Micro-Computed Tomography Detection of Gold Nanoparticle-Labelled Mesenchymal Stem Cells in the Rat Subretinal Layer
Abstract
:1. Introduction
2. Results
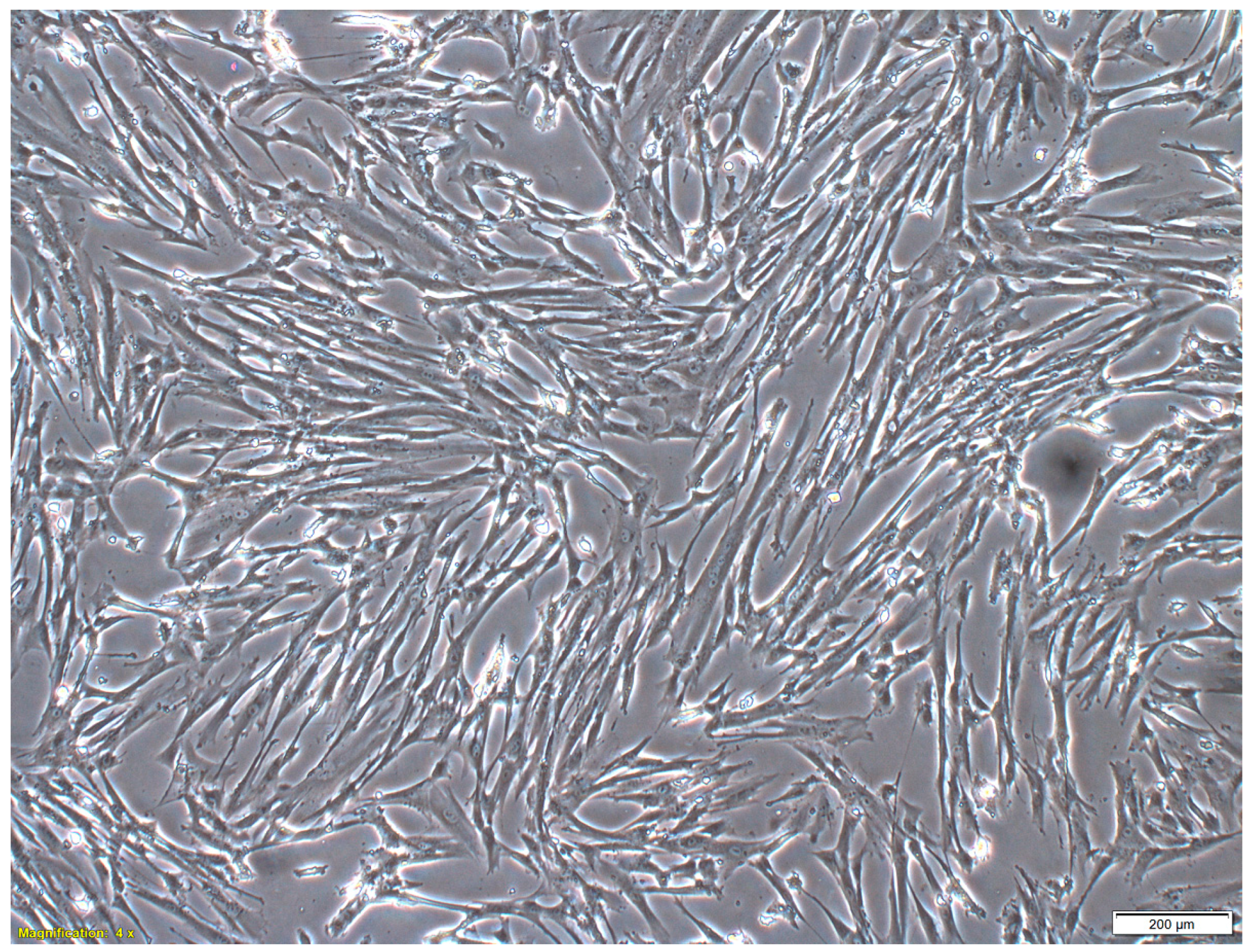
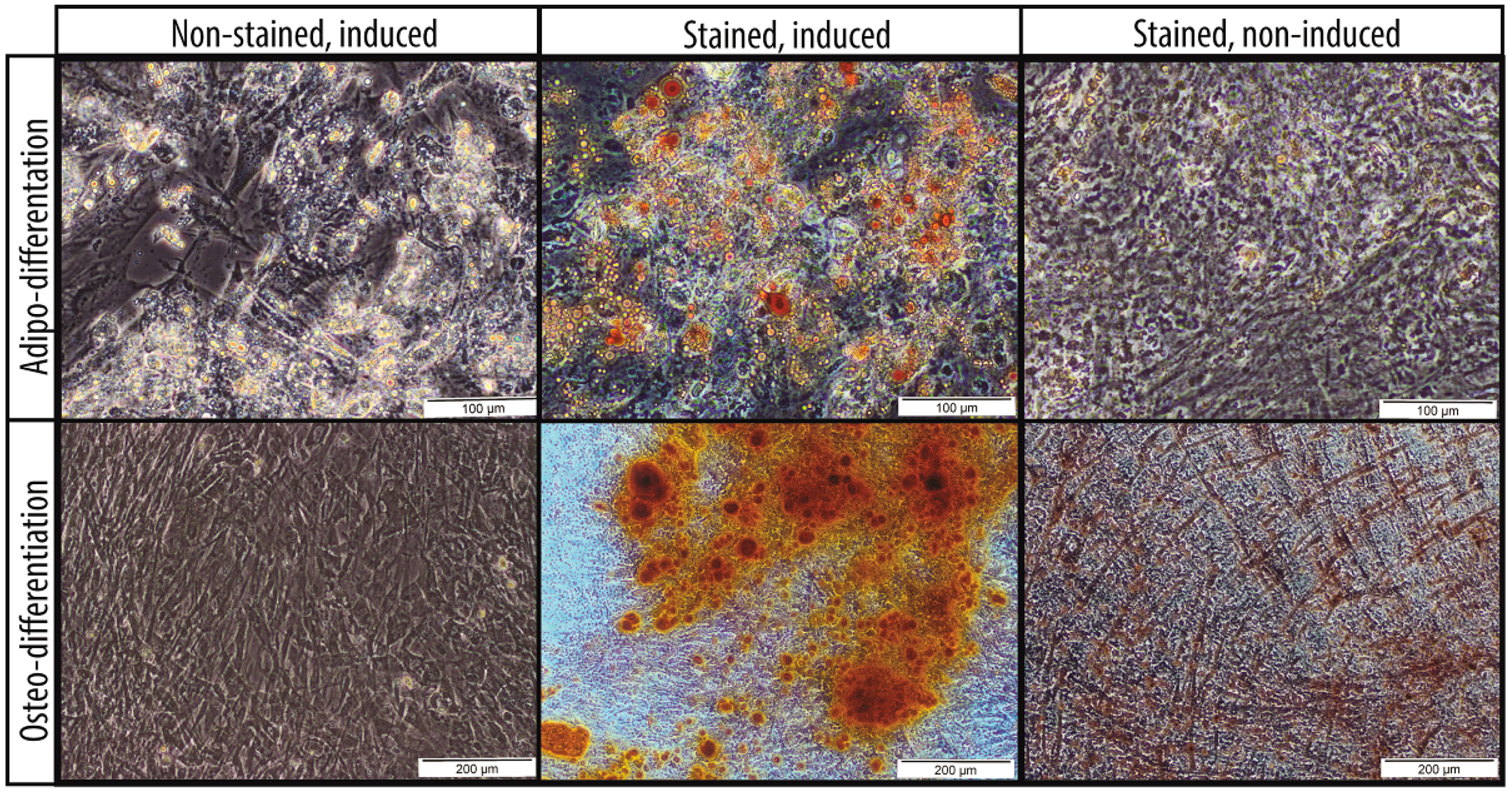
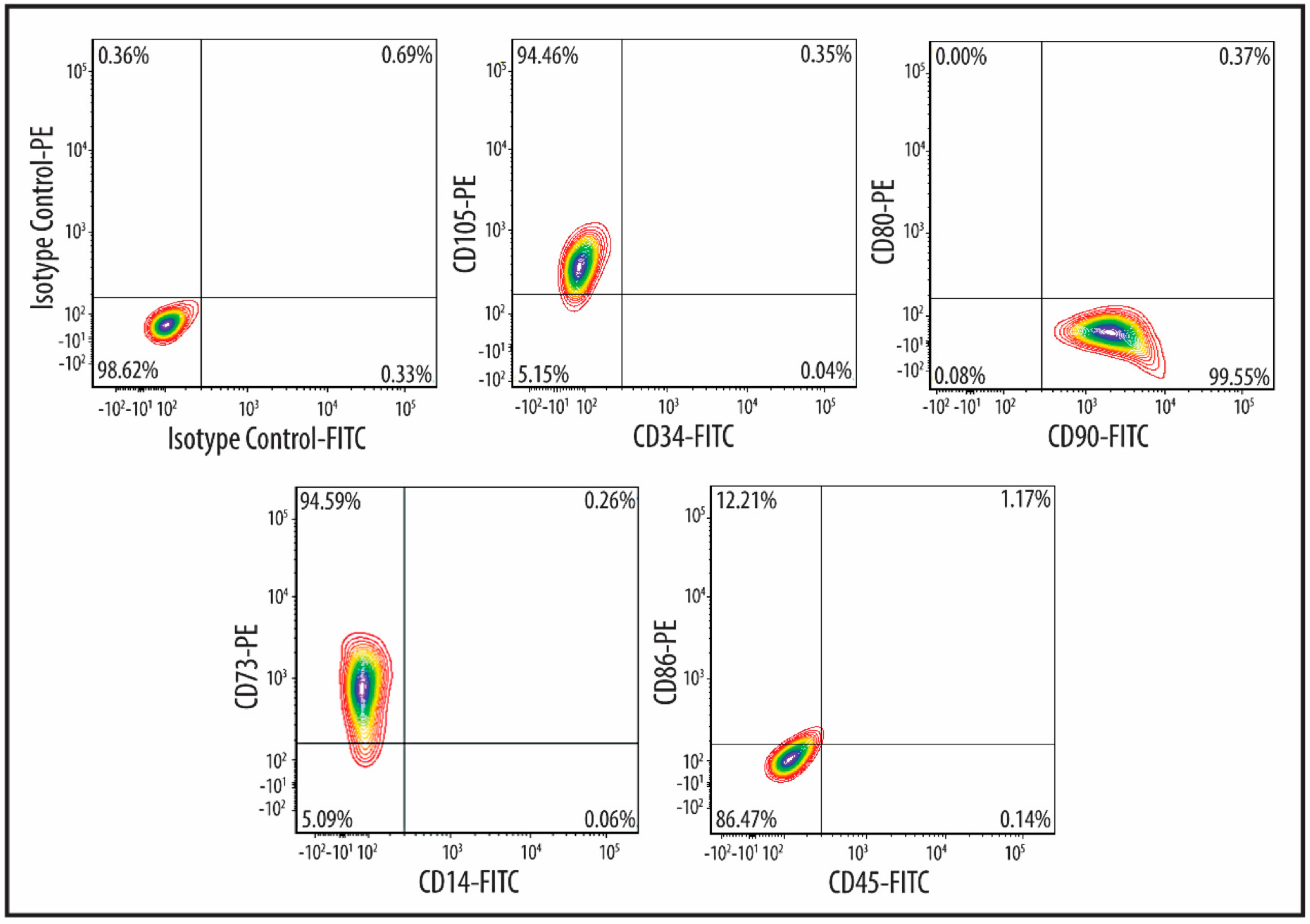
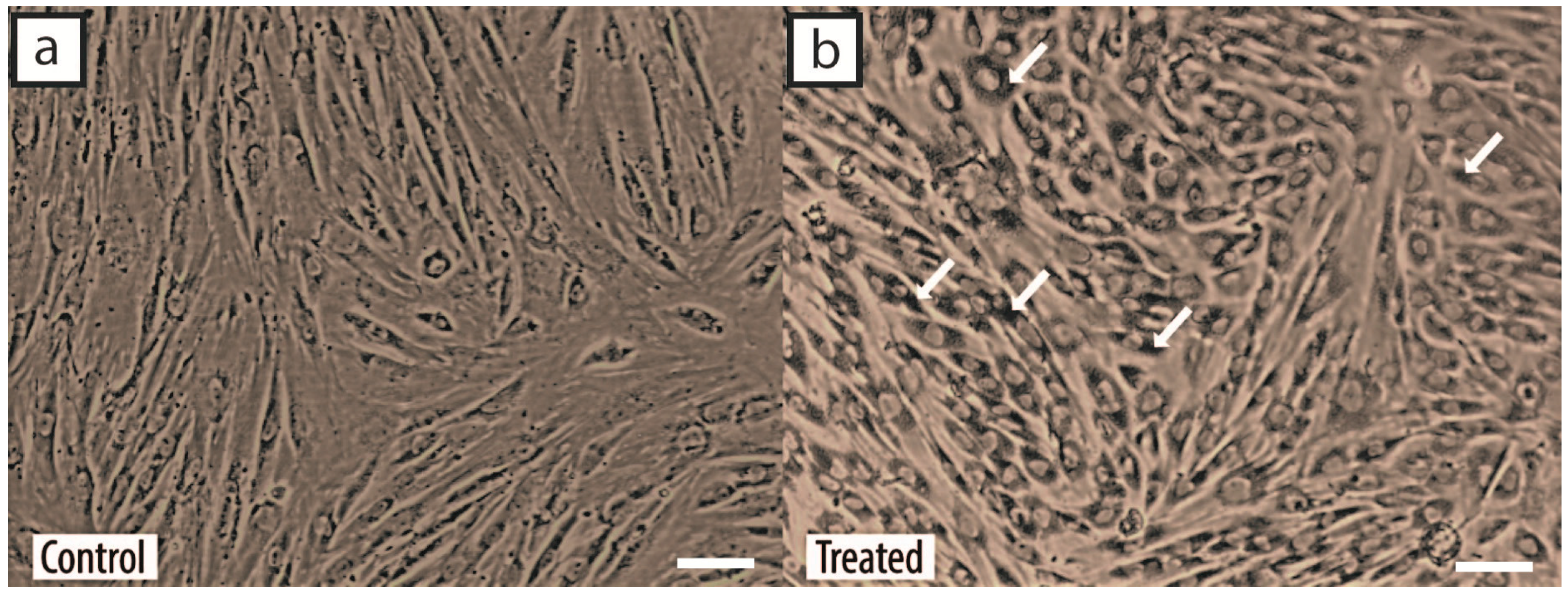
2.1. Characteristics of MSCs
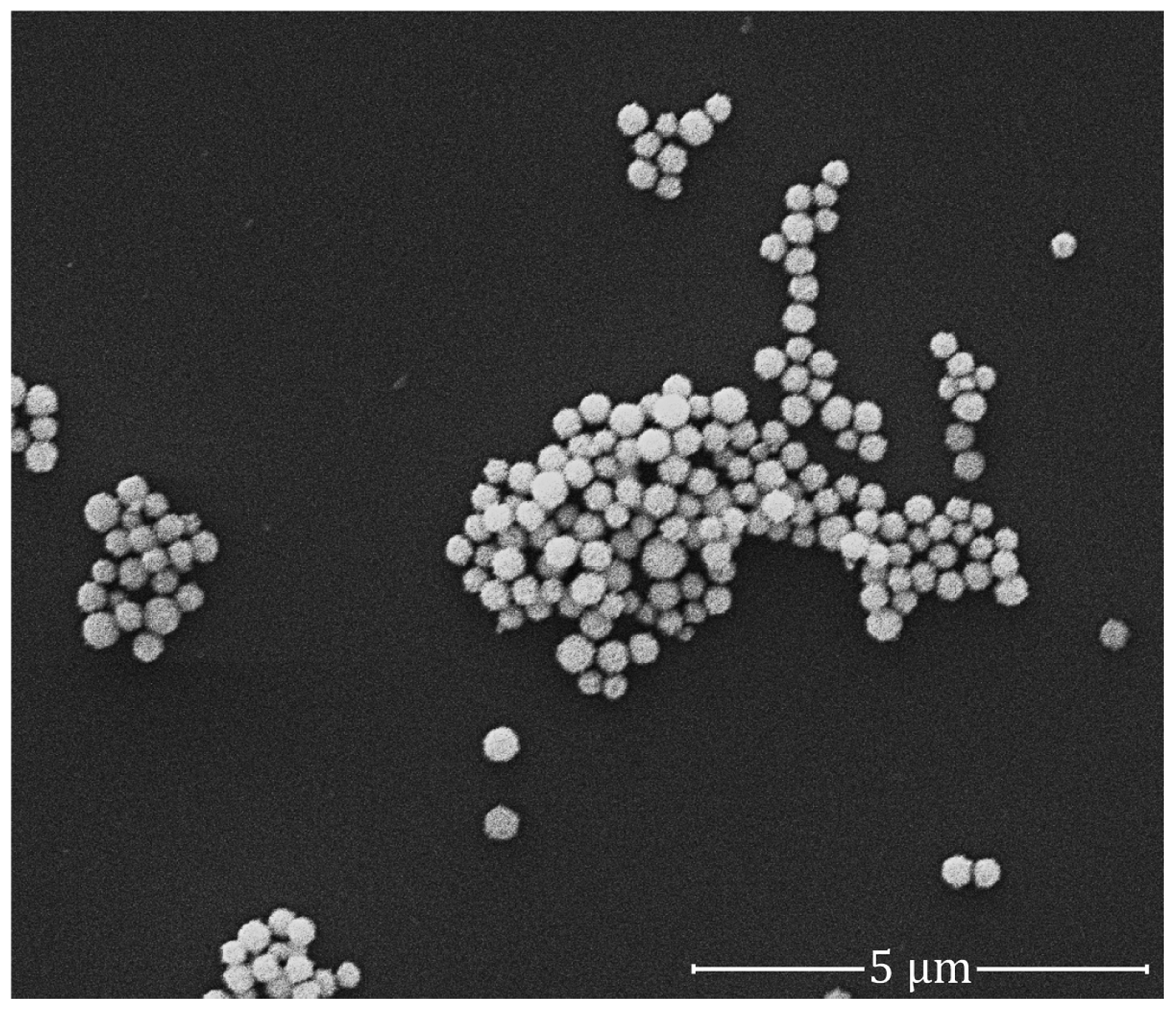
2.2. Quality of GNPs
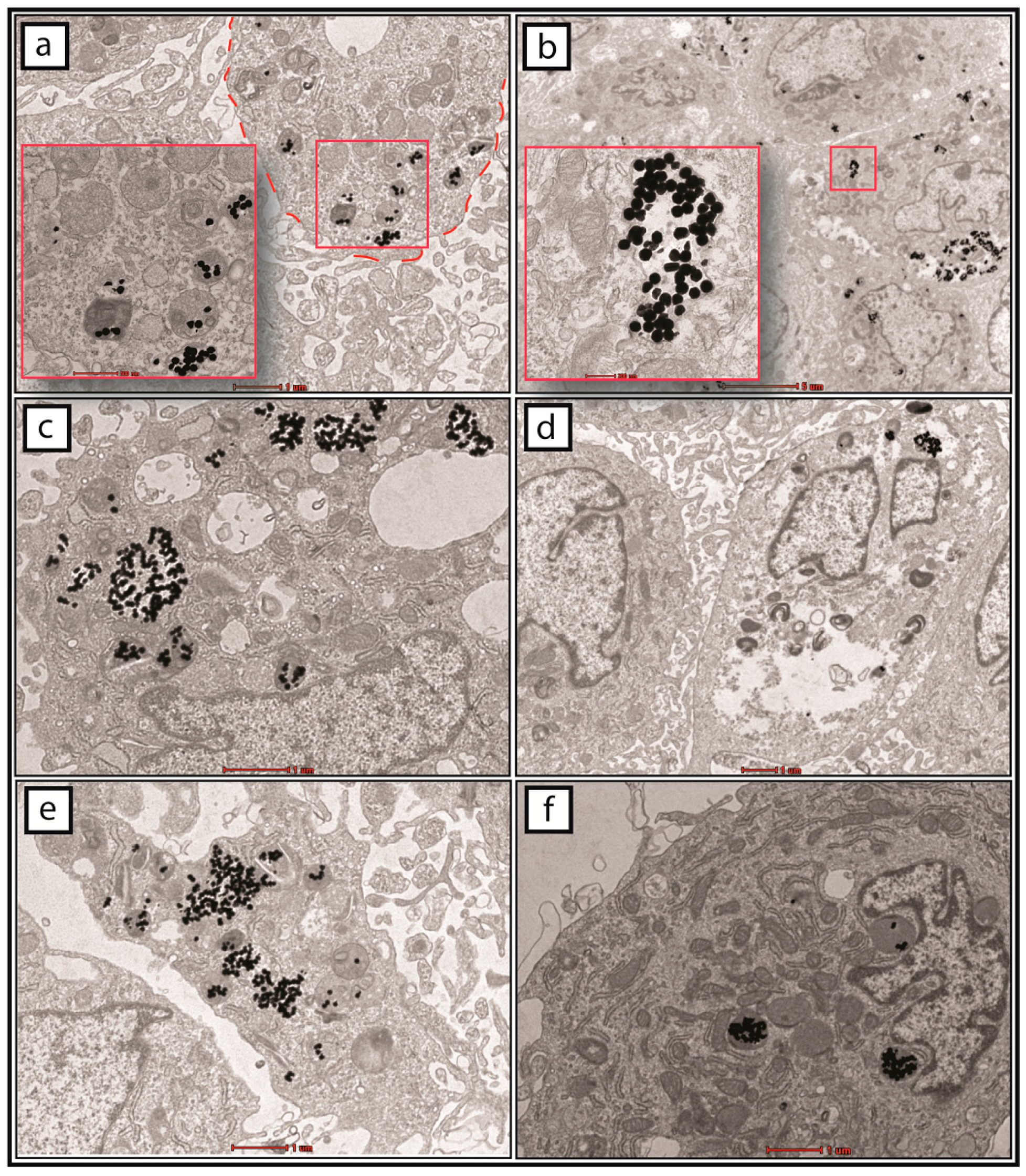
2.3. Cellular Uptake of GNPs by MSCs
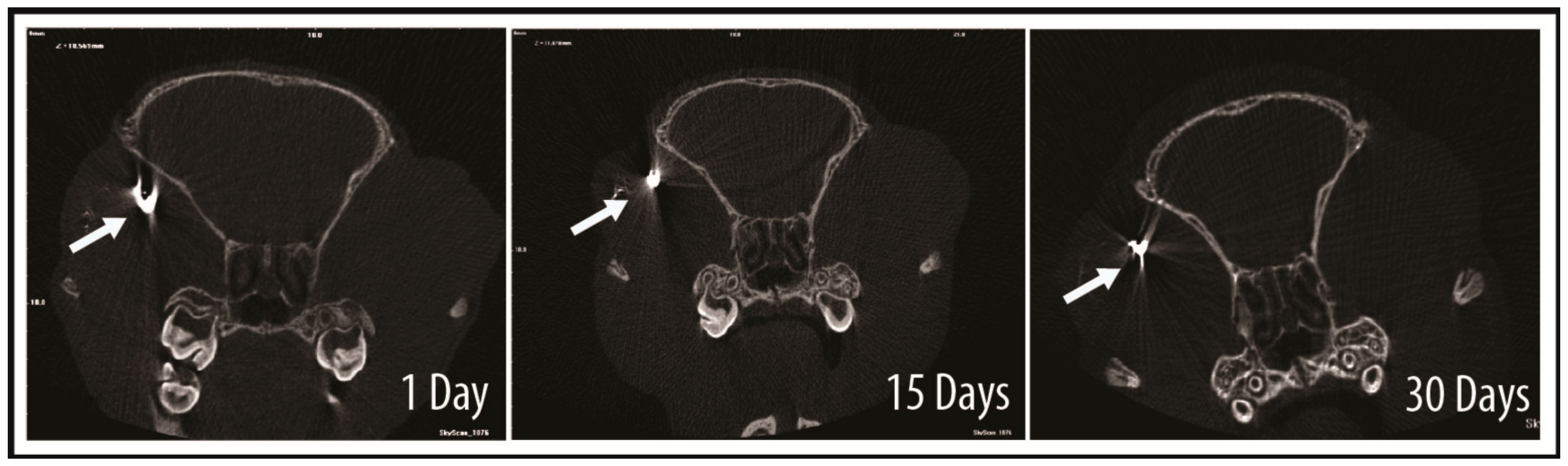
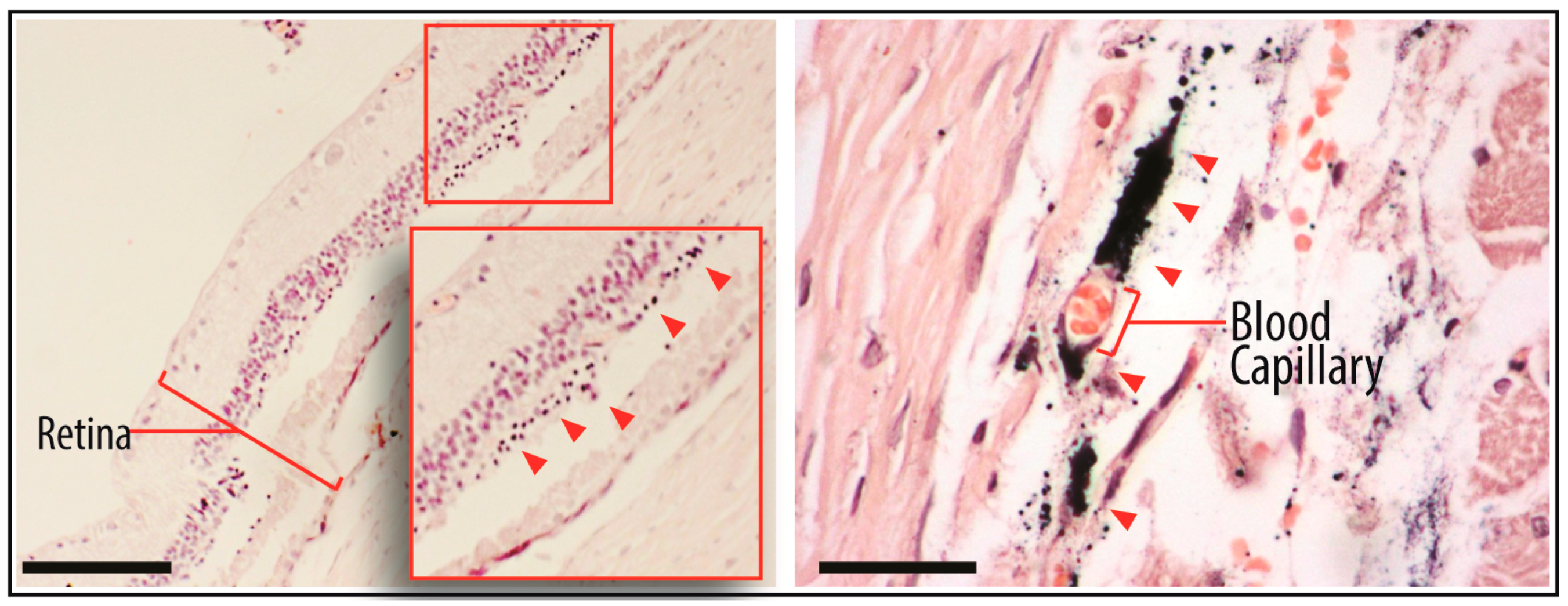
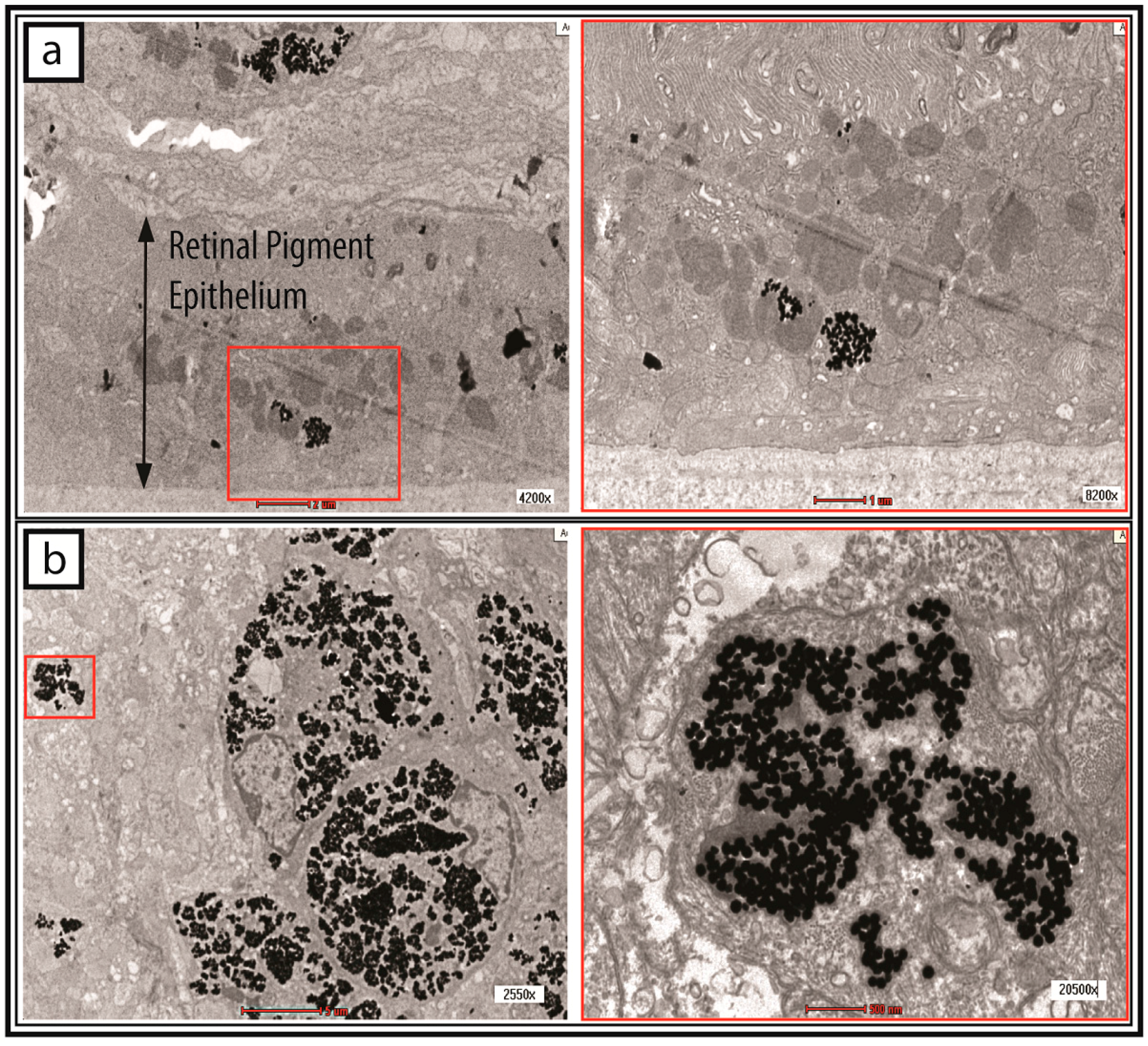
2.4. Tracking of GNP-Labelled MSCs by Micro-CT
3. Discussion
4. Materials and Methods
4.1. MSC Culture Conditions
4.2. MSC Characterization
4.3. GNP Labelling: Determination of the Quality of Colloidal GNPs
4.4. GNP Labelling: Cell Incubation with GNPs
4.5. GNP Lableling: Determination of the Cellular Uptake of GNPs
4.6. Subretinal Injection of GNP-Labelled MSCs
4.7. Micro-CT of Treated Rats
4.8. TEM of Injected MSCs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Figueroa, F.E.; Carrión, F.; Villanueva, S.; Khoury, M. Mesenchymal stem cell treatment for autoimmune diseases: A critical review. Biol. Res. 2016, 45, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; Hare, J.M. Use of mesenchymal stem cells for therapy of cardiac disease. Circ. Res. 2015, 116, 1413–1430. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.; Annett, G.; Wirthlin, L.; Olson, S.; Bauer, G.; Nolta, J. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen. Med. 2010, 5, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Cho, S.G. Clinical applications of mesenchymal stem cells. Korean J. Intern. Med. 2013, 28, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Leow, S.N.; Luu, C.D.; Hairul Nizam, M.H.; Mok, P.; Ruhaslizan, R.; Wong, H.S.; Wan Abdul Halim, W.; Ng, M.H.; Ruszymah, B.; Chowdhury, S.R.; et al. Safety and efficacy of human Wharton’s Jelly-derived mesenchymal stem cells therapy for retinal degeneration. PLoS ONE 2015, 10, e0128973. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.S.; Park, J.B.; Kim, H.S.; Kim, D.S.; Park, D.J.; Kang, S.J. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med. J. 2011, 52, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Barrera, J.; Wong, V.W.; Maan, Z.N.; Whittam, A.J.; Januszyk, M.; Gurtner, G.C. Stem cells in wound healing: The future of regenerative medicine? A Mini-Review. Gerontology 2015, 62, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Luk, F.; Dahlke, M.H.; Hoogduijn, M.J. The life and fate of mesenchymal stem cells. Front. Immunol. 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.M.; Leng Teo, G.S. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Bulte, J.W.M. Seeing stem cells at work in vivo. Stem Cell Rev. Rep. 2014, 10, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Bhirde, A.; Xie, J.; Swierczewska, M.; Chen, X.; Carpenter, M.K.; Frey-Vasconcells, J.; Rao, M.S.; Marin-Garcia, J.; Goldenthal, M.J.; Hart, L.S.; et al. Nanoparticles for cell labeling. Nanoscale 2011, 3, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Ricles, L.M.; Nam, S.Y.; Treviño, E.A.; Emelianov, S.Y.; Suggs, L.J. A dual gold nanoparticle system for mesenchymal stem cell tracking. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 8220–8230. [Google Scholar] [CrossRef] [PubMed]
- Mok, P.L.; Leong, C.F.; Cheong, S.K. Cellular mechanisms of emerging applications of mesenchymal stem cells. Malays. J. Pathol. 2013, 35, 17–32. [Google Scholar] [PubMed]
- Ricles, L.M.; Nam, S.Y.; Sokolov, K.; Emelianov, S.Y.; Suggs, L.J. Function of mesenchymal stem cells following loading of gold nanotracers. Int. J. Nanomed. 2011, 6, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Murph, S.; Jacobs, S.; Liu, J.; Hu, T.C.; Siegfired, M.; Serkiz, S.M.; Hudson, J. Manganese-gold nanoparticles as an MRI positive contrast agent in mesenchymal stem cell labeling. J. Nanopart. Res. 2012, 14, 658. [Google Scholar] [CrossRef]
- Kohl, Y.; Gorjup, E.; Katsen-Globa, A.; Büchel, C.; von Briesen, H.; Thielecke, H. Effect of gold nanoparticles on adipogenic differentiation of human mesenchymal stem cells. J. Nanopart. Res. 2011, 13, 6789–6803. [Google Scholar] [CrossRef]
- Choi, S.Y.; Song, M.S.; Ryu, P.D.; Lam, A.T.; Joo, S.W.; Lee, S.Y. Gold nanoparticles promote osteogenic differentiation in human adipose-derived mesenchymal stem cells through the Wnt/β-catenin signaling pathway. Int. J. Nanomed. 2015, 10, 4383–4392. [Google Scholar]
- Kang, S.; Bhang, S.H.; Hwang, S.; Yoon, J.K.; Song, J.; Jang, H.K.; Kim, S.; Kim, B.S. Mesenchymal stem cells aggregate and deliver gold nanoparticles to tumors for photothermal therapy. ACS Nano 2015, 9, 9678–9690. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, F.; Mirabelli, P.; Gorrese, M.; Scalia, G.; Gemei, M.; Mariotti, E.; di Noto, R.; Martinelli, P.; Fortunato, G.; Paladini, D.; et al. Polychromatic flow cytometry analysis of CD34+ hematopoietic stem cells in cryopreserved early preterm human cord blood samples. Cytom. Part A 2011, 79, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jinde, K.; Endoh, M.; Sakai, H. Clinical significance of costimulatory molecules CD80/CD86 expression in IgA nephropathy. Kidney Int. 2004, 65, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Accomasso, L.; Gallina, C.; Turinetto, V.; Giachino, C. Stem cell tracking with nanoparticles for regenerative medicine purposes: An overview. Stem Cells Int. 2016, 2016, 7920358. [Google Scholar] [CrossRef] [PubMed]
- Meir, R.; Motiei, M.; Popovtzer, R. Gold nanoparticles for in vivo cell tracking. Nanomedicine 2014, 9, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Huefner, A.; Septiadi, D.; Wilts, B.D.; Patel, I.I.; Kuan, W.; Fragniere, A.; Barker, R.A.; Mahajan, S. Gold nanoparticles explore cells: Cellular uptake and their use as intracellular probes. Methods 2014, 68, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D. Intracellular uptake, transport, and processing of gold nanostructures. Mol. Membr. Biol. 2010, 27, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, L.; Yeh, J.; Wu, X.; Cao, Z.; Wang, Y.A.; Zhang, M.; Yang, L.; Mao, H. Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-PγMPS copolymer coating. Biomaterials 2010, 31, 5397–5407. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Xu, H.; Zeng, Y.; Wang, Y.; Yin, Z.Q. Human bone marrow stromal cells can differentiate to a retinal pigment epithelial phenotype when co-cultured with pig retinal pigment epithelium using a transwell system. Cell. Physiol. Biochem. 2013, 31, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Vossmerbaeumer, U.; Ohnesorge, S.; Kuehl, S.; Haapalahti, M.; Kluter, H.; Jonas, J.B.; Thierse, H.J.; Bieback, K. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy 2009, 11, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Q.; Wang, Y.; Wang, Y.Q. Differentiation of human bone marrow-derived mesenchymal stem cells into neural-like cells by co-culture with retinal pigmented epithelial cells. Int. J. Ophthalmol. 2010, 3, 23–27. [Google Scholar] [PubMed]
- Kevany, B.M.; Palczewski, K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 2010, 25, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Ding, Y.; Caberoy, N.; Alvarado, G.; Wang, F.; Chen, R.; Li, W. ABCF1 extrinsically regulates retinal pigment epithelial cell phagocytosis Running title: ABCF1 is a novel phagocytosis ligand. Mol. Biol. Cell. 2015, 26, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cai, J.; Liu, B.; Zhong, Y.; Qin, Y. Cellular magnetic resonance imaging contrast generated by the ferritin heavy chain genetic reporter under the control of a Tet-On switch. Stem Cell Res. Ther. 2015, 6, 207. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.M.; Moss, D.; Williams, S.R.; Murray, P.; Taylor, A. Overexpression of the MRI reporter genes ferritin and transferrin receptor affect iron homeostasis and produce limited contrast in mesenchymal stem cells. Int. J. Mol. Sci. 2015, 16, 15481–15496. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, Y.; Mardor, Y.; Feinberg, M.S.; Landa, N.; Miller, L.; Daniels, D.; Ocherashvilli, A.; Holbova, R.; Yosef, O.; Barbash, I.M.; et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 2007, 116, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.C.; Gunst, P.R.; Cascio, W.E.; Kypson, A.P.; Muller-Borer, B.J. Labeling and imaging mesenchymal stem cells with quantum dots. Methods Mol. Biol. 2012, 906, 199–210. [Google Scholar] [PubMed]
- Fan, J.; Li, W.; Hung, W.; Hung, W.; Chen, C.; Yeh, J. Cytotoxicity and differentiation effects of gold nanoparticles to human bone marrow mesenchymal stem cells. Biomed. Eng. Appl. Basis Commun. 2011, 23, 141–152. [Google Scholar] [CrossRef]
- Sivan, P.P.; Syed, S.; Mok, P.L.; Higuchi, A.; Murugan, K.; Alarfaj, A.A.; Munusamy, M.A.; Hamat, R.H.; Umezawa, A.; Kumar, S. Stem Cell Therapy for Treatment of Ocular Disorders. Stem Cells Int. 2016, 2016, 8304879. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Z.; Qiao, H.; Chen, L.; Fan, Y. Effect of Gold/Fe3O4 Nanoparticles on biocompatibility and neural differentiation of rat olfactory bulb neural stem cells. J. Nanomater. 2013, 2013, 867426. [Google Scholar]
- Paviolo, C.; Haycock, J.W.; Yong, J.; Yu, A.; McArthur, S.L.; Stoddart, P.R. Plasmonic properties of gold nanoparticles can promote neuronal activity. In Proceedings of the SPIE International Society for Optics and Photonics, San Diego, CA, USA, 25–29 August 2013; Jansen, E.D., Thomas, R.J., Eds.; p. 85790C.
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Kharlamov, A.; Perrish, A.; Gabinsky, J. Silica-gold nanoparticles and mesenchymal stem cells versus composite ferro-magnetic approach for management of atherosclerotic plaque and artery remodeling. Circulation 2011, 124, A8303. [Google Scholar]
- Plasmonic Photothermal and Stem Cell Therapy of Atherosclerosis versus Stenting (NANOM PCI). Available online: https://clinicaltrials.gov/ct2/show/NCT01436123 (accessed on 30 July 2016).
- Sarkar, D.; Vemula, P.K.; Zhao, W.; Gupta, A.; Karnik, R.; Karp, J.M. Engineered mesenchymal stem cells with self-assembled vesicles for systemic cell targeting. Biomaterials 2010, 31, 5266–5274. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Borenstein, J.; Toner, M.; Takayama, S. Micro and Nanoengineering of the Cell Microenvironment; Artech House Publishers: Norwood, MA, USA, 2008. [Google Scholar]
- Qureshi, S. β-lactamase: An ideal reporter system for monitoring gene expression in live eukaryotic cells. BioTechniques 2007, 42, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Smale, S. Chloramphenicol acetyltransferase assay. Cold Spring Harb. Protoc. 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Ude, C.; Shamsul, B.; Ng, M.; Chen, H.C.; Norhamdan, M.Y.; Aminuddin, B.S.; Ruszymah, B.H.I. Bone marrow and adipose stem cells can be tracked with PKH26 until post staining passage 6 in in vitro and in vivo. Tissue Cell 2012, 44, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, C.; Festin, R.; Tufveson, G.; Tötterman, T.H. Ex vivo PKH26-labelling of lymphocytes for studies of cell migration in vivo. Scand. J. Immunol. 1997, 45, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Huang, L.; Wei, W.; Chen, X.; Zhang, X.; Zhang, X. Real-time imaging and tracking of ultrastable organic dye nanoparticles in living cells. Biomaterials 2016, 93, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, M.L.; Clarke, D.T. Single molecule fluorescence detection and tracking in mammalian cells: The state-of-the-art and future perspectives. Int. J. Mol. Sci. 2012, 11, 14742–14765. [Google Scholar] [CrossRef] [PubMed]
- Muthana, M.; Kennerley, A.J.; Hughes, R.; Fagnano, E.; Richardson, J.; Paul, M.; Murdoch, C.; Wright, F.; Payne, C.; Lythgoe, M.F.; et al. Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat. Commun. 2015, 6, 8009. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E. Types of imaging, part 2: An overview of fluorescence microscopy. Anat. Rec. 2012, 295, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Progatzky, F.; Dallman, M.J.; Lo Celso, C. From seeing to believing: Labelling strategies for in vivo cell-tracking experiments. Interface Focus 2013, 3, 20130001. [Google Scholar] [CrossRef] [PubMed]
- Edmundson, M.; Capeness, M.; Horsfall, L. Exploring the potential of metallic nanoparticles within synthetic biology. New Biotechnol. 2014, 3, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kawazoe, N.; Chen, G. Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis. Biomaterials 2015, 54, 226–236. [Google Scholar] [CrossRef] [PubMed]
| Cell Labelling Technology | Advantages/Disadvantages | References |
|---|---|---|
| Viral or non-viral reporter gene systems | Advantages: Allows monitoring of co-expressed genes. | [49] |
| Disadvantages: Labour-intensive and time-consuming in the preparation of transduced cell clones. Radioactive substance is required in a conventional chloramphenicol acetyl transferase reporter system, which is potentially hazardous. | [16,50] | |
| Free organic dyes e.g., PKH26, carboxyfluorescein succinimidyl ester (CFSE) | Advantages: Simple cell-labelling protocol. Long-term cell tracking in both in vitro and in vivo systems, e.g., PKH26. | [51] |
| Disadvantages: Possible transfer of dye from labelled to unlabelled cells. | [52] | |
| Organic dye nanoparticles | Advantages: Suitable for living-cell imaging as it demonstrates high fluorescence intensity, large Stokes shift, photostability, and emission in the near-infrared range. | [53] |
| Disadvantages: High tendency of organic dye to stick to the cell substrate. | [54] | |
| Superparamagnetic iron oxide nanoparticles (SPIO) | Advantages: Direct tissue targeting of SPIO-labelled cells is feasible with use of an appropriate magnetic field. | [55] |
| Disadvantages: Requires cross-linking with a membrane-translocating signal peptide (e.g., HIV-1 Tat protein) or co-incubation with transfection agents to facilitate cellular uptake. | [25] | |
| Semiconductor quantum dots | Advantages: Photostable, possesses size-controlled fluorescence, and the emitted fluorescence has a long lifetime. | [56] |
| Disadvantages: High cost of reagents; generation of free radicals may cause cellular toxicity. | [57,58] | |
| Noble metallic nanoparticles e.g., gold nanoparticles | Advantages: Simple cell-labelling protocol and no acute cellular toxicity demonstrated. | [59] |
| Disadvantages: Different shapes and sizes could affect stem cell differentiation potential. | [16,40] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mok, P.L.; Leow, S.N.; Koh, A.E.-H.; Mohd Nizam, H.H.; Ding, S.L.S.; Luu, C.; Ruhaslizan, R.; Wong, H.S.; Halim, W.H.W.A.; Ng, M.H.; et al. Micro-Computed Tomography Detection of Gold Nanoparticle-Labelled Mesenchymal Stem Cells in the Rat Subretinal Layer. Int. J. Mol. Sci. 2017, 18, 345. https://doi.org/10.3390/ijms18020345
Mok PL, Leow SN, Koh AE-H, Mohd Nizam HH, Ding SLS, Luu C, Ruhaslizan R, Wong HS, Halim WHWA, Ng MH, et al. Micro-Computed Tomography Detection of Gold Nanoparticle-Labelled Mesenchymal Stem Cells in the Rat Subretinal Layer. International Journal of Molecular Sciences. 2017; 18(2):345. https://doi.org/10.3390/ijms18020345
Chicago/Turabian StyleMok, Pooi Ling, Sue Ngein Leow, Avin Ee-Hwan Koh, Hairul Harun Mohd Nizam, Suet Lee Shirley Ding, Chi Luu, Raduan Ruhaslizan, Hon Seng Wong, Wan Haslina Wan Abdul Halim, Min Hwei Ng, and et al. 2017. "Micro-Computed Tomography Detection of Gold Nanoparticle-Labelled Mesenchymal Stem Cells in the Rat Subretinal Layer" International Journal of Molecular Sciences 18, no. 2: 345. https://doi.org/10.3390/ijms18020345






