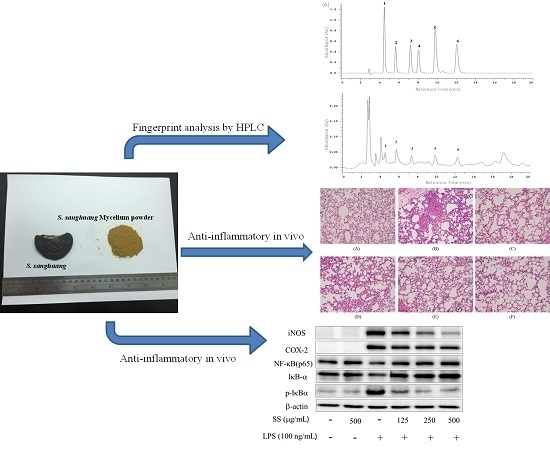
Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium
Abstract
:1. Introduction
2. Results
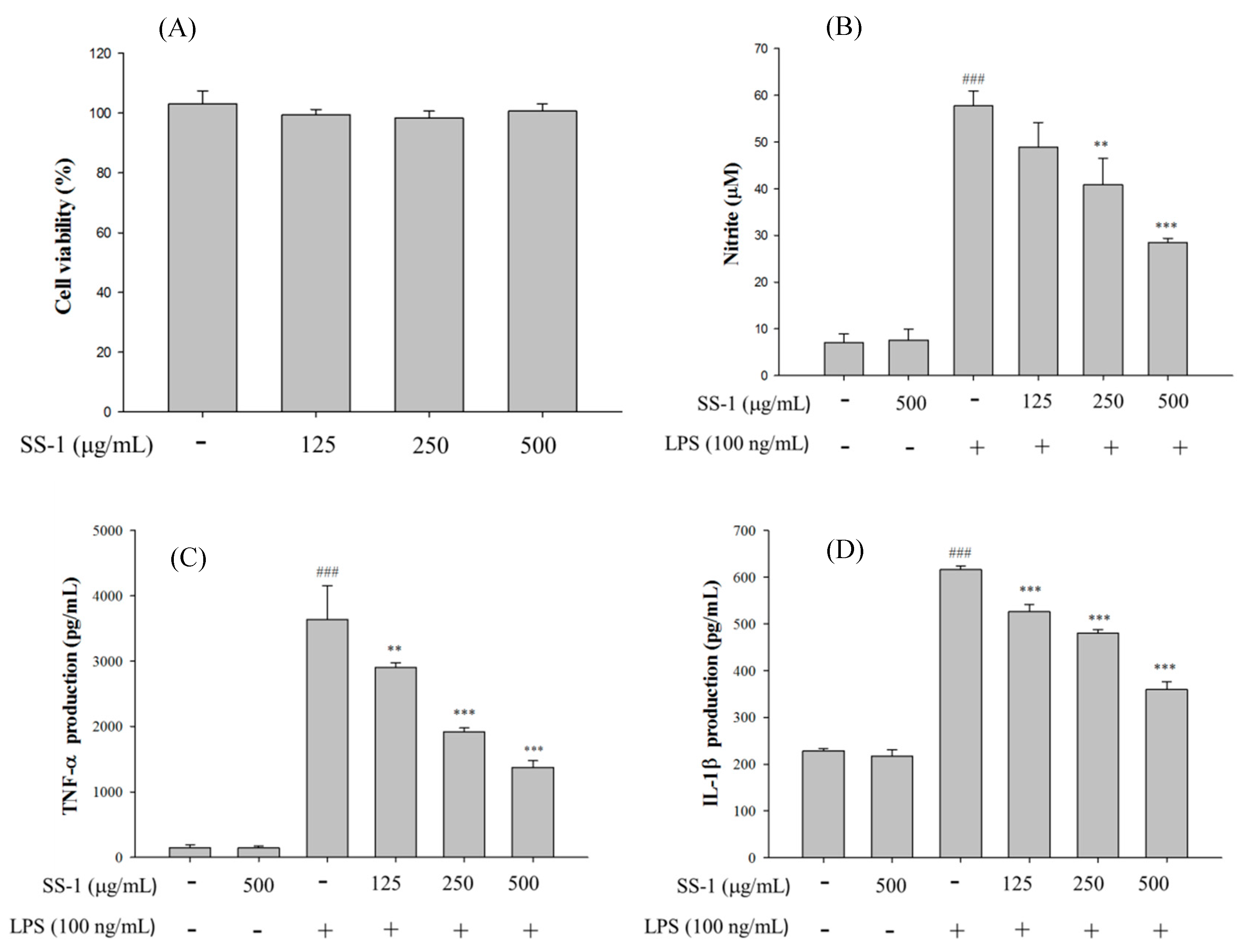
2.1. Cytotoxicity and NO Inhibition
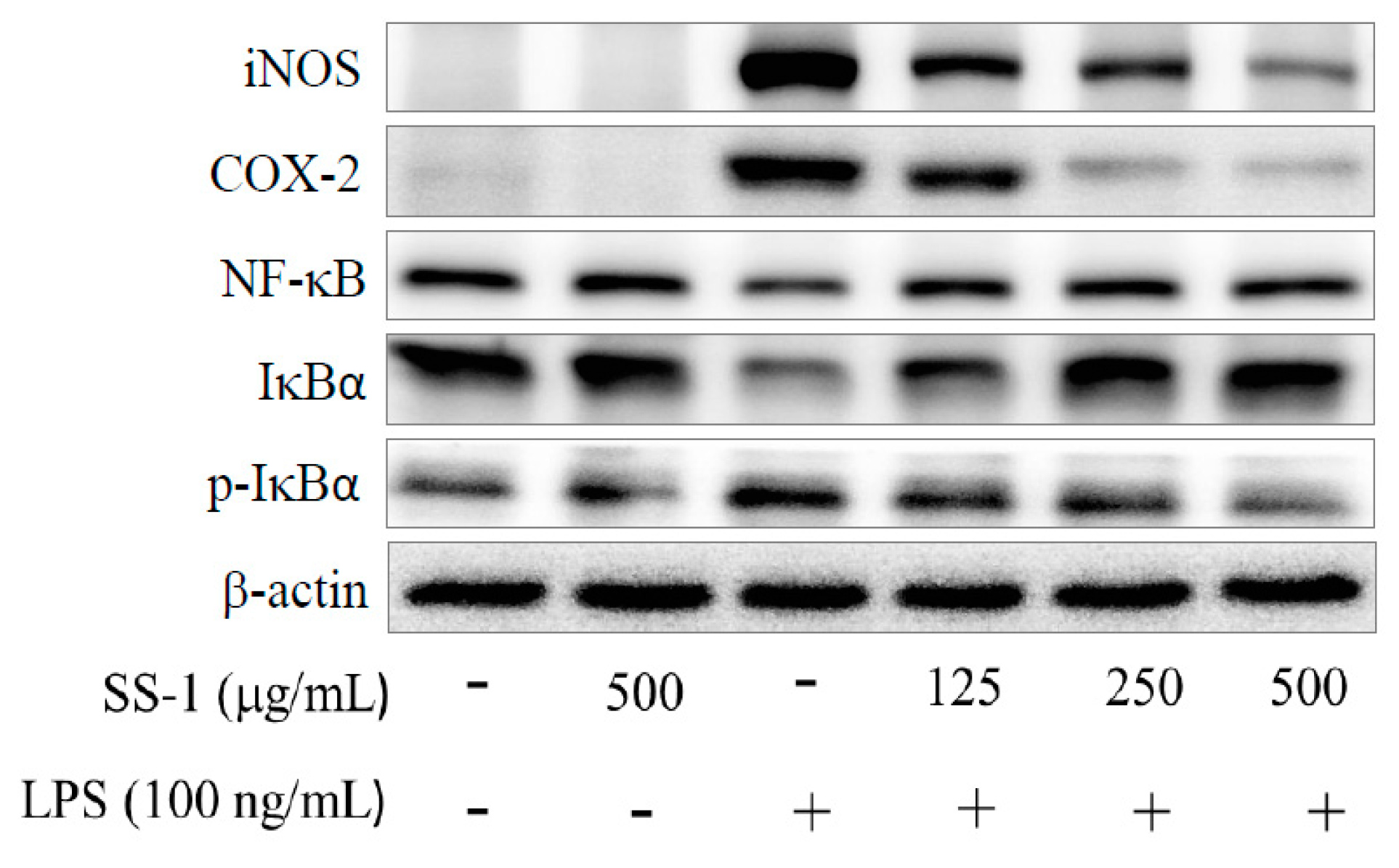
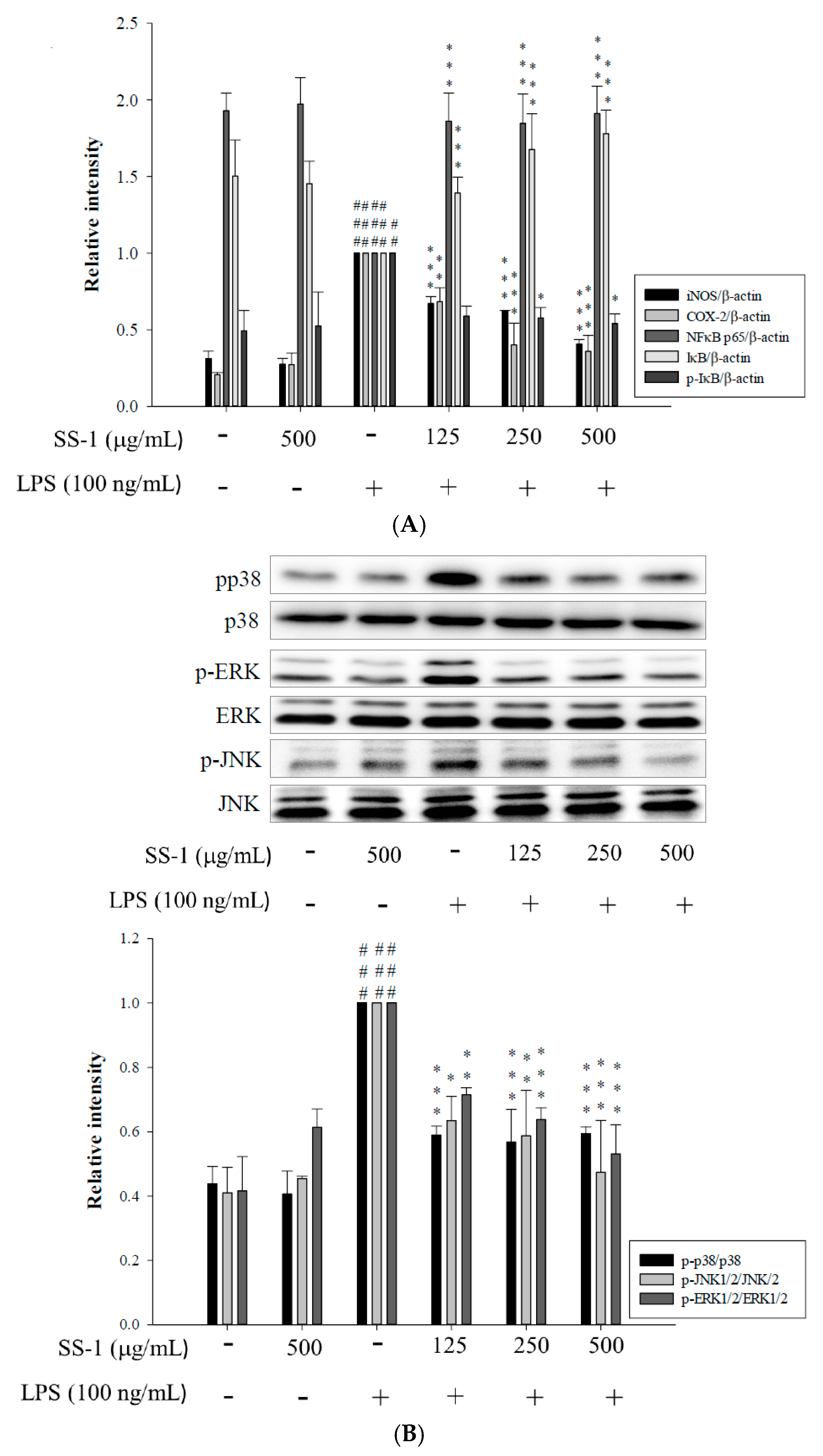
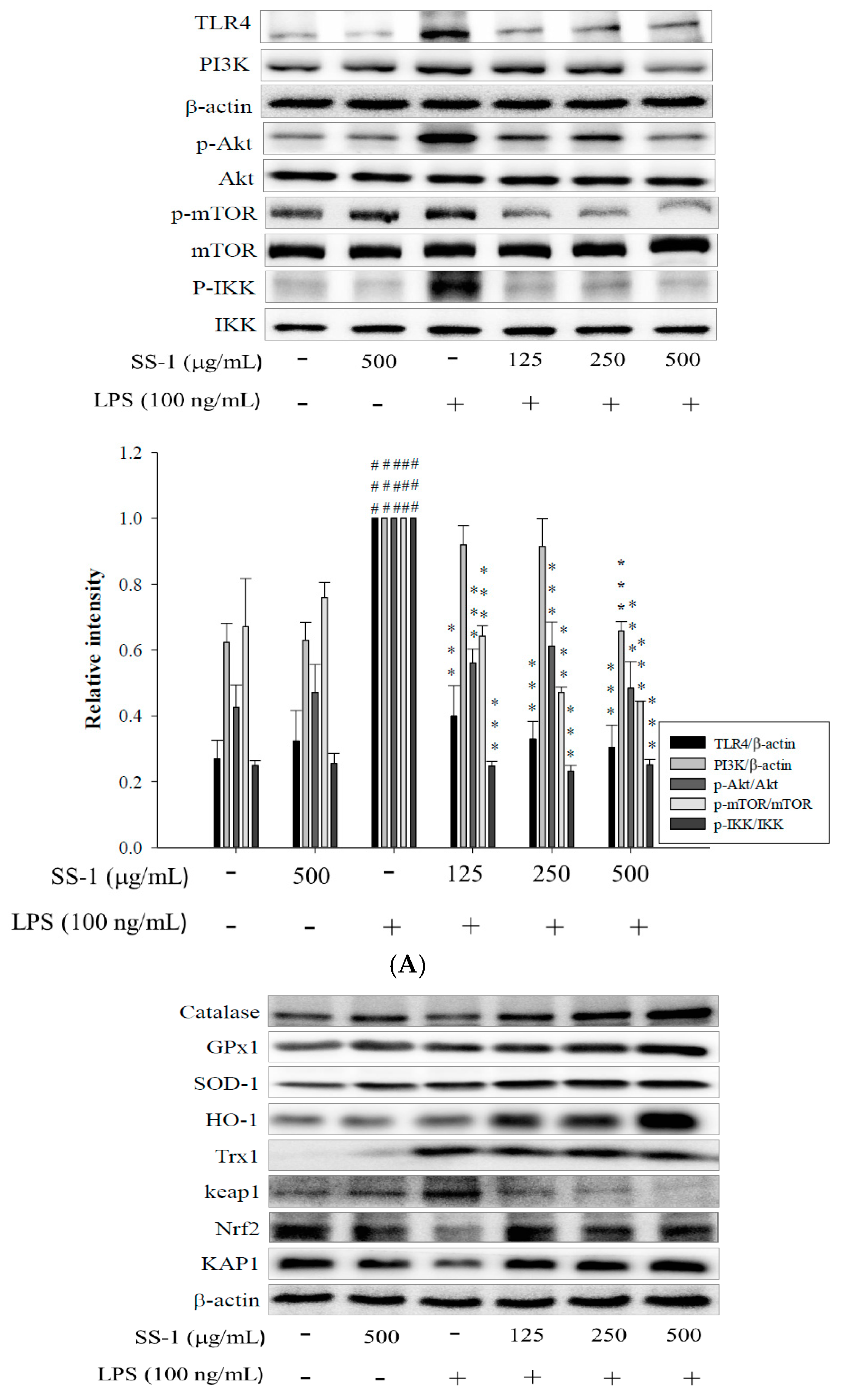
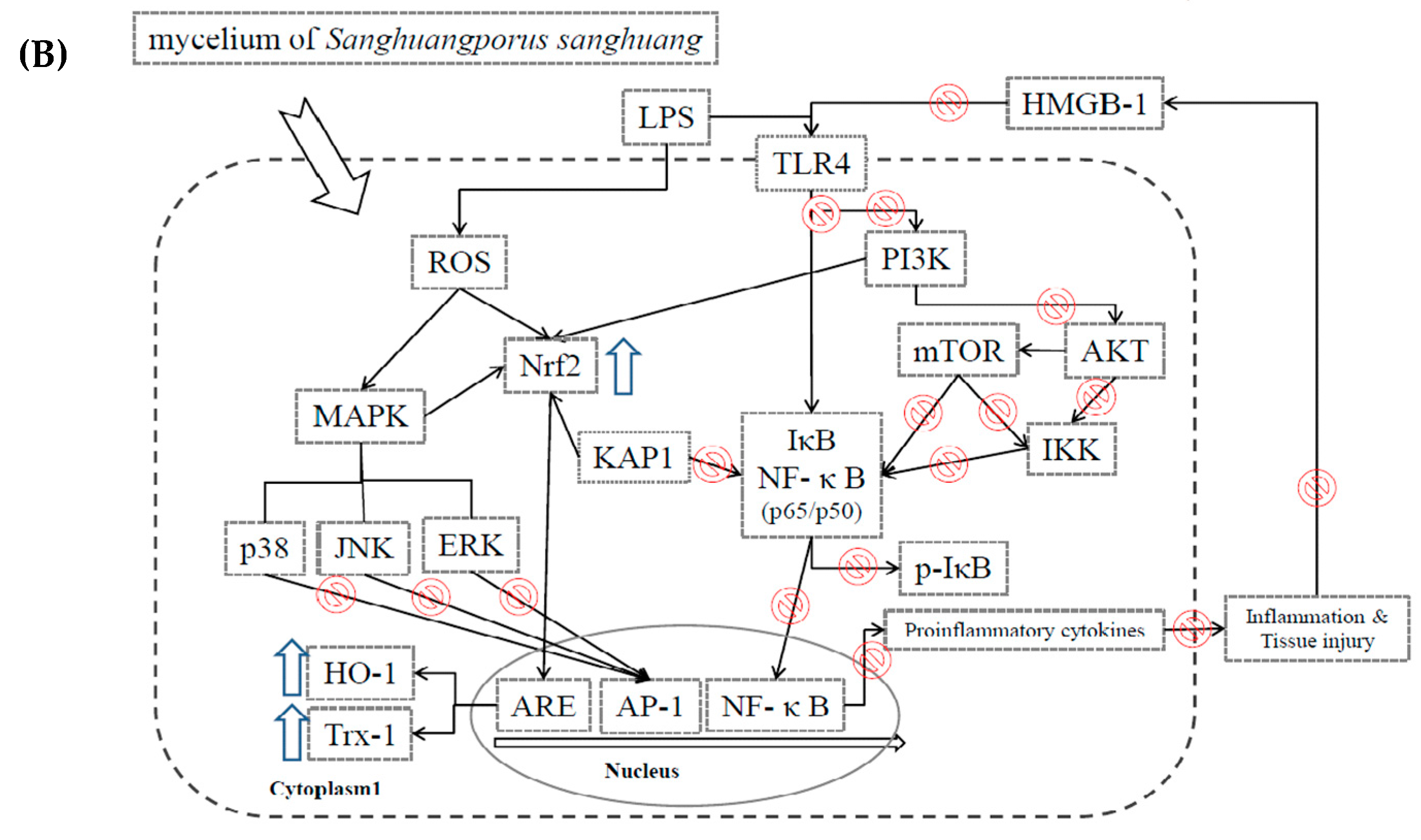
2.2. Effects of SS-1 on LPS-Induced iNOS, COX-2, NF-κB, MAPK and TLR4/PI3K/Akt/mTOR/IKKβ Protein Expressions in Macrophages
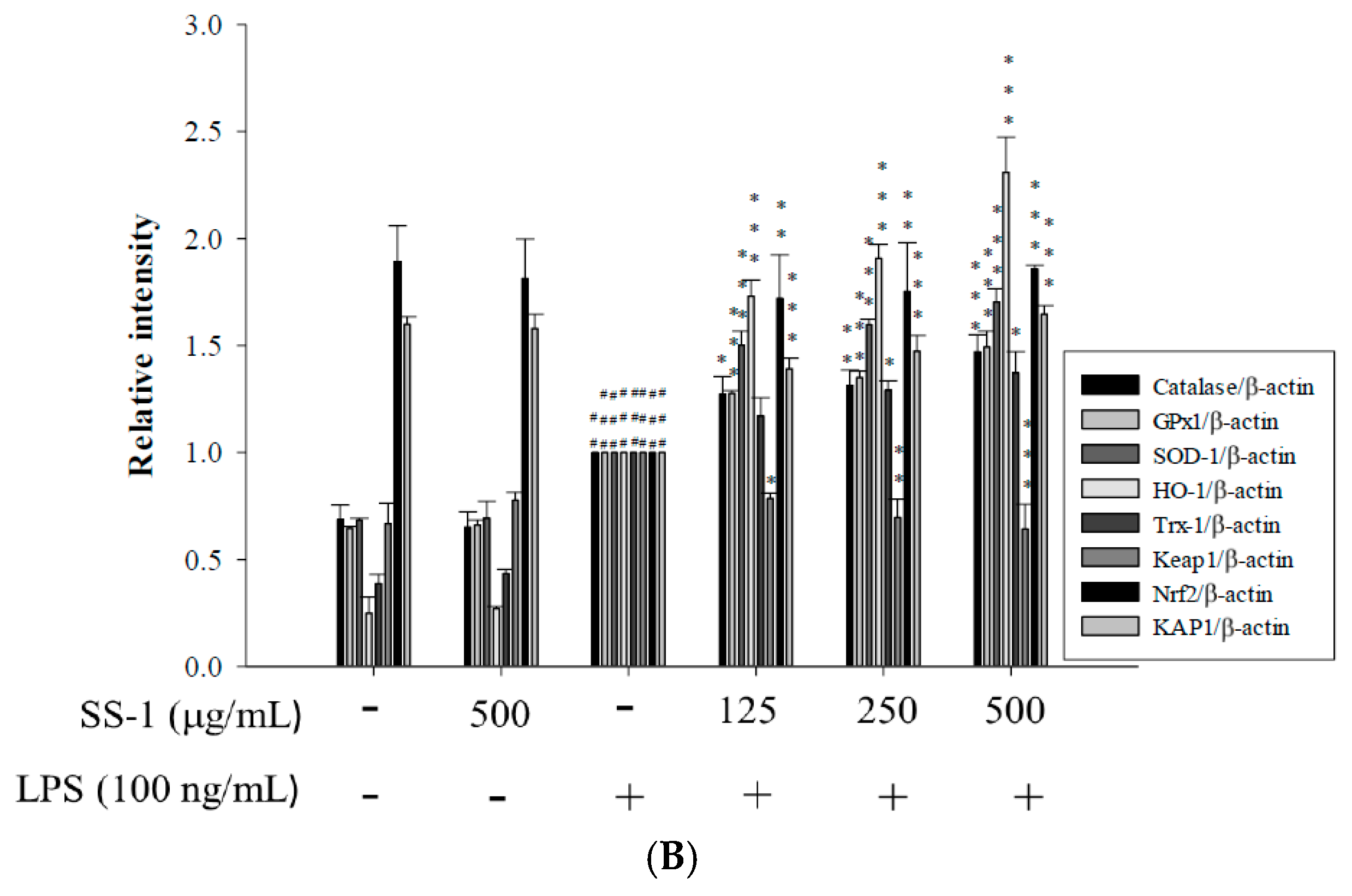
2.3. Effects of SS-1 on LPS-Induced Antioxidative Enzymes and HO-1, Trx-1, KAP1/Nrf2 Protein Expressions in Macrophage
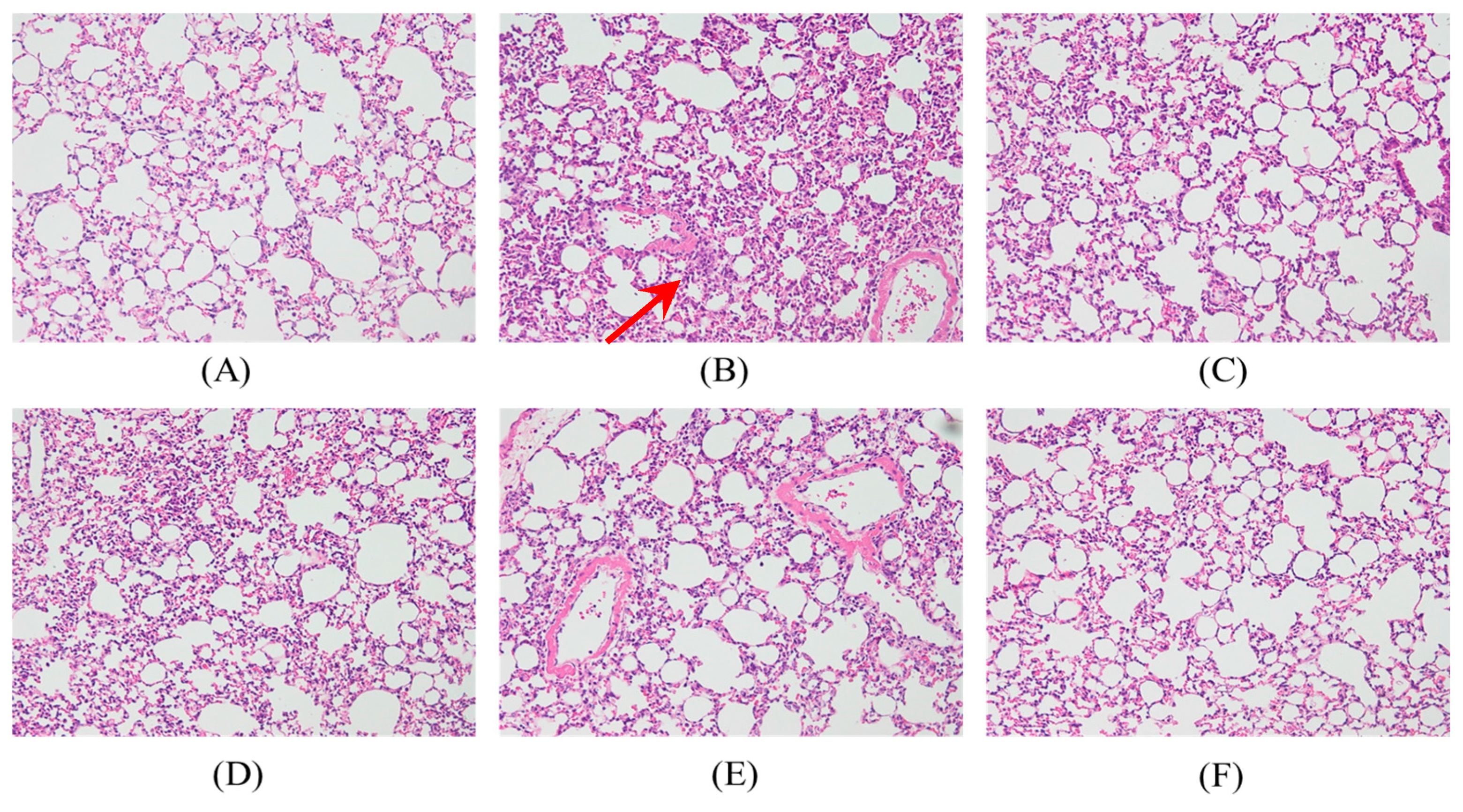
2.4. Effects of SS-1 on LPS-Mediated Lung Histopathologic Changes
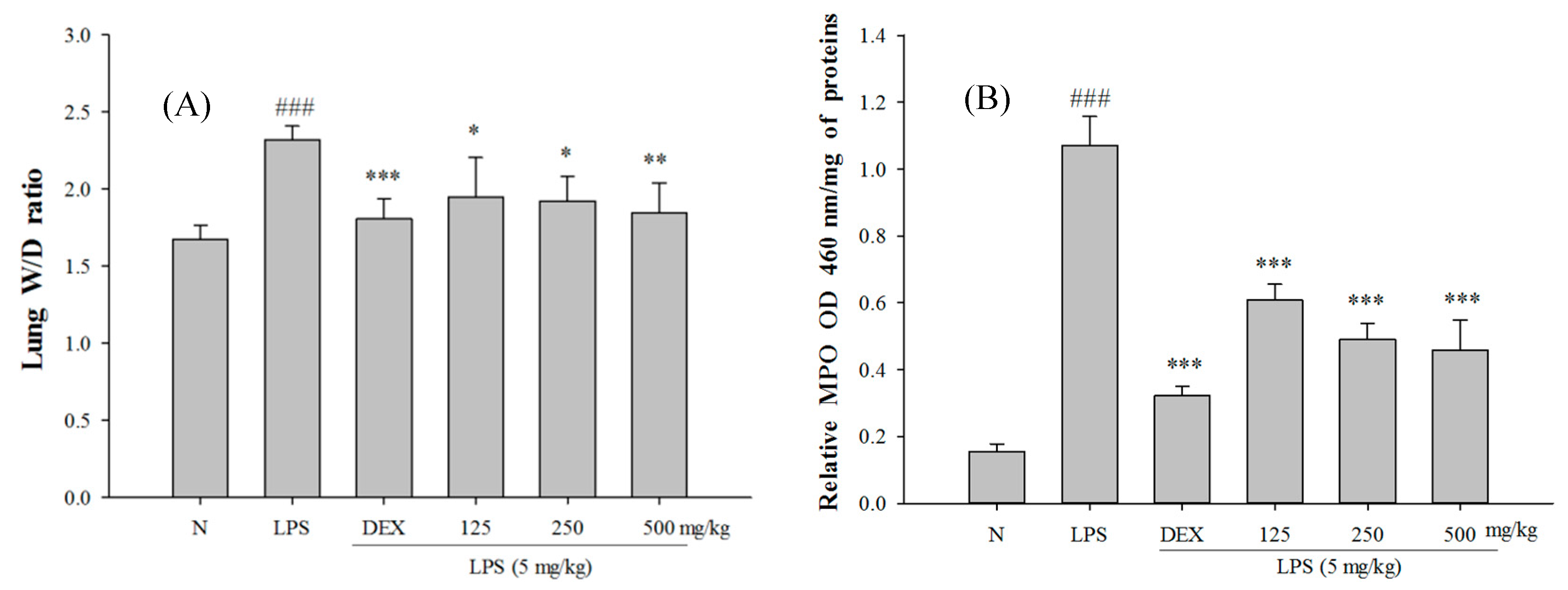
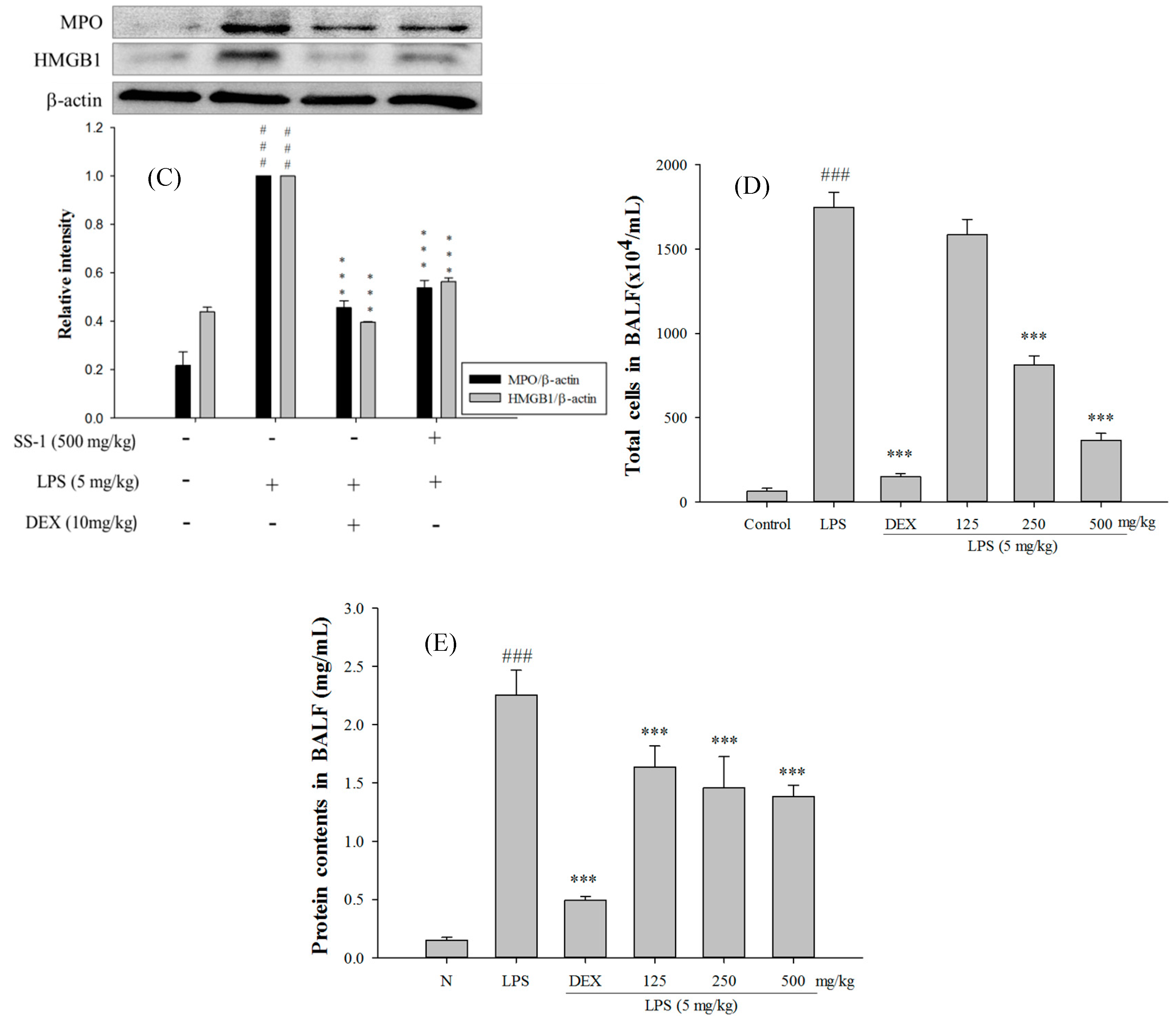
2.5. SS-1 Attenuates Pulmonary Edema and Reduces Cellular Counts and Proteins in BALF in LPS-Induced ALI Mice
2.6. Effect of SS-1 on BALF Cytokine Levels and iNOS, COX-2 Protein Expressions in LPS-Induced ALI Mice
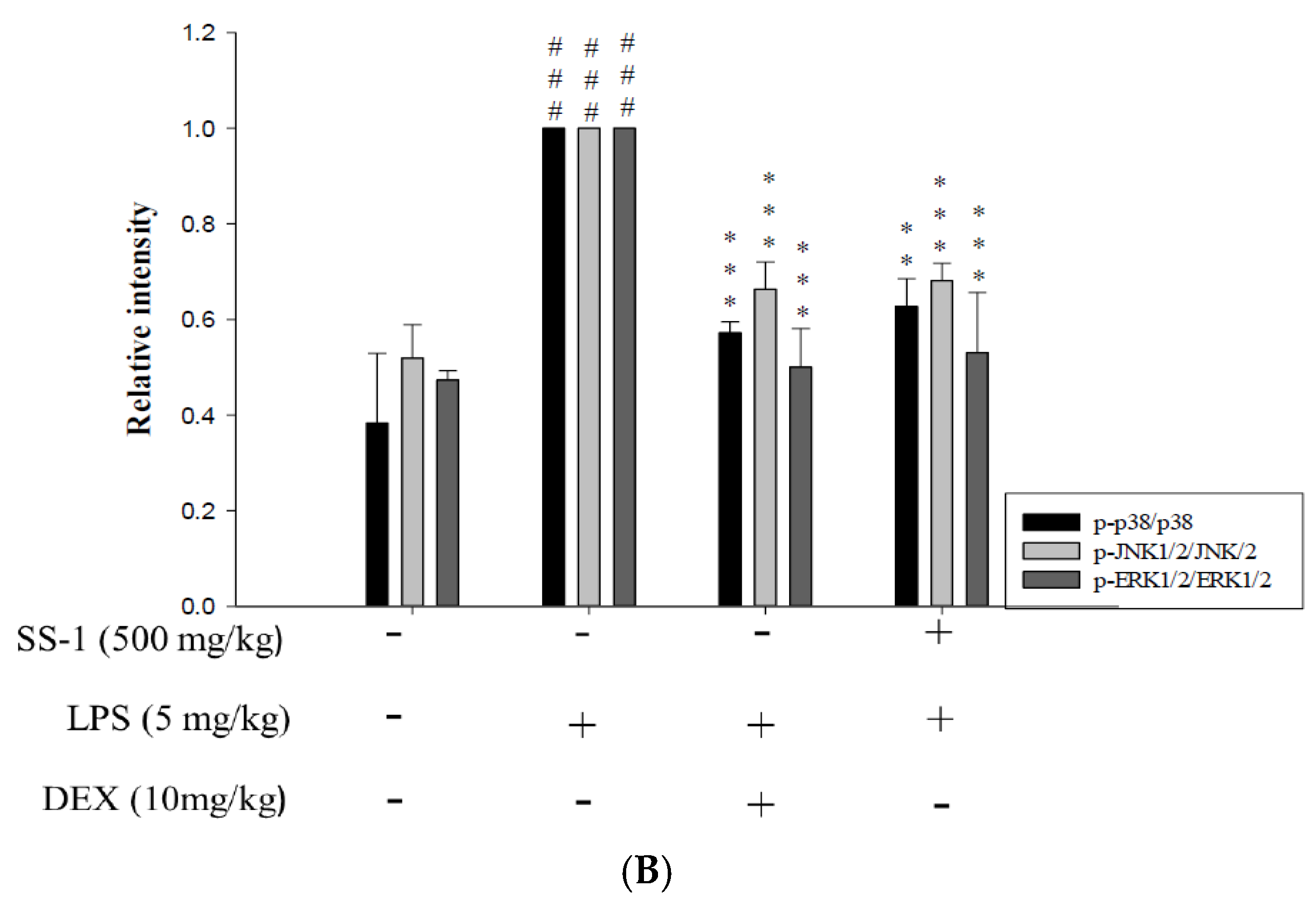
2.7. Effects of SS-1 on NF-κB and MAPK Activation in LPS Induced ALI Mice
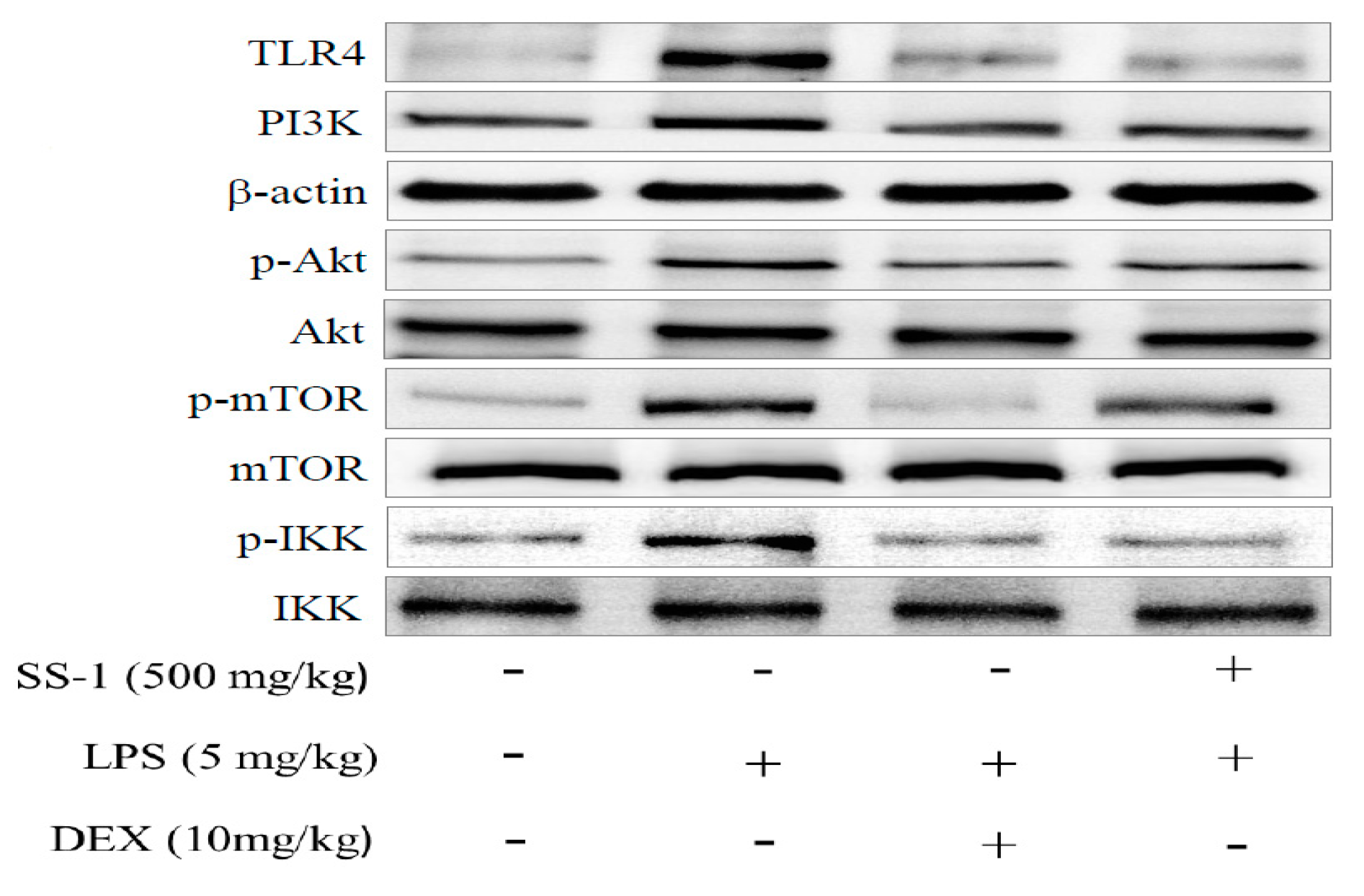
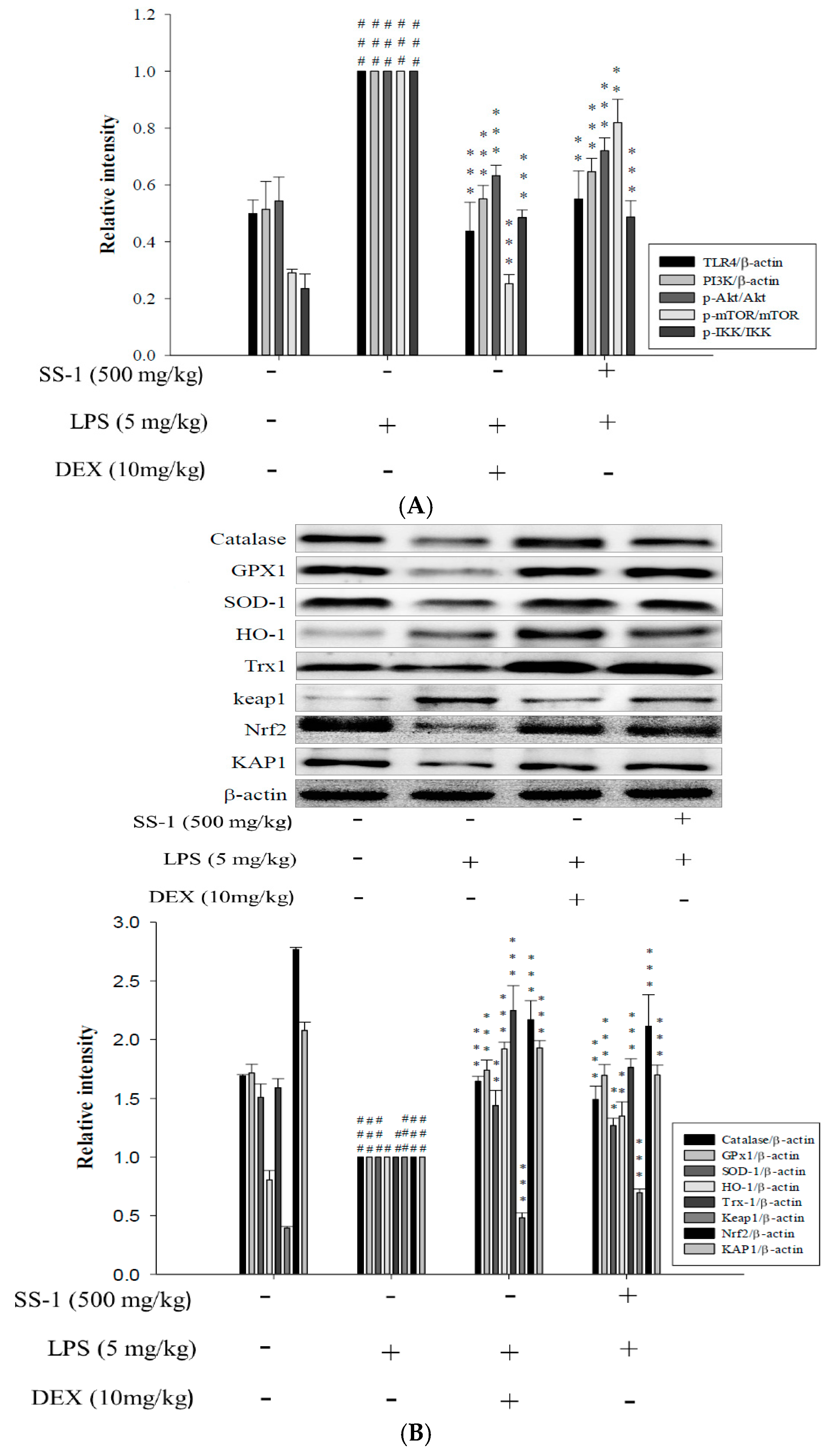
2.8. Effect of SS-1 on PI3K/Akt/mTOR /IKKβ Pathway Activation in LPS Induced ALI Mice
2.9. Effects of SS-1 on LPS-Induced Antioxidative Enzymes and HO-1/Nrf2 Protein Expressions in ALI Mice
2.10. HPLC Profile of SS-1
3. Discussion
4. Materials and Methods
4.1. Source of Material
4.2. Sample Extraction
4.3. Cell Culture
4.4. Cytotoxicity and NO Production
4.5. Animals
4.6. Model of LPS Induced ALI
4.7. Bronchoalveolar Lavage Fluid (BALF), Total Cell Count and Protein Analysis
4.8. TNF-α, IL-6, and IL-1β Cytokines in BALF
4.9. Myeloperoxidase (MPO) Activity Assay
4.10. Lung Wet/Dry Weight Ratio
4.11. H&E Staining
4.12. Protein Extraction and Western Blot Analysis
4.13. Fingerprint Analysis by HPLC
4.14. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Y.; Wu, H.; Nie, Y.C.; Chen, J.L.; Su, W.W.; Li, P.B. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-κB pathway. Int. Immunopharmacol. 2011, 11, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Li, B.; Hu, Y.; Li, X.; Li, J.; Zhang, H. Protective effects of scoparone against lipopolysaccharide-induced acute lung injury. Int. Immunopharmacol. 2014, 23, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.L.; Chen, C.S.; Hsu, C.W.; Li, M.H.; Chang, H.; Tsai, S.H.; Chu, S.J. Therapeutic effects of baicalin on lipopolysaccharide-induced acute lung injury in rats. Am. J. Chin. Med. 2008, 36, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Lin, Y.C.; Wang, H.M.; Chou, T.C. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J. Ethnopharmacol. 2014, 153, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Yang, M.L.; Tsai, C.H.; Li, Y.C.; Lin, Y.J.; Kuan, Y.H. Ginkgo biloba leaves extract (EGb-761) attenuates lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and NF-κB-dependent matrix metalloproteinase-9 pathway. Phytomedicine 2013, 20, 303–309. [Google Scholar] [CrossRef] [PubMed]
- El Mezayen, R.; El Gazzar, M.; Seeds, M.C.; McCall, C.E.; Dreskin, S.C.; Nicolls, M.R. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol. Lett. 2007, 1, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Deng, Y.; Xie, Y.; Liu, H.; Gong, F. Expression and significance of HMGB1, TLR4 and NF-κB p65 in human epidermal tumors. BMC Cancer 2013, 26, 311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Zhang, X.Y.; Xu, L.H.; Ouyang, D.Y.; Liu, K.P.; Pan, H.; He, J.; He, X.H. Ginsenoside Rg1 regulates innate immune responses in macrophages through differentially modulating the NF-κB and PI3K/Akt/mTOR pathways. Int. Immunopharmacol. 2014, 23, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, C.; Tian, G.; Zhu, M.; Bu, W.; Chen, J.; Hou, X.; Di, L.; Jia, X.; Dong, Z.; et al. Organic acid component from Taraxacum mongolicum Hand.-Mazz alleviates inflammatory injury inlipopolysaccharide-induced acute tracheobronchitis of ICR mice through TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2016, 34, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choo, Y.Y.; Tae, N.; Min, B.S.; Lee, J.H. The anti-inflammatory effect of 3-deoxysappanchalcone is mediated by inducing heme oxygenase-1 via activating the Akt/mTOR pathway in murine macrophages. Int. Immunopharmacol. 2014, 22, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, J.; Wu, Y.F.; Lou, J.; Mao, Y.Y.; Shen, H.H.; Chen, Z.H. mTOR and autophagy in regulation of acute lung injury: A review and perspective. Microbes Infect. 2014, 16, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Qiang, M. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Wang, T.C.; Lee, Y.C.; Shen, P.T.; Chang, J.Y.; Yeh, T.K.; Huang, C.H.; Chang, H.H.; Cheng, S.Y.; Lin, C.Y.; et al. Novel Nrf2/ARE Activator, trans-Coniferylaldehyde, Induces a HO-1-Mediated Defense Mechanism through a Dual p38α/MAPKAPK-2 and PK-N3 Signaling Pathway. Chem. Res. Toxicol. 2015, 28, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Nakaso, K.; Yano, H.; Fukuhara, Y.; Takeshima, T.; Wada-Isoe, K.; Nakashima, K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003, 546, 181–184. [Google Scholar] [CrossRef]
- Iyengar, S.; Farnham, P.J. KAP1 Protein: An Enigmatic Master Regulator of the Genome. J. Biol. Chem. 2011, 286, 26267–26276. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, A.; Nishikawa, K.; Kawatani, Y.; Mimura, J.; Hosoya, T.; Harada, N.; Yamamato, M.; Itoh, K. The novel Nrf2-interacting factor KAP1 regulates susceptibility to oxidative stress by promoting the Nrf2-mediated cytoprotective response. Biochem. J. 2011, 436, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.J.; Li, J.R.; Zhu, Z.G.; Kong, H.Y.; Jin, H.; Zhang, J.Y.; Tian, Y.X.; Li, Z.H.; Wu, X.Y.; Zhang, J.J.; et al. Praeruptorin D and E attenuate lipopolysaccharide/hydrochloric acid induced acute lung injury in mice. Eur. J. Pharmacol. 2013, 710, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Ho, Y.L.; Chen, C.Y.; Hsieh, W.T.; Chang, Y.S.; Huang, G.J. Lobeline improves acute lung injury via nuclear factor-κB-signaling pathway and oxidative stress. Respir. Physiol. Neurobiol. 2016, 225, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Yang, J.J.; Yang, M.L.; Li, Y.C.; Kuan, Y.H. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free Radic. Biol. Med. 2014, 69, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chang, H.Y.; Deng, J.S.; Chen, J.J.; Huang, S.S.; Lin, I.H.; Kuo, W.L.; Chao, W.; Huang, G.J. Hispolon from Phellinus linteus induces G0/G1 cell cycle arrest and apoptosis in NB4 human leukaemia cells. Am. J. Chin. Med. 2013, 41, 1439–1457. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Huang, S.S.; Deng, J.S. Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo. PLoS ONE 2012, 7, e35922. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Dai, Y.C.; Hattori, T.; Yu, T.W.; Wang, D.M.; Parmasto, E.; Chang, H.Y.; Shin, S.Y. Species clarification for the medicinally valuable ‘sanghuang’ mushroom. Bot. Stud. 2012, 53, 135–149. [Google Scholar]
- Zhou, L.W.; Vlasák, J.; Decock, C.; Assefa, A.; Stenlid, J.; Abate, D.; Wu, S.H.; Dai, Y.C. Global diversity and taxonomy of the Inonotus linteus complex (Hymenochaetales, Basidiomycota): Sanghuangporus gen. nov., Tropicoporus excentrodendri and T. guanacastensis gen. et spp. nov., and 17 new combinations. Fungal Divers. 2016, 77, 335–347. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Lee, S.; Cho, J.; Ze, K.; Sung, J.; Kim, Y.C. Mycelial culture of Phellinus linteus protectsprimary cultured rat hepatocytes against hepatotoxins. J. Ethnopharmacol. 2004, 95, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.W.; Wu, J.B.; Wu, Y.C. Chemistry and biology of Phellinus linteus. BioMedicine 2013, 3, 106–113. [Google Scholar] [CrossRef]
- Shie, P.H.; Wang, S.Y.; Lay, H.L.; Huang, G.J. 4,7-dimethoxy-5-methyl-1,3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-κB and induction HO-1 in RAW264.7 cells. Int. Immunopharmacol. 2016, 31, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, H.; Yang, T.; Ye, Y.; Shan, J.; Yin, Z.; Luo, L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 2010, 41, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, B.; Chen, X.J.; Xu, D.Q.; Wang, Y.X.; Dong, H.Y.; Ma, S.R.; Sun, R.H.; Hui, Y.P.; Li, Z.C. Osthole protects lipopolysaccharide-induced acute lung injury in mice by preventing down-regulation of angiotensin-converting enzyme 2. Eur. J. Pharm. Sci. 2013, 48, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.K.; Yang, C.Y.; Tsai, T.H.; Hsieh, C.L. Moutan cortex radices improves lipopolysaccharide-induced acute lung injury in rats through anti-inflammation. Phytomedicine 2012, 19, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Chen, C.C.; Huang, C.J.; Sung, P.J.; Huang, S.S.; Kuo, Y.H. Methanol extract of Antrodia camphorata protects against lipopolysaccharide-induced acute lung injury by suppressing NF-κB and MAPK pathways in mice. J. Agric. Food Chem. 2014, 62, 5321–5329. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhu, L.; Wang, J.; He, H.; Chang, X.; Gao, J.; Shumin, W.; Yan, T. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2015, 168, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gui, Y.S.; Tian, X.L.; Cai, B.Q.; Wang, D.T.; Zhang, D.; Zhao, H.; Xu, K.F. Inactivation of mammalian target of rapamycin (mTOR) by rapamycin in a murine model of lipopolysaccharide-induced acute lung injury. Chin. Med. J. 2011, 124, 3112–3117. [Google Scholar] [PubMed]
- Li, K.C.; Ho, Y.L.; Hsieh, W.T.; Huang, S.S.; Chang, Y.S.; Huang, G.J. Apigenin-7-Glycoside Prevents LPS-Induced Acute Lung Injury via Downregulation of Oxidative Enzyme Expression and Protein Activation through Inhibition of MAPK Phosphorylation. Int. J. Mol. Sci. 2015, 16, 1736–1754. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Yin, H.; Fang, M.; Xiang, Y.; Yuan, C.L.; Zheng, G.Y.; Yang, H.; Xiong, P.; Chen, G.; Gong, F.L. Heme oxygenase-1 upregulation significantly inhibits TNF-α and Hmgb1releasing and attenuates lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2008, 8, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 4, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Shie, P.H.; Huang, S.S.; Deng, J.S.; Huang, G.J. Spiranthes sinensis Suppresses Production of Pro-Inflammatory Mediators by Down-Regulating the NF-κB Signaling Pathway and Up-Regulating HO-1/Nrf2 Anti-Oxidant Protein. Am. J. Chin. Med. 2015, 43, 969–989. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, N.; Shukla, S.; Kanwal, R.; Srivastava, J.K.; Gupta, S. Induction of heme oxygenase-1by chamomile protects murine macrophages against oxidative stress. Life Sci. 2012, 90, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Choi, H.J.; Lee, D.S.; Oh, H.; Kim, Y.C.; Moon, J.Y.; Park, W.H.; Park, S.D.; Kim, J.E. Amomum Tsao-ko suppresses lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages via Nrf2-dependent heme oxygenase-1 expression. Am. J. Chin. Med. 2014, 42, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.S.; Huang, S.S.; Lin, T.H.; Lee, M.M.; Kuo, C.C.; Sung, P.J.; Hou, W.C.; Huang, G.J.; Kuo, Y.H. The analgesic and anti-inflammatory bioactivities of eburicoic acid and dehydroeburicoic acid isolated from Antrodia camphorata on the inflammatory mediator expression in mice. J. Agric. Food Chem. 2013, 61, 5064–5071. [Google Scholar] [CrossRef] [PubMed]
- San, Z.; Fu, Y.; Li, W.; Zhou, E.; Li, Y.; Song, X.; Wang, T.; Tian, Y.; Wei, Z.; Yao, M.; et al. Protective effect of taraxasterol on acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2014, 19, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Lewis, J.B.; McCloud, V.V.; Shaw, M.; Omata, Y.; Lockwood, P.E.; Messer, R.L.; Hansen, J.M. Effect of mercury(II) on Nrf2, thioredoxin reductase-1 and thioredoxin-1 in human monocytes. Dent. Mater. 2008, 24, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Sueblinvong, V.; Mills, S.T.; Neujahr, D.C.; Go, Y.M.; Jones, D.P.; Guidot, D.M. Nuclear Thioredoxin-1 Overexpression Attenuates Alcohol-Mediated Nrf2 Signaling and Lung Fibrosis. Alcohol. Clin. Exp. Res. 2016, 40, 1846–1856. [Google Scholar] [CrossRef] [PubMed]
- Yayeh, T.; Hong, M.; Jia, Q.; Lee, Y.C.; Kim, H.J.; Hyun, E.; Kim, T.W.; Rhee, M.H. Pistaciachinensis inhibits NO production and upregulates HO-1 induction via PI3K/Akt pathway in LPS stimulated macrophage cells. Am. J. Chin. Med. 2012, 40, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Kamitani, S.; Togi, S.; Ikeda, O.; Nakasuji, M.; Sakauchi, A.; Sekine, Y.; Muromoto, R.; Oritani, K.; Matsuda, T. Krüppel-associated box-associated protein 1 negatively regulates TNF-α-induced NF-κB transcriptional activity by influencing the interactions among STAT3, p300, and NF-κB/p65. J. Immunol. 2011, 187, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Tsuruma, R.; Ohbayashi, N.; Kamitani, S.; Ikeda, O.; Sato, N.; Muromoto, R.; Sekine, Y.; Oritani, K.; Matsuda, T. Physical and functional interactions between STAT3 and KAP1. Oncogene 2008, 27, 3054–3059. [Google Scholar] [CrossRef] [PubMed]
- Gegotek, A.; Niklinski, J.; Charkiewicz, R.; Bielawska, K.; Kozlowski, M.; Skrzydlewska, E. Relationships between level of lipid peroxidation products and expression of Nrf2 and its activators/ inhibitors in non-small cell lung cancer tissue. Free Radic. Biol. Med. 2014, 75 (Suppl. 1), S31. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-C.; Deng, J.-S.; Huang, S.-S.; Wu, S.-H.; Chen, C.-C.; Lin, W.-R.; Lin, H.-Y.; Huang, G.-J. Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium. Int. J. Mol. Sci. 2017, 18, 347. https://doi.org/10.3390/ijms18020347
Lin W-C, Deng J-S, Huang S-S, Wu S-H, Chen C-C, Lin W-R, Lin H-Y, Huang G-J. Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium. International Journal of Molecular Sciences. 2017; 18(2):347. https://doi.org/10.3390/ijms18020347
Chicago/Turabian StyleLin, Wang-Ching, Jeng-Shyan Deng, Shyh-Shyun Huang, Sheng-Hua Wu, Chin-Chu Chen, Wan-Rong Lin, Hui-Yi Lin, and Guan-Jhong Huang. 2017. "Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium" International Journal of Molecular Sciences 18, no. 2: 347. https://doi.org/10.3390/ijms18020347