochemical localization of zinc transporter 3 in the ependyma of the mouse spinal cord. Neurosci. Lett. 2003, 342, 81–84. [Google Scholar] [CrossRef]
- Bader, S.; Diener, M. Novel aspects of cholinergic regulation of colonic ion transport. Pharmacol. Res. Perspect. 2015, 3, e00139. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, A.; Kuramoto, H.; Kadowaki, M. 5-HT activates nitric oxide-generating neurons to stimulate chloride secretion in guinea pig distal colon. Am. J. Physiol. 1998, 275, G829–G834. [Google Scholar] [PubMed]
- Schleiffer, R.; Raul, F. Nitric oxide and the digestive system in mammals and non-mammalian vertebrates. Comp. Biochem. Physiol. 1997, 118A, 965–974. [Google Scholar] [CrossRef]
- Page, A.J.; O’Donnell, T.A.; Cooper, N.J.; Young, R.L.; Blackshaw, L.A. Nitric oxide as an endogenous peripheral modulator of visceral sensory neuronal function. J. Neurosci. 2009, 29, 7246–7255. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Alonso, P.; Martinez-Fong, D.; Pazos-Salazar, N.G.; Brambila, E.; Gonzalez-Barrios, J.A.; Mejorada, A.; Flores, G.; Millan-Perez Peña, L.; Rubio, H.; Leon-Chavez, B.A. The increase in zinc levels and upregulation of zinc transporters are mediated by nitric oxide in the cerebral cortex after transient ischemia in the rat. Brain Res. 2008, 1200, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Maret, W.; Cuajungco, M.P. Zinc and excitotoxic brain injury: A new model. Neuroscientist 2004, 10, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Giblin, L.J.; Krezel, A.; McAdoo, D.J.; Muelle, R.N.; Zeng, Y.; Balajim, R.V.; Masalha, R.; Thompson, R.B.; Fierke, C.A.; et al. Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp. Neurol. 2006, 198, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Yeiser, E.C.; Vanlandingham, J.W.; Levenson, C.W. Moderate zinc deficiency increases cell death after brain injury in the rat. Nutr. Neurosci. 2002, 5l, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Devirgiliis, C.; Zalewski, P.D.; Perozzi, G.; Murgia, C. Zinc fluxes and zinc transporters genes in chronic diseases. Mutation Res. 2007, 622, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Sandgren, K.; Ekblad, E. Increased expression of nitric oxide synthase in cultured neurons from adult rat colonic submucous ganglia. Auton. Neurosci. 2004, 114, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Arciszewski, M.B.; Ekblad, E. Effects of vasoactive intestinal petide and galanin on survival of cultured porcine myenteric neurons. Regul. Pept. 2005, 125, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Holmes, F.E.; Mahoney, S.A.; Wynick, D. Use of genetically engineered transgenic mice to investigate the role of galanin in the peripheral nervous system after injury. Neuropeptides 2005, 39, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kasparek, M.S.; Fatima, J.; Iqbal, C.W.; Duenes, J.A.; Sarr, M.G. Role of VIP and Substance P in NANC innervation in the longitudinal smooth muscle of the rat jejunuminfluence of extrinsic denervation. J. Surg. Res. 2007, 141, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, A.; Lindig, T.M.; Schrödl, F.; Neuhuber, W.; Ditterich, D.; Rexer, M.; Rupprecht, H. Morphology of enkephalin-immunoreactive myenteric neurons in the human gut. Histochem. Cell Biol. 2005, 123, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Matsuyama, H.; Shiina, T.; Takewaki, T.; Furness, J.B. Tachykinins and their functions in the gastrointestinal tract. Cell. Mol. Life Sci. 2008, 65, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Chimienti, F. Zinc, pancreatic islet cell function and diabetes: New insights into an old story. Nutr. Res. Rev. 2013, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, P.D.; Truong-Tran, A.Q.; Grosser, D.; Jayaram, L.; Murgia, C.; Ruffin, R.E. Zinc metabolism in airway epithelium and airway inflammation: Basic mechanisms and clinical targets: A review. Pharmacol. Ther. 2005, 105, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Phuong Nguyen, G. Iron deficiency, zinc, magnesium, vitamin deficiencies in Crohn's disease: Substitute or not? Dig. Dis. 2016, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Song, M.; Higuchi, L.M.; Richter, J.M.; Chan, A.T. Zinc intake and risk of Crohn’s disease and ulcerative colitis: A prospective cohort study. Int. J. Epidemiol. 2015, 44, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Nakamura, M.; Fujii, H.; Tamano, H. Synaptic Zn2+ homeostasis and its significance. Metallomics 2013, 5, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.H.; Sensi, S.L.; Koh, J.Y. Zn2+: A novel ionic mediator of neural injury in brain disease. Trends Pharmacol. Sci. 2000, 21, 395–401. [Google Scholar] [CrossRef]
- Marger, L.; Schubert, C.R.; Bertrand, D. Zinc: An underappreciated modulatory factor of brain function. Biochem. Pharmacol. 2014, 91, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A. Significance of Zn2+ signaling in cognition: Insight from synaptic Zn2+ dyshomeostasis. J. Trace Elem. Med. Biol. 2014, 28, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Troche, C.; Aydemir, T.B.; Cousins, R.J. Zinc transporter Slc39a14 regulates inflammatory signaling associated with hypertrophic adiposity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E258–E268. [Google Scholar] [CrossRef] [PubMed]
- Sturniolo, G.C.; Fries, W.; Mazzon, E.; di Leo, V.; Barollo, M.; D’inca, R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J. Lab. Clin. Med. 2002, 139, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Massimi, M.; Conti Devirgiliis, L.; Mengheri, E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J. Nutr. 2008, 138, 1664–1670. [Google Scholar] [PubMed]
- Ranaldi, G.; Ferruzza, S.; Canali, R.; Leoni, G.; Zalewski, P.D.; Sambuy, Y.; Perozzi, G.; Murgia, C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J. Nutr. Biochem. 2013, 24, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.M.; Danscher, G.; Schrøder, H.D.; Suh, S.W. Depletion of vesicular zinc in dorsal horn of spinal cord causes increased neuropathic pain in mice. Biometals 2008, 21, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Miampamba, M.; Chery-Croze, S.; Gorry, F.; Berger, F.; Chayvialle, J.A. Inflammation of the colonic wall induced by formalin as a model of acute visceral pain. Pain 1994, 57, 327–334. [Google Scholar] [CrossRef]
- Gonkowski, S.; Burliński, P.; Skobowiat, C.; Majewski, M.; Całka, J. Inflammation- and axotomy-induced changes in galanin-like immunoreactive (GAL-LI) nerve structures in the porcine descending colon. Acta Vet. Hung. 2010, 58, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Burliński, P.J. Inflammation- and axotomy-induced changes in cocaine- and amphetamine-regulated transcript peptide-like immunoreactive (CART-LI) nervous structures in the porcine descending colon. Pol. J. Vet. Sci. 2012, 15, 517–524. [Google Scholar] [PubMed]
| Enteric Plexus | MP | OSP | ISP | |||
|---|---|---|---|---|---|---|
| C Group | I Group | C Group | I Group | C Group | I Group | |
| PGP+/ZnT3+ 1 | 42.3 ± 4.7 | 84 ± 3.9 | 43.5 ± 6.8 | 85.6 ± 2.0 | 48.6 ± 4.8 | 79.0 ± 3.2 |
| ZnT3+/VAChT+ 2 | 85.0 ± 5.8 | 41.0 ± 2.7 | 82.0 ± 5.9 | 31.3 ± 2.8 | 90.0 ± 7.2 | 34.0 ± 3.0 |
| ZnT3+/VAChT+/CGRP+ 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZnT3+/VAChT+/GAL+ 3 | 0 | 5.4 ± 1.5 | 0 | 4.7 ± 0.9 | 65.2 ± 1.2 | 4.7 ± 1.3 |
| ZnT3+/VAChT+/LENK+ 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZnT3+/VAChT+/NOS+ 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZnT3+/VAChT+/NPY+ 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZnT3+/VAChT+/SOM+ 3 | 2.9 ± 1.4 | 0 | 7.4 ± 1.4 | 1.1 ± 0.8 | 24.1 ± 1.2 | 4.1 ± 0.8 |
| ZnT3+/VAChT+/SP+ 3 | 1.2 ± 0.5 | 4.2 ± 1.2 | 4.6 ± 1.6 | 2.8 ± 1.4 | 55.1 ± 1.3 | 6.8 ± 1.5 |
| ZnT3+/VAChT+/VIP+ 3 | 2.9 ± 1.4 | 11.2 ± 1.9 | 1.4 ± 0.8 | 3.8 ± 1.4 | 33.0 ± 3.6 | 7.3 ± 1.6 |
| ZnT3+/VAChT− 2 | 15.0 ± 5.6 | 59.0 ± 2.7 | 18.0 ± 5.9 | 68.7 ± 2.8 | 10.0 ± 7.2 | 66.0 ± 3.0 |
| ZnT3+/VAChT−/CGRP+ 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZnT3+/VAChT−/GAL+ 3 | 11.9 ± 1.5 | 30.2 ± 3.4 | 0 | 36.3 ± 7.5 | 2.8 ± 0.1 | 54.7 ± 7.4 |
| ZnT3+/VAChT−/LENK+ 3 | 0.5 ± 0.2 | 41.2 ± 2.7 | 0 | 0 | 0 | 0 |
| ZnT3+/VAChT−/NOS+ 3 | 17.1 ± 1.1 | 57.0 ± 4.0 | 7.4 ± 1.3 | 10.3 ± 2.4 | 0 | 0 |
| ZnT3+/VAChT−/NPY+ 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ZnT3+/VAChT−/SOM+ 3 | 3.4 ± 1.3 | 0 | 1.4 ± 0.6 | 43.0 ± 3.6 | 3.7 ± 1.0 | 38.6 ± 1.5 |
| ZnT3+/VAChT−/SP+ 3 | 1.1 ± 0.6 | 32.2 ± 0.4 | 4.7 ± 1.8 | 9.5 ± 2.4 | 4.1 ± 1.2 | 36.9 ± 1.5 |
| ZnT3+/VAChT−/VIP+ 3 | 3.9 ± 0.3 | 52.1 ± 2.8 | 4.6 ± 1.6 | 20.7 ± 9.7 | 2.9 ± 1.3 | 51.4 ± 3.5 |
| Primary Antibody | ||||
| Antisera | Code | Host Species | Dilution | Supplier |
| PGP9.5 | 7863-2004 | Mouse | 1:2000 | Biogenesis Inc., Poole, UK; www.biogenesis.co.uk |
| ZnT3 | - | Rabbit | 1:600 | Gift from prof. Palmiter, University of Washington Seattle, WA, USA |
| NOS | N2280 | Mouse | 1: 2000 | Sigma-Aldrich, Saint Louis, MS, USA; www.sigma-aldrich.com |
| VIP | 9535-0504 | Mouse | 1: 2000 | Biogenesis Inc. |
| SP | 8450-0505 | Rat | 1:300 | Biogenesis Inc. |
| SOM | 8330-0009 | Rat | 1: 100 | Biogenesis Inc. |
| LENK | 4140-0355 | Mouse | 1: 1000 | Biogenesis Inc. |
| VAChT | H-V007 | Goat | 1: 2000 | Phoenix, Pharmaceuticals, INC., Belmont, CA, USA; www.phoenixpeptide.com |
| NPY | NZ1115 | Rat | 1:300 | Biomol Research Laboratories Inc., Plymouth, PA, USA |
| GAL | T-5036 | Guinea pig | 1:1000 | Peninsula Labs., San Carlos, CA, USA; see Bachem AG; www.bachem.com |
| CGRP | T-5027 | Guinea pig | 1:1000 | Peninsula Labs. |
| Secondary Antibodies | ||||
| Reagent | Dilution | Supplier | ||
| FITC-conjugated donkey-anti-mouse IgG (H+L) | 1:800 | Jackson, 715-095-151, West Grove, PA, USA | ||
| FITC-conjugated donkey-anti-rat IgG (H+L) | 1:800 | Jackson, 712-095-153 | ||
| FITC-conjugated donkey-anti-guinea pig IgG (H+L) | 1:1000 | Jackson, 706-095-148 | ||
| FITC-conjugated donkey-anti-goat IgG (H+L) | 1:1000 | Jackson, 705-096-147 | ||
| Biotinylated goat anti-rabbit immunoglobulins | 1:1000 | DAKO, E 0432, Carpinteria, CA, USA | ||
| Biotin conjugated F(ab)’ fragment of affinity Purified anti-rabbit IgG (H+L) | 1:1000 | BioTrend, 711-1622, Cologne, Germany | ||
| AMCA-conjugated donkey-anti-mouse IgG (H+L) | 1:50 | Jackson, 715-155-151 | ||
| AMCA-conjugated donkey-anti-rat IgG (H+L) | 1:50 | Jackson, 715-155-153 | ||
| AMCA-conjugated donkey-anti-goat IgG (H+L) | 1:50 | Jackson, 705-156-147 | ||
| CY3-conjugated Streptavidin | 1:9000 | Jackson, 016-160-084 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonkowski, S.; Rowniak, M.; Wojtkiewicz, J. Zinc Transporter 3 (ZnT3) in the Enteric Nervous System of the Porcine Ileum in Physiological Conditions and during Experimental Inflammation. Int. J. Mol. Sci. 2017, 18, 338. https://doi.org/10.3390/ijms18020338
Gonkowski S, Rowniak M, Wojtkiewicz J. Zinc Transporter 3 (ZnT3) in the Enteric Nervous System of the Porcine Ileum in Physiological Conditions and during Experimental Inflammation. International Journal of Molecular Sciences. 2017; 18(2):338. https://doi.org/10.3390/ijms18020338
Chicago/Turabian StyleGonkowski, Sławomir, Maciej Rowniak, and Joanna Wojtkiewicz. 2017. "Zinc Transporter 3 (ZnT3) in the Enteric Nervous System of the Porcine Ileum in Physiological Conditions and during Experimental Inflammation" International Journal of Molecular Sciences 18, no. 2: 338. https://doi.org/10.3390/ijms18020338