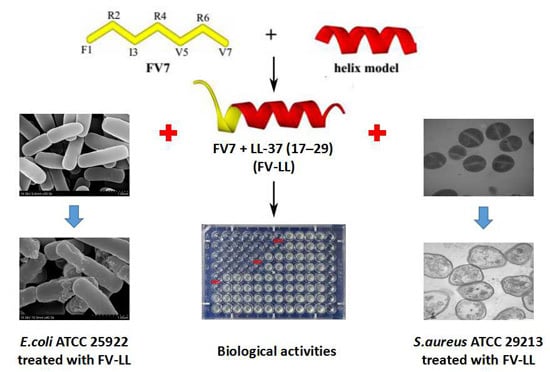
High Specific Selectivity and Membrane-Active Mechanism of Synthetic Cationic Hybrid Antimicrobial Peptides Based on the Peptide FV7
Abstract
:1. Introduction
2. Results
2.1. Peptide Design and Characterization
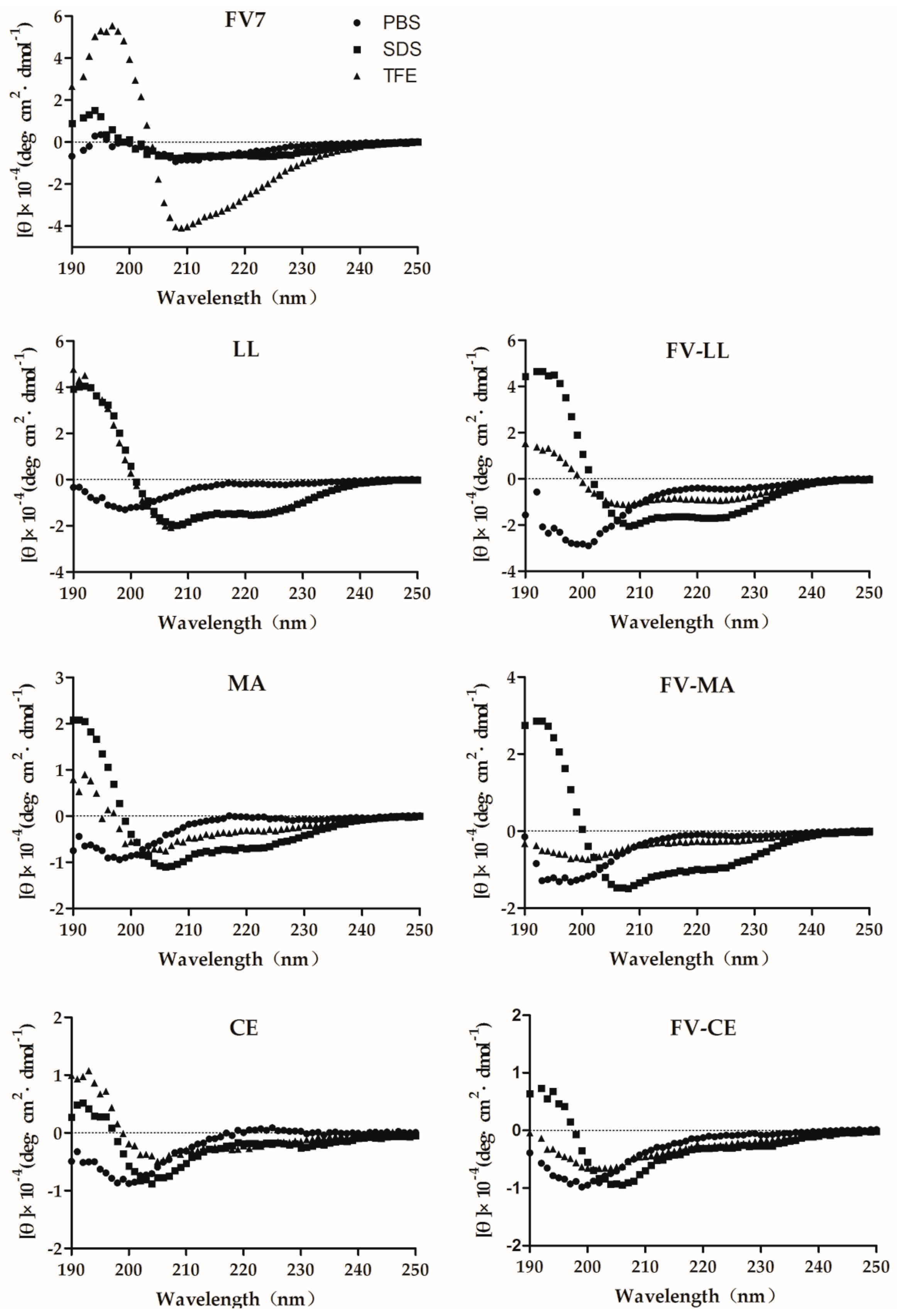
2.2. The Hybrid Peptides Have Typical α-Helix Structures
2.3. FV-LL Is the Optimal Hybrid Peptide Compared with the Parental Peptides and Other Peptides in the Antimicrobial Activities
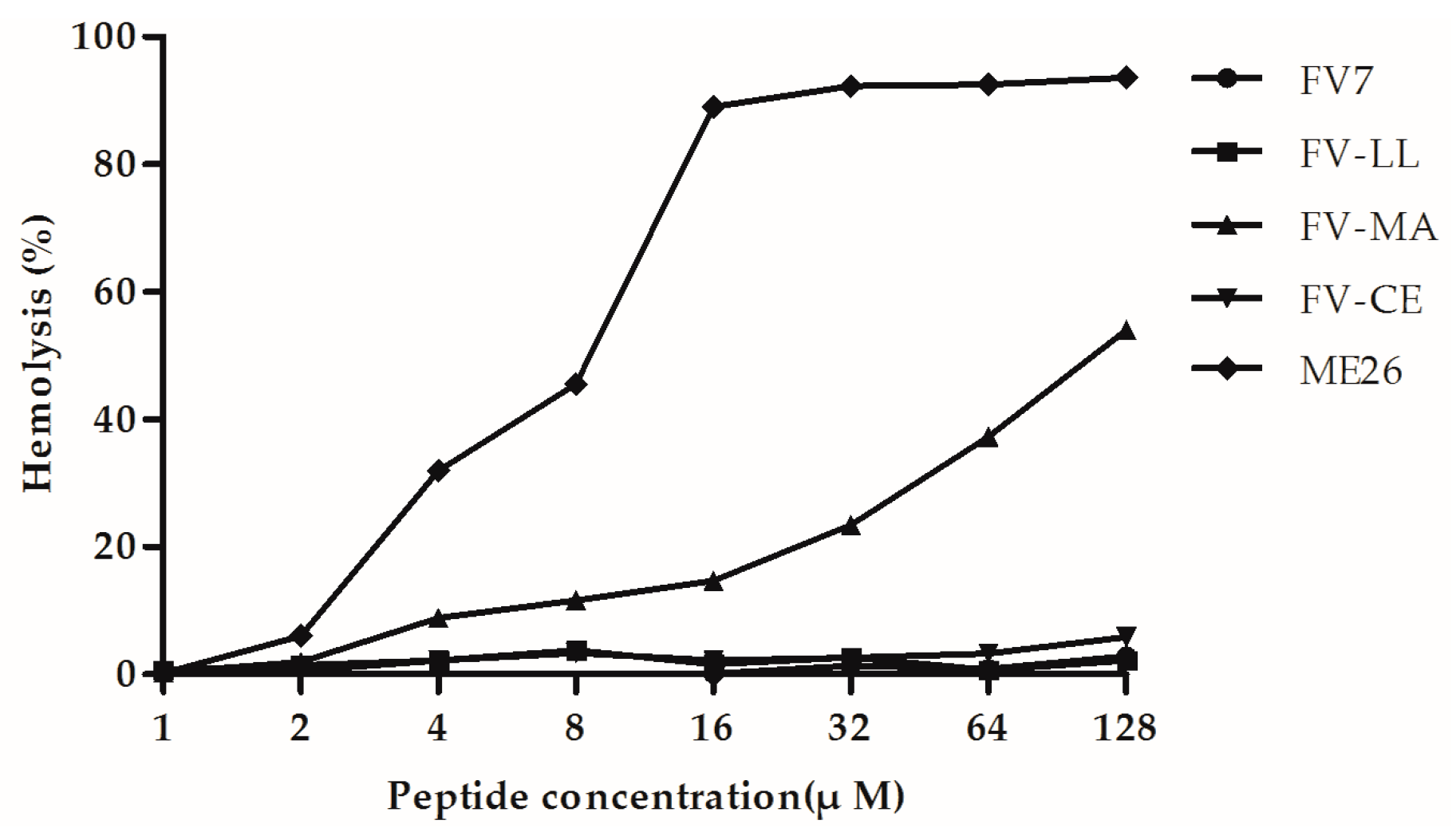
2.4. The Hybrid Peptides, Particularly FV-LL, Had Lower Hemolytic Activity Than the Control Peptide Melittin (ME26)
2.5. FV-LL Was the Most Resistant of the Peptides in Stability Studies
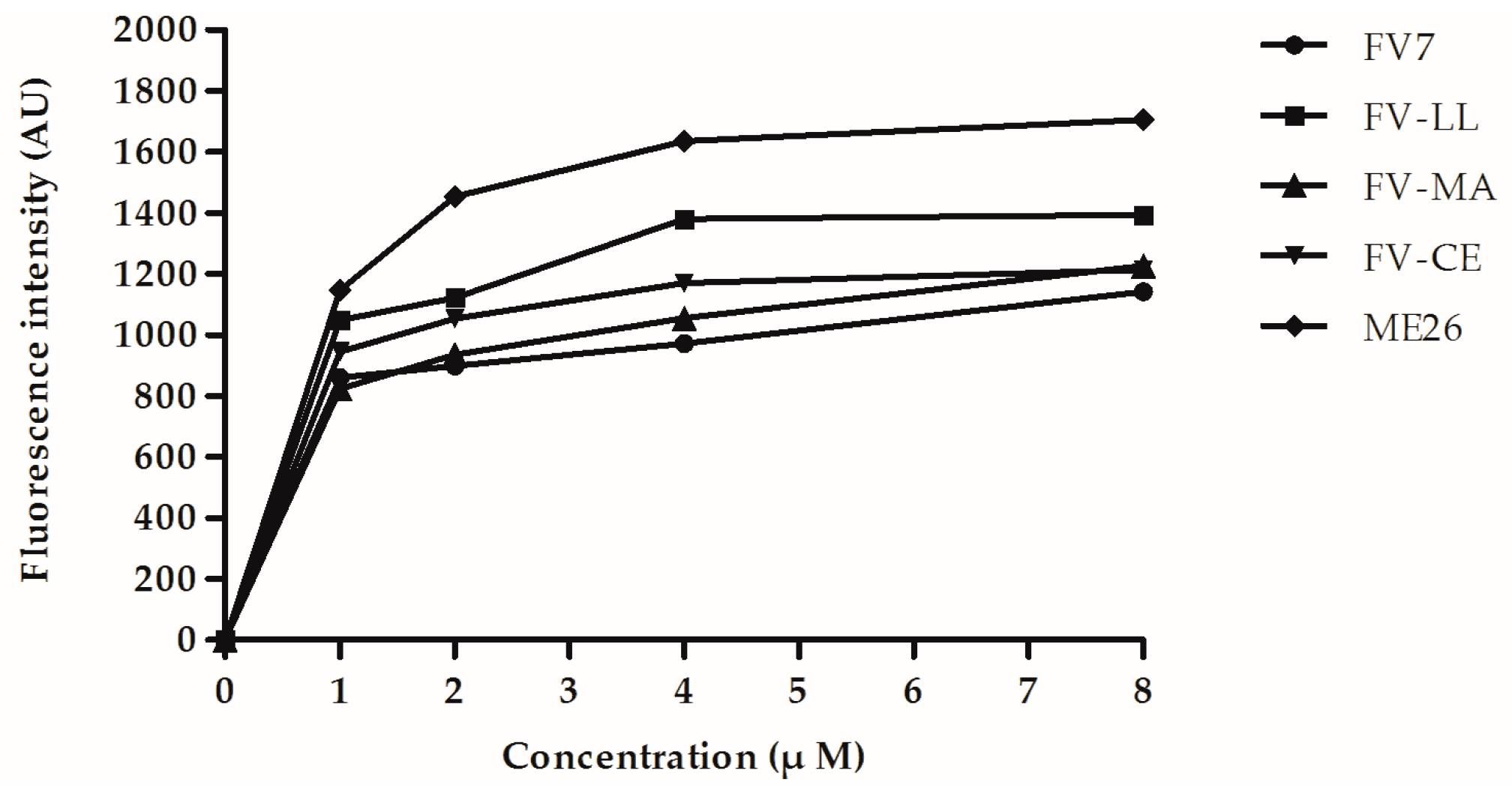
2.6. The Hybrid Peptides Increased Outer and Inner Membrane Permeabilities
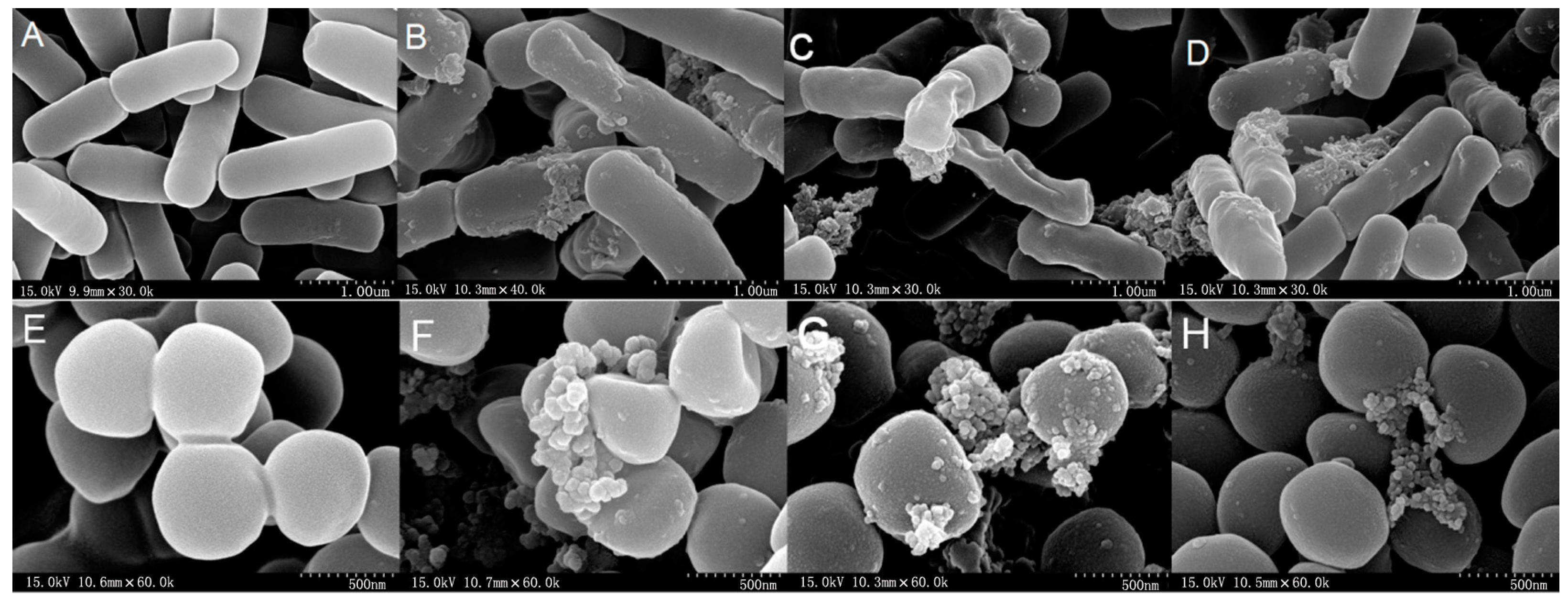
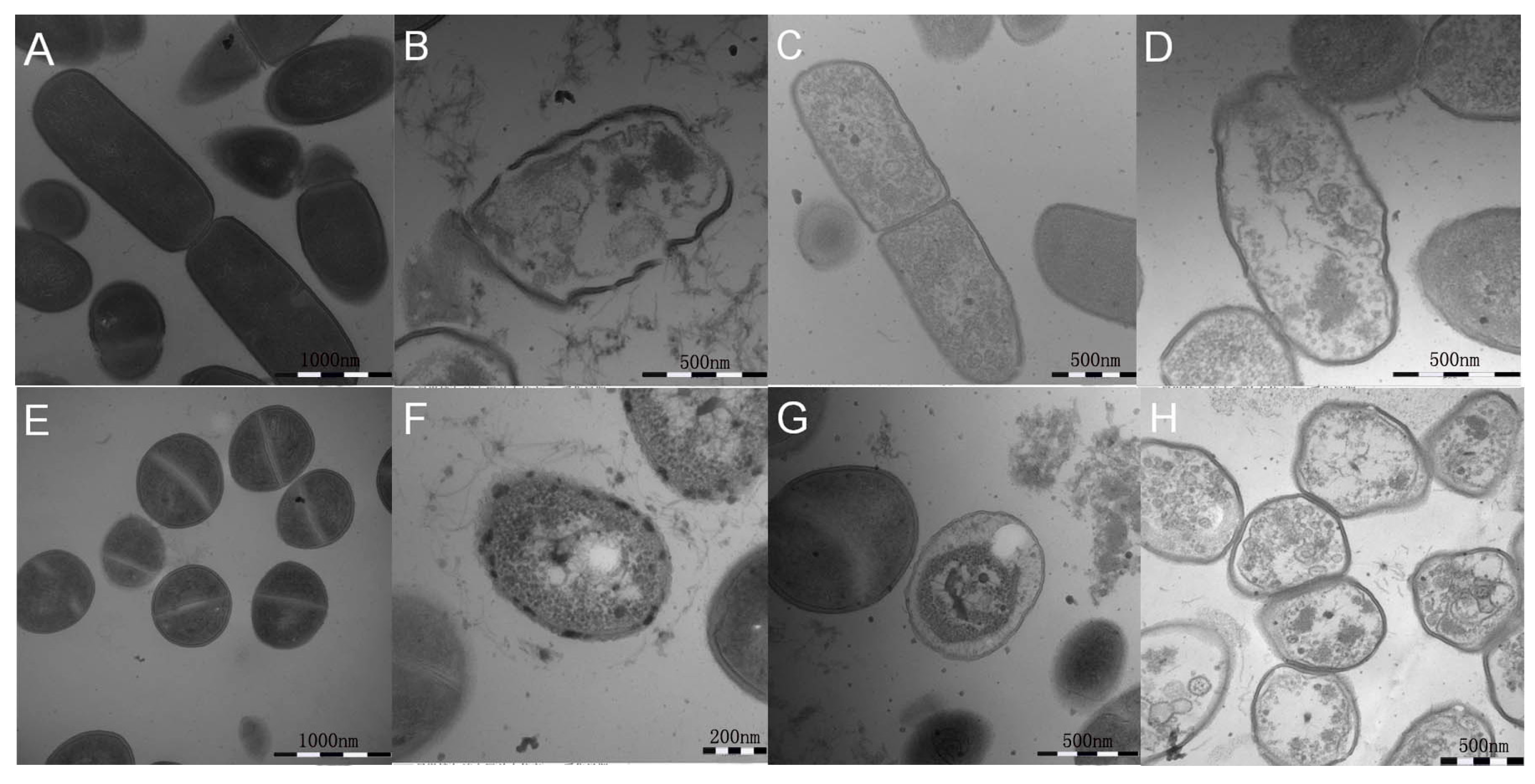
2.7. All Hybrid Peptides Caused a Certain Degree of Membrane Damage in Scanning Electron Microscopy (SEM) and Transmission Electron Eicroscopy (TEM), Particularly FV-LL
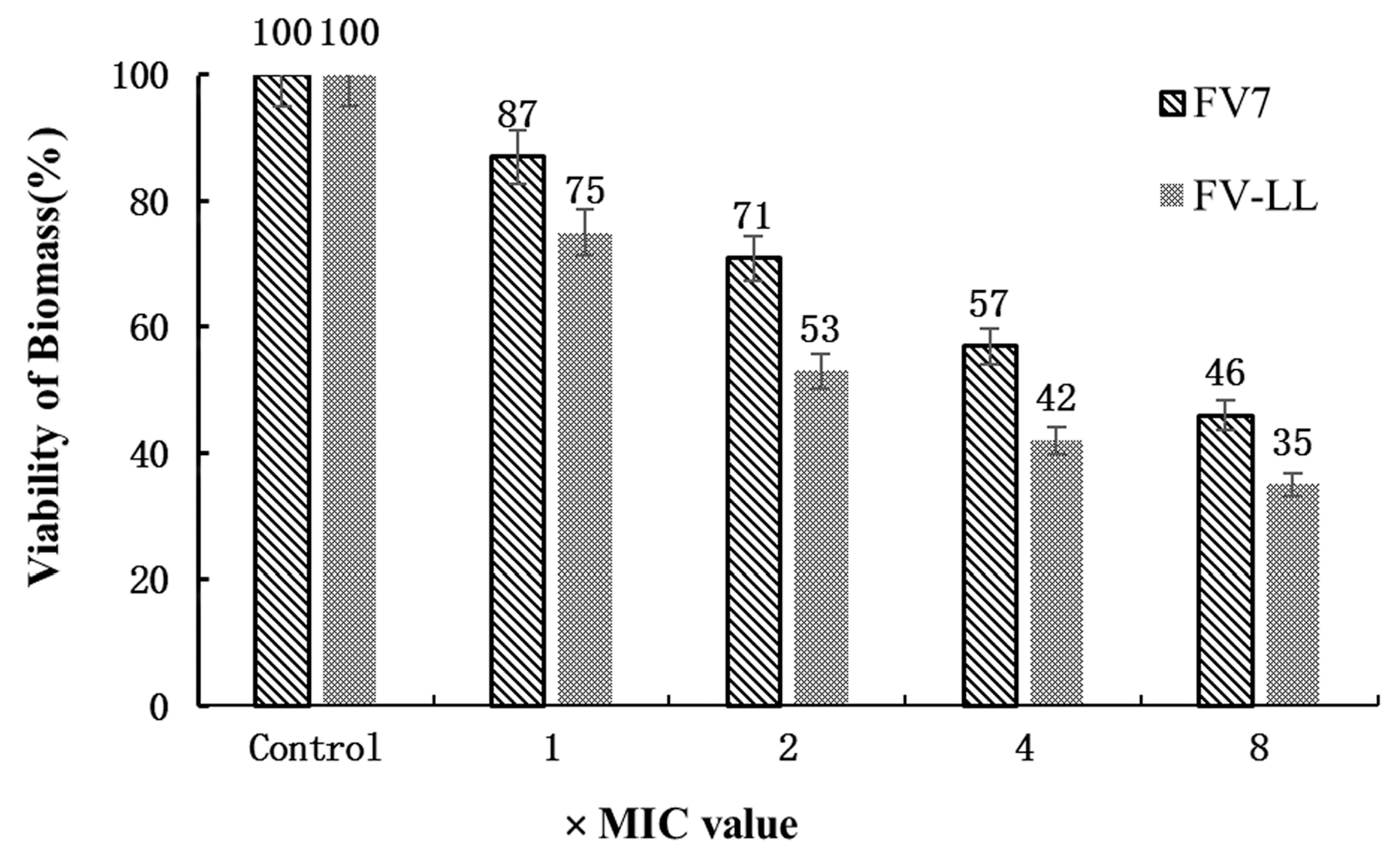
2.8. FV-LL Was More Effective in Inhibition of Biofilm Formation Compared with FV7
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sequence Analysis of the Peptides
4.3. Synthesis of the Peptides
4.4. Circular Dichroism (CD) Spectra
4.5. Antimicrobial Assays
4.6. Measurement of Hemolytic Activity
4.7. Salt and Heat Sensitivity
4.8. Outer Membrane Permeability Assay
4.9. Evaluation of the Inner Membrane Permeability
4.10. Scanning Electron Microscopy (SEM) Characterization
4.11. Transmission Electron Microscopy (TEM) Characterization
4.12. Effect of Peptides on Biofilm Formation
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [PubMed]
- Cherkasov, A.; Hilpert, K.; Jenssen, H.; Fjell, C.D.; Waldbrook, M.; Mullaly, S.C.; Volkmer, R.; Hancock, R.E. Use of artificial intelligence in the design of small peptide antibiotics effective against a broad spectrum of highly antibiotic-resistant superbugs. ACS Chem. Biol. 2009, 4, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Easton, D.M.; Nijnik, A.; Mayer, M.L.; Hancock, R.E. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009, 27, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, K.; Volkmer-Engert, R.; Walter, T.; Hancock, R.E. High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 2005, 23, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Patrzykat, A.; Zhang, L.; Mendoza, V.; Iwama, G.K.; Hancock, R.E. Synergy of histone-derived peptides of coho salmon with lysozyme and flounder pleurocidin. Antimicrob. Agents Chemother. 2001, 45, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Sahl, H. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.P.S.; Hancock, R.E.W. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Brinkløv, S.; Kalko, E.K.V.; Surlykke, A. Intense echolocation calls from two ”whispering” bats, Artibeus jamaicensis and Macrophyllum macrophyllum (Phyllostomidae). J. Exp. Biol. 2009, 212, 11–20. [Google Scholar] [CrossRef]
- Zanetti, M. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 2005, 7, 179–196. [Google Scholar] [PubMed]
- Zhang, L.; Falla, T.J. Antimicrobial peptides: Therapeutic potential. Expert Opin. Pharmacother. 2006, 7, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wei, D.; Yan, P.; Zhu, X.; Shan, A.; Bi, Z. Characterization of cell selectivity, physiological stability and endotoxin neutralization capabilities of α-helix-based peptide amphiphiles. Biomaterials 2015, 52, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dong, N.; Wang, Z.; Ma, Z.; Zhang, L.; Ma, Q.; Shan, A. Design of imperfectly amphipathic α-helical antimicrobial peptides with enhanced cell selectivity. Acta Biomater. 2014, 10, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Y.; Wiradharma, N.; Yang, Y.Y. Strategies employed in the design and optimization of synthetic antimicrobial peptide amphiphiles with enhanced therapeutic potentials. Adv. Drug Deliv. Rev. 2014, 78, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Smith, C.; Wu, H.; Teng, P.; Shi, Y.; Padhee, S.; Jones, T.; Nguyen, A.M.; Cao, C.; Yin, H. Short Antimicrobial Lipo-α/γ-AA Hybrid Peptides. Chembiochem 2015, 15, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Ubhayasekera, W.; Gallo, R.L.; Marcos, J.F. Parallel evaluation of antimicrobial peptides derived from the synthetic PAF26 and the human LL37. Biochem. Biophys. Res. Commun. 2007, 356, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.G.; Dong, T.T.; Teng, D.; Yang, Y.L.; Wang, J.H. Design and characterization of novel hybrid peptides from LFB15(W4,10), HP(2–20), and cecropin A based on structure parameters by computer-aided method. Appl. Microbiol. Biotechnol. 2009, 82, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- De, L.F.-N.C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar]
- Xu, W.; Zhu, X.; Tan, T.; Li, W.; Shan, A. Design of Embedded-Hybrid Antimicrobial Peptides with Enhanced Cell Selectivity and Anti-Biofilm Activity. PLoS ONE 2014, 9, e98935. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef] [PubMed]
- Mehrnejad, F.; Naderi-Manesh, H.; Ranjbar, B. The structural properties of magainin in water, TFE/water, and aqueous urea solutions: Molecular dynamics simulations. Proteins Struct. Funct. Bioinform. 2007, 67, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A.; Thwaite, J.E.; Ulaeto, D.O.; Atkins, T.P.; Atkins, H.S. Design and characterization of novel hybrid antimicrobial peptides based on cecropin A, LL-37 and magainin II. Peptides 2012, 33, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Ubach, J.; Boman, A.; Wåhlin, B.; Wade, D.; Merrifield, R.B.; Boman, H.G. Shortened cecropin A-melittin hybrids Significant size reduction retains potent antibiotic activity. FEBS Lett. 1992, 296, 190–194. [Google Scholar] [CrossRef]
- Chicharro, C.; Granata, C.; Lozano, R.; Andreu, D.; Rivas, L. N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1–7)M(2–9), a cecropin-melittin hybrid peptide. Antimicrob. Agents Chemother. 2001, 45, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, A.; Cirioni, O.; Kamysz, W.; D’Amato, G.; Silvestri, C.; Simona, D.P.M.; Lukasiak, J.; Scalise, G. In vitro activity and killing effect of the synthetic hybrid cecropin A-melittin peptide CA(1–7)M(2–9)NH(2) on methicillin-resistant nosocomial isolates of Staphylococcus aureus and interactions with clinically used antibiotics. Diagn. Microbiol. Infect. Dis. 2004, 38, 115–118. [Google Scholar] [CrossRef]
- Cavallarin, L.; Andreu, D.; San, S.B. Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol. Plant Microbe Interact. 1998, 11, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.S.; Reddy, A.S.N. Inhibition of fungal and bacterial plant pathogens by synthetic peptides: In vitro growth inhibition, interaction between peptides and inhibition of disease progression. Mol. Plant Microbe Interact. 2000, 13, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hammer, J.; Pate, M.; Zhang, Y.; Zhu, F.; Zmuda, E.; Blazyk, J. Antimicrobial activities and structures of two linear cationic peptide families with various amphipathic β-sheet and α-helical potentials. Antimicrob. Agents Chemother. 2006, 49, 4957–4964. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.; Kaur, J.; Ahmad, E.; Khan, R.H.; Jain, S.K. Structural characteristics of thermostable immunogenic outer membrane protein from Salmonella enterica serovar Typhi. Appl. Microbiol. Biotechnol. 2014, 98, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Vasil, A.; Gera, L.; Vasil, M.; Hodges, R.S. Rational design of α-Helical antimicrobial peptides to target gram-negative pathogens, acinetobacter baumannii and pseudomonas aeruginosa and the development of a systemic animal model to evaluate efficacy. Biopolymers 2011, 77, 425–426. [Google Scholar]
- Xue, R.; Wang, S.; Qi, H.; Song, Y.; Wang, C.; Li, F. Structure analysis of the fourth transmembrane domain of Nramp1 in model membranes. Biochim. Biophys. Acta 2008, 1778, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, J.; Gao, H.; Wang, Z.; Dong, N.; Ma, Q.; Shan, A. Antimicrobial properties and membrane-active mechanism of a potential α-helical antimicrobial derived from cathelicidin PMAP-36. PLoS ONE 2014, 9, e86364. [Google Scholar] [CrossRef] [PubMed]
- Scocchi, M.; Zelezetsky, I.; Benincasa, M.; Gennaro, R.; Mazzoli, A.; Tossi, A. Structural aspects and biological properties of the cathelicidin PMAP-36. FEBS J. 2005, 272, 4398–4406. [Google Scholar] [CrossRef] [PubMed]
- Dathe, M.; Wieprecht, T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta 1999, 1462, 71–87. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, X.; Xu, L.; Wang, Y. Design of hybrid β-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials 2013, 34, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Pitts, B.; Hamilton, M.A.; Zelver, N.; Stewart, P.S. A microtiter-plate screening method for biofilm disinfection and removal. J. Microbiol. Methods 2003, 54, 269–276. [Google Scholar] [CrossRef]
- Huang, J.; Hao, D.; Chen, Y.; Xu, Y.; Tan, J.; Huang, Y.; Li, F.; Chen, Y. Inhibitory effects and mechanisms of physiological conditions on the activity of enantiomeric forms of an α-helical antibacterial peptide against bacteria. Peptides 2011, 32, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.U.; Brauser, A.; Olak, C.; Brezesinski, G.; Goldmann, T.; Gutsmann, T.; Andrä, J. Lipopolysaccharide interaction is decisive for the activity of the antimicrobial peptide NK-2 against Escherichia coli and Proteus mirabilis. Biochem. J. 2010, 427, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Saar-Dover, R.; Bitler, A.; Nezer, R.; Shmuel-Galia, L.; Firon, A.; Shimoni, E.; Trieu-Cuot, P.; Shai, Y. D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B Streptococcus by increasing the cell wall density. PLoS Pathog. 2012, 8, e1002891. [Google Scholar] [CrossRef]
- Sato, H.; Feix, J.B. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog. Lipid Res. 2012, 51, 149–177. [Google Scholar] [CrossRef] [PubMed]
- ExPASy Proteomics Server. Available online: http://www.expasy.org/tools/protparam.html (accessed on 22 October 2015).
- Antimicrobial Peptide Database. Available online: http://aps.unmc.edu/AP/main.php (accessed on 22 October 2015).
- Jpred 3. Available online: http://www.compbio.dundee.ac.uk/www-jpred/index.html (accessed on 22 October 2015).
- I-TASSER. Available online: http://zhanglab.ccmb.med.umich.edu/I-TASSER/ (accessed on 22 October 2015).
- Herzog, I.M.; Feldman, M.; Eldar-Boock, A.; Satchi-Fainaro, R.; Fridman, M. Design of membrane targeting tobramycin-based cationic amphiphiles with reduced hemolytic activity. Med. Chem. Commun. 2013, 4, 120–124. [Google Scholar] [CrossRef]
- Han, F.F.; Gao, Y.H.; Luan, C.; Xie, Y.G.; Liu, Y.F.; Wang, Y.Z. Comparing bacterial membrane interactions and antimicrobial activity of porcine lactoferricin-derived peptides. J. Dairy Sci. 2013, 96, 3471–3487. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed]


| Peptide | Sequence | Design 1 | Theoretical Mw | Measured Mw 2 | Charge | H 3 |
|---|---|---|---|---|---|---|
| FV7 | FRIRVRV-NH2 | FV7 | 945.18 | 946.24 | +4 | 0.57 |
| LL | FKRIVQRIKDFLR-NH2 | LL-37 (17–29) | 1719.106 | 1718.14 | +4 | 0.46 |
| MA | AKKFGKAFVGEIM-NH2 | magainin 2 (9–21) | 1425.749 | 1424.78 | +2 | 0.53 |
| CE | KWKLFKKI-NH2 | cecropin A (1–8) | 1090.405 | 1089.45 | +4 | 0.50 |
| FV-LL | FRIRVRV-FKRIVQRIKDFLR-NH2 | FV7 + LL-37 (17–29) | 2646.27 | 2645.31 | +7 | 0.50 |
| FV-MA | FRIRVRV-AKKFGKAFVGEIM-NH2 | FV7 + magainin 2 (9–21) | 2352.92 | 2351.96 | +5 | 0.55 |
| FV-CE | FRIRVRV-AKKFGKAFVGEIM-NH2 | FV7 + cecropin A (1–8) | 2017.58 | 2016.62 | +7 | 0.53 |
| MIC 1 (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| FV7 | LL | MA | CE | FV-LL | FV-MA | FV-CE | ME26 | |
| Gram-negative bacteria | ||||||||
| E. coli ATCC 25922 | 16 | 16 | 32 | 64 | 1 | 8 | 2 | 2 |
| E. coli UB 1005 | 32 | 16 | >64 | >64 | 4 | 8 | 2 | 2 |
| P. aeruginosa ATCC 27853 | 16 | 8 | >64 | >64 | 4 | 8 | 2 | 2 |
| P. aeruginosa PAO1 | 32 | 16 | >64 | >64 | 2 | 2 | 2 | 2 |
| S. typhimurium ATCC 14028 | 16 | 32 | >64 | >64 | 2 | 4 | 4 | 4 |
| S. typhimurium ATCC 7731 | 32 | 16 | >64 | >64 | 2 | 4 | 4 | 4 |
| Gram-positive bacteria | ||||||||
| S. aureus ATCC 29213 | 64 | 16 | >64 | >64 | 2 | 8 | 8 | 8 |
| S. faecalis ATCC 29212 | 32 | 32 | >64 | >64 | 4 | 16 | 1 | 1 |
| B. subtilis CMCC 63501 | 16 | 16 | >64 | >64 | 4 | 4 | 1 | 1 |
| S. epidermidis ATCC12228 | 32 | 8 | >64 | >64 | 1 | 8 | 0.5 | 0.5 |
| Peptide | Control 1 | NaCl 2 | KCl 2 | NH4Cl 2 | MgCl2 2 | ZnCl2 2 | FeCl3 2 | CaCl2 2 | Mix 3 | Heat (100 °C) |
|---|---|---|---|---|---|---|---|---|---|---|
| E. coli ATCC 25922 | ||||||||||
| FV7 | 16 | 32 | 64 | >128 | 32 | 32 | 128 | 64 | 128 | 16 |
| FV-LL | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| FV-MA | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 16 |
| FV-CE | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 2 | 2 | 2 |
| ME26 | 2 | 4 | 2 | 2 | 4 | 4 | 2 | 2 | 4 | 2 |
| S. aureus ATCC 29213 | ||||||||||
| FV7 | 64 | 128 | 64 | 64 | 64 | 32 | 64 | 64 | 128 | 64 |
| FV-LL | 2 | 2 | 2 | 2 | 4 | 2 | 4 | 2 | 4 | 2 |
| FV-MA | 8 | 32 | 8 | 8 | 8 | 8 | 8 | 16 | 16 | 8 |
| FV-CE | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 2 | 4 | 4 |
| ME26 | 8 | 4 | 4 | 2 | 2 | 2 | 2 | 4 | 4 | 8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, T.; Wu, D.; Li, W.; Zheng, X.; Li, W.; Shan, A. High Specific Selectivity and Membrane-Active Mechanism of Synthetic Cationic Hybrid Antimicrobial Peptides Based on the Peptide FV7. Int. J. Mol. Sci. 2017, 18, 339. https://doi.org/10.3390/ijms18020339
Tan T, Wu D, Li W, Zheng X, Li W, Shan A. High Specific Selectivity and Membrane-Active Mechanism of Synthetic Cationic Hybrid Antimicrobial Peptides Based on the Peptide FV7. International Journal of Molecular Sciences. 2017; 18(2):339. https://doi.org/10.3390/ijms18020339
Chicago/Turabian StyleTan, Tingting, Di Wu, Weizhong Li, Xin Zheng, Weifen Li, and Anshan Shan. 2017. "High Specific Selectivity and Membrane-Active Mechanism of Synthetic Cationic Hybrid Antimicrobial Peptides Based on the Peptide FV7" International Journal of Molecular Sciences 18, no. 2: 339. https://doi.org/10.3390/ijms18020339