Azacitidine for Front-Line Therapy of Patients with AML: Reproducible Efficacy Established by Direct Comparison of International Phase 3 Trial Data with Registry Data from the Austrian Azacitidine Registry of the AGMT Study Group
Abstract
:1. Introduction
2. Results
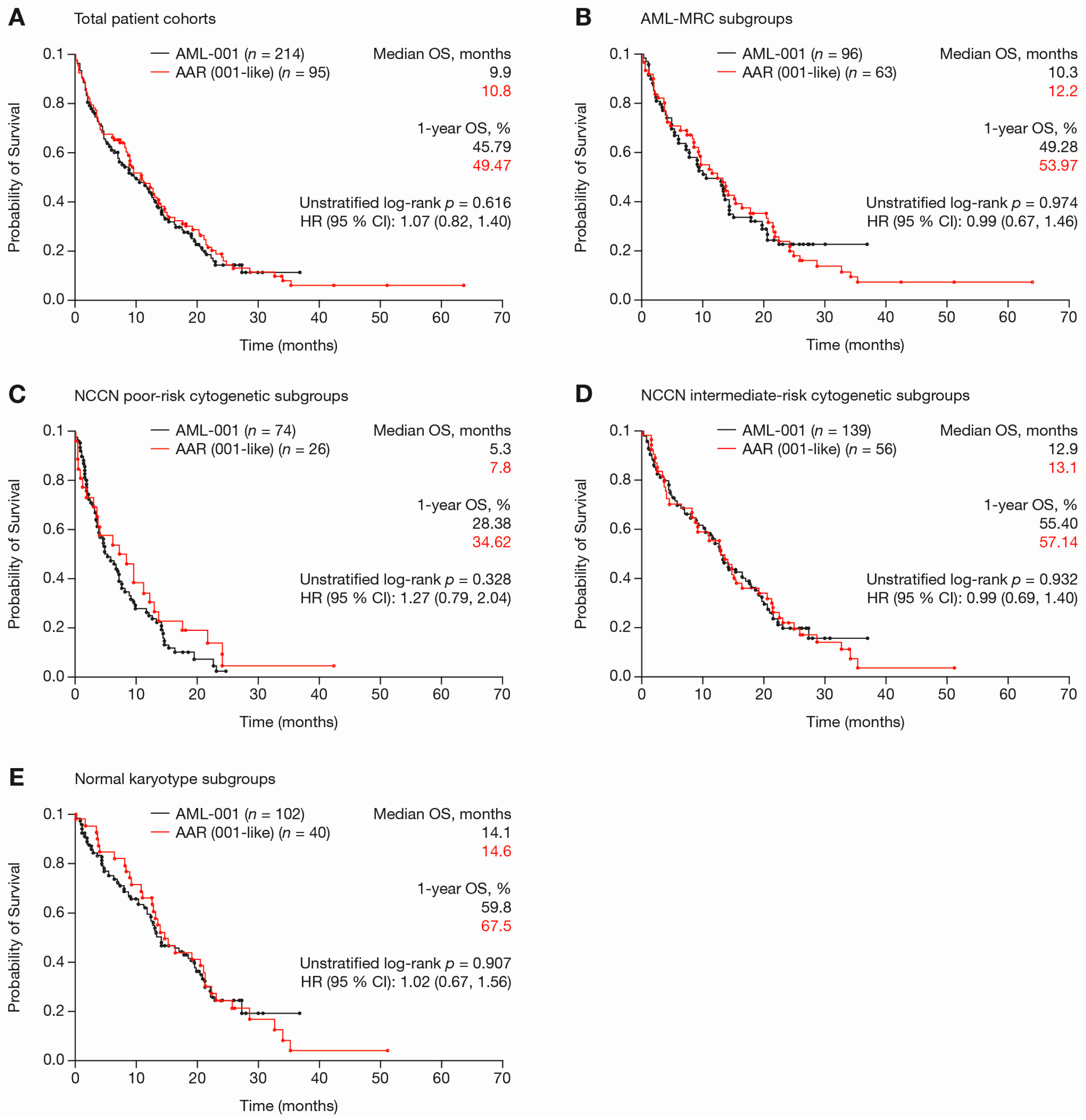
2.1. Clinical Trial versus Registry Subsets
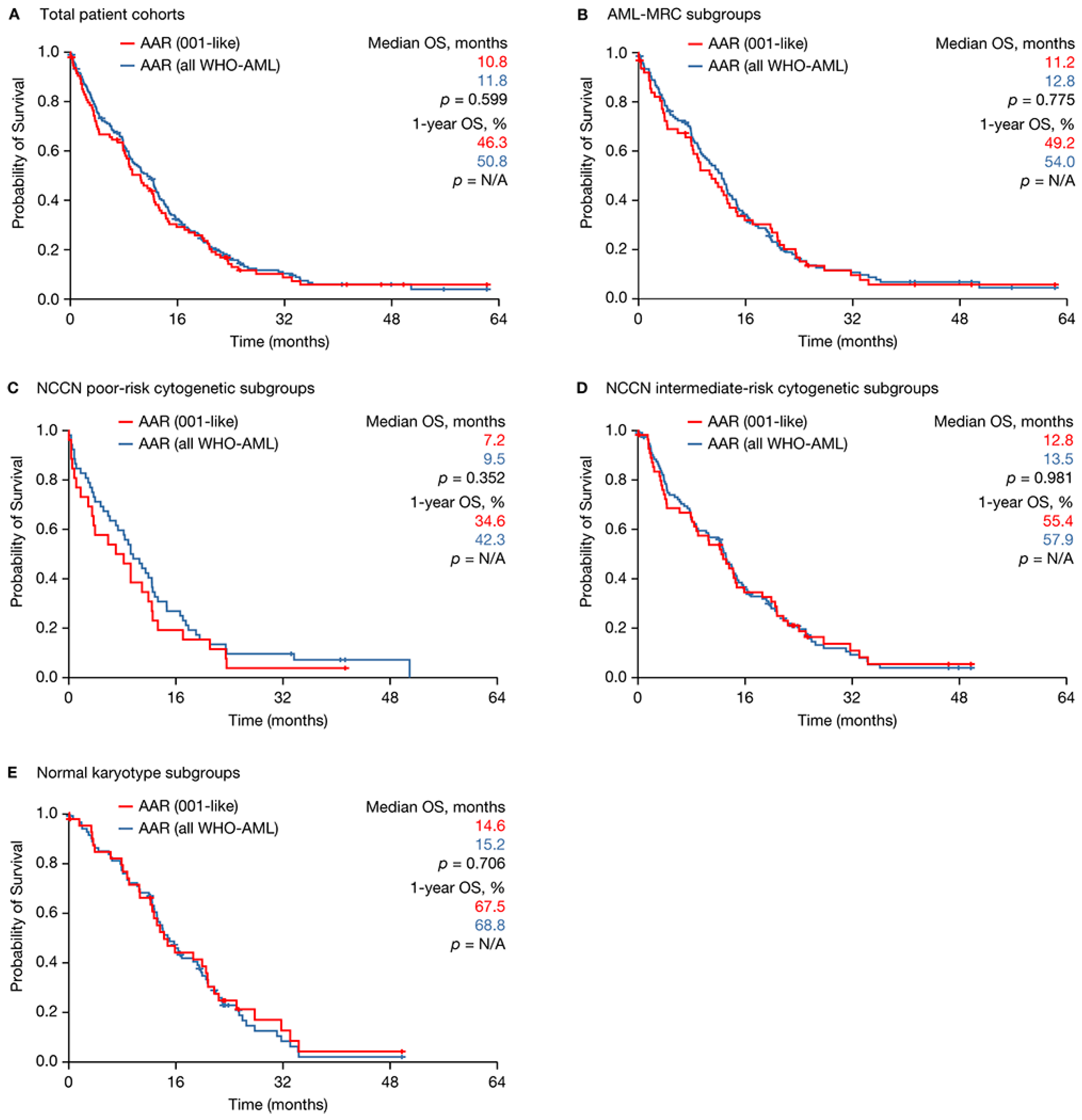
2.2. Registry Subsets Meeting Clinical Trial Inclusion Criteria versus All WHO-AML Patients
3. Discussion
4. Materials and Methods
4.1. Design and Aim of the Study
4.2. Setting of the Study
4.3. Definitions and Endpoints
4.4. Characteristics of Participants
4.5. Processes, Interventions and Comparisons
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAR | Austrian Azacitidine Registry |
| AE | Adverse event |
| AGMT | Arbeitsgemeinschaft Medikamentöse Tumortherapie |
| AHRQ | Agency for Healthcare Research and Quality |
| AML | Acute myeloid leukaemia |
| ANC | Absolute neutrophil count |
| AZA | Azacitidine |
| BM | Bone marrow |
| BSC | Best supportive care |
| CI | Confidence interval |
| CMML | Chronic myelomonocytic leukaemia |
| CR | Complete response |
| CRi | Morphologic CR with incomplete blood count recovery |
| ECOG-PS | Eastern Cooperative Oncology Group Performance Status |
| eCRF | Electronic case report form |
| EFS | Event-free survival |
| EMA | European Medicines Agency |
| HSCT | Hematopoietic stem cell transplantation |
| Hb | Haemoglobin |
| HR | Hazards ratio |
| IWG | International Working Group |
| KM | Kaplan–Meier |
| MDS | Myelodysplastic syndromes |
| MPN | Myeloproliferative neoplasm |
| MRC | Myelodysplasia-related changes |
| NCCN | National Comprehensive Cancer Network |
| NOS | Not otherwise specified |
| ORR | Overall response rate |
| OS | Overall survival |
| PLT | Platelet |
| PR | Partial response |
| pts | Patients |
| RBC | Red blood cell |
| RCA | Recurrent cytogenetic abnormalities |
| RFS | Relapse-free survival |
| SD | Standard deviation |
| t-AML | Therapy-related AML |
| TD | Transfusion dependence |
| TEAE | Treatment-emergent adverse event |
| TI | Transfusion independence |
| vs. | Versus |
| WBC | White blood cell |
| WHO | World Health Organisation |
References
- Klepin, H.D.; Balducci, L. Acute myelogenous leukemia in older adults. Oncologist 2009, 14, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and acute myeloid leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef] [PubMed]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Giles, F.J.; Borthakur, G.; Ravandi, F.; Faderl, S.; Verstovsek, S.; Thomas, D.; Wierda, W.; Ferrajoli, A.; Kornblau, S.; Pierce, S.; et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br. J. Haematol. 2007, 136, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.; Hörstedt, A.S.; Johansson, B.; Antunovic, P.; Billström, R.; Derolf, Å.; Hulegårdh, E.; Lehmann, S.; Möllgård, L.; Nilsson, C.; et al. Incidence and prognostic significance of karyotypic subgroups in older patients with acute myeloid leukemia: The swedish population-based experience. Blood Cancer J. 2014, 4, e188. [Google Scholar] [CrossRef] [PubMed]
- Oran, B.; Weisdorf, D.J. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica 2012, 97, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Gattermann, N.; Germing, U.; Sanz, G.; List, A.F.; Gore, S.; Seymour, J.F.; et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Silverman, L.R.; McKenzie, D.R.; Peterson, B.L.; Holland, J.F.; Backstrom, J.T.; Beach, C.L.; Larson, R.A. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the cancer and leukemia group B. J. Clin. Oncol. 2006, 24, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, L.; Stauder, R.; Burgstaller, S.; Schreder, M.; Tinchon, C.; Pfeilstocker, M.; Steinkirchner, S.; Melchardt, T.; Mitrovic, M.; Girschikofsky, M.; et al. Azacitidine in patients with who-defined AML—Results of 155 patients from the Austrian azacitidine registry of the AGMT-study group. J. Hematol. Oncol. 2013, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, L.; Burgstaller, S.; Girschikofsky, M.; Linkesch, W.; Stauder, R.; Pfeilstöcker, M.; Schreder, M.; Tinchon, C.; Sliwa, T.; Lang, A.; et al. Azacitidine in 302 patients with who-defined acute myeloid leukemia: Results from the Austrian azacitidine registry of the AGMT-study group. Ann. Hematol. 2014, 93, 1825–1838. [Google Scholar] [PubMed]
- Pleyer, L.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Sill, H.; Schlick, K.; Thaler, J.; Halter, B.; Hherndl-Spandl, S.; Zebisch, A.; et al. Azacitidine front-line in 339 patients with myelodysplastic syndromes and acute myeloid leukaemia: Comparison of French-American-British and World Health Organization classifications. J. Hematol. Oncol. 2016, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, L.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Linkesch, W.; Pfeilstöcker, M.; Autzinger, E.M.; Tinchon, C.; Sliwa, T.; Lang, A.; et al. Azacitidine in patients with acute myeloid leukemia: Assessing the potential negative impact of elevated baseline white blood cell count on outcome. Blood 2014, 124. abstract 3683. [Google Scholar]
- Pleyer, L.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Sill, H.; Schlick, K.; Thaler, J.; Halter, B.; Machherndl-Spandl, S.; Zebisch, A.; et al. Azacitidine in acute myeloid leukemia with >30% bone marrow blasts and <15 g/L white blood cell count: Results from the Austrian Azacitidine Registry of the AGMT study group versus randomized controlled phase III clinical trial data. Blood 2015, 126. abstract 2515. [Google Scholar]
- Pleyer, L.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Linkesch, W.; Pfeilstöcker, M.; Autzinger, E.M.; Tinchon, C.; Sliwa, T.; Lang, A.; et al. Azacitidine in patients with treatment-related acute myeloid leukemia: Retrospective analysis of the Austrian Azacitidine Registry. Blood 2014, 124. abstract 2284. [Google Scholar]
- Pleyer, L.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Linkesch, W.; Pfeilstöcker, M.; Autzinger, E.M.; Tinchon, C.; Sliwa, T.; Lang, A.; et al. Azacitidine in acute myeloid leukemia: Comparison of patients with AML-MRF vs. AML-NOS enrolled in the Austrian Azacitidine Registry. Blood 2014, 124. abstract 3681. [Google Scholar]
- Pleyer, L.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Linkesch, W.; Pfeilstöcker, M.; Autzinger, E.M.; Tinchon, C.; Sliwa, T.; Lang, A.; et al. Azacitidine in patients with acute myeloid leukemia: Impact of intermediate-risk vs. high-risk cytogenetics on patient outcomes. Blood 2014, 124. abstract 955. [Google Scholar]
- Pleyer, L.; Germing, U.; Sperr, W.R.; Linkesch, W.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Schreder, M.; Pfeilstocker, M.; Lang, A.; et al. Azacitidine in CMML: Matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leuk. Res. 2014, 38, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, L.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Linkesch, W.; Pfeilstöcker, M.; Autzinger, E.M.; Tinchon, C.; Sliwa, T.; Lang, A.; et al. Azacitidine in patients with relapsed/refractory acute myeloid leukemia: Retrospective analysis of the Austrian Azacitidine Registry. Blood 2014, 124. abstract 943. [Google Scholar]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs. conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Mosenifar, Z. Population issues in clinical trials. Proc. Am. Thorac. Soc. 2007, 4, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Denson, A.C.; Mahipal, A. Participation of the elderly population in clinical trials: Barriers and solutions. Cancer Control 2014, 21, 209–214. [Google Scholar] [PubMed]
- Bernal, T.; Martinez-Camblor, P.; Sanchez-Garcia, J.; de Paz, R.; Luno, E.; Nomdedeu, B.; Ardanaz, M.T.; Pedro, C.; Amigo, M.L.; Xicoy, B.; et al. Effectiveness of azacitidine in unselected high-risk myelodysplastic syndromes: Results from the Spanish registry. Leukemia 2015, 29, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Dinmohamed, A.G.; van Norden, Y.; Visser, O.; Posthuma, E.F.M.; Huijgens, P.C.; Sonneveld, P.; van de Loosdrecht, A.A.; Jongen-Lavrencic, M. Effectiveness of azacitidine for the treatment of higher-risk myelodysplastic syndromes in daily practice: Results from the Dutch population-based PHAROS MDS registry. Leukemia 2015, 29, 2449–2451. [Google Scholar] [CrossRef] [PubMed]
- Ostgard, L.S.; Norgaard, M.; Sengelov, H.; Medeiros, B.C.; Kjeldsen, L.; Overgaard, U.M.; Severinsen, M.T.; Marcher, C.W.; Jensen, M.K.; Norgaard, J.M. Improved outcome in acute myeloid leukemia patients enrolled in clinical trials: A national population-based cohort study of danish intensive chemotherapy patients. Oncotarget 2016, 7, 72044–72056. [Google Scholar] [PubMed]
- Gahn, B.; Haase, D.; Unterhalt, M.; Drescher, M.; Schoch, C.; Fonatsch, C.; Terstappen, L.W.; Hiddemann, W.; Buchner, T.; Bennett, J.M.; et al. De novo aml with dysplastic hematopoiesis: Cytogenetic and prognostic significance. Leukemia 1996, 10, 946–951. [Google Scholar] [PubMed]
- Miesner, M.; Haferlach, C.; Bacher, U.; Weiss, T.; Macijewski, K.; Kohlmann, A.; Klein, H.U.; Dugas, M.; Kern, W.; Schnittger, S.; et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: A comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “aml with myelodysplasia-related changes” (AML-MRC). Blood 2010, 116, 2742–2751. [Google Scholar] [PubMed]
- Food and Drug Administration. Guidance for Industry and Food and Drug Administration Staff—Postmarket Surveillance under Section 522 of the Federal Food, Drug and Cosmetic Act. 2016. Available online: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm268141.pdf (accessed on 10 February 2017). [Google Scholar]
- Juliusson, G.; Lazarevic, V.; Horstedt, A.S.; Hagberg, O.; Hoglund, M. Acute myeloid leukemia in the real world: Why population-based registries are needed. Blood 2012, 119, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Lawyer, P. Improving Health Care Value: The Case for Disease Registries. (The Boston Consulting Group). 2011. Available online: http://2eic.com/sites/default/files/bcg_-_registries_can_add_health_care_value.pdf (accessed on 11 January 2017).
- Noe, L.; Larson, L.; Trotter, J. Utilizing Patient Registries to Support Health Economics Research: Integrating Observational Data with Economic Analyses, Models, and Other Applications. Available online: https://www.ispor.org/news/articles/oct05/patient_registr.asp (accessed on 11 January 2017).
- Stark, N.J. Registry Studies: Why and How? Available online: http://clinicaldevice.typepad.com/cdg_whitepapers/2011/07/registry-studies-why-and-how.html (accessed on 11 January 2017).
- European Medicines Agency. Patient Registries. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000658.jsp (accessed on 21 September 2016).
- Thepot, S.; Itzykson, R.; Seegers, V.; Recher, C.; Raffoux, E.; Quesnel, B.; Delaunay, J.; Cluzeau, T.; Marfaing Koka, A.; Stamatoullas, A.; et al. Azacitidine in untreated acute myeloid leukemia. A report on 149 patients. Am. J. Hematol. 2014, 89, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Dinmohamed, A.G.; van Norden, Y.; Visser, O.; Posthuma, E.F.; Huijgens, P.C.; Sonneveld, P.; van de Loosdrecht, A.A.; Jongen-Lavrencic, M. The use of medical claims to assess incidence, diagnostic procedures and initial treatment of myelodysplastic syndromes and chronic myelomonocytic leukemia in The Netherlands. Leuk. Res. 2015, 39, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Ostgard, L.S.; Norgaard, J.M.; Severinsen, M.T.; Sengelov, H.; Friis, L.; Jensen, M.K.; Nielsen, O.J.; Norgaard, M. Data quality in the Danish national acute leukemia registry: A hematological data resource. Clin. Epidemiol. 2013, 5, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Dinmohamed, A.G.; Brink, M.; Visser, O.; Jongen-Lavrencic, M. Population-based analyses among 184 patients diagnosed with large granular lymphocyte leukemia in The Netherlands between 2001 and 2013. Leukemia 2016, 30, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Seymour, J.F.; Butrym, A.; Willemze, R.; Selleslag, D.; Jang, J.H.; Cavenagh, J.; Kumar, R.; Schuh, A.C.; Candoni, A.; et al. Overall survival in older patients with newly diagnosed acute myeloid leukemia (AML) with >30% bone marrow blasts treated with azacitidine by cytogenetic risk status: Results of the AZA-AML-001 study. Blood 2014, 124. abstract 621. [Google Scholar]
- Seymour, J.F.; Döhner, H.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Cavenagh, J.D.; Kumar, R.; Schuh, A.C.; Candoni, A.; et al. Azacitidine (AZA) versus conventional care regimens (CCR) in older patients with newly diagnosed acute myeloid leukemia (>30% bone marrow blasts) with morphologic dysplastic changes: A subgroup analysis of the AZA-AML-001 trial. Blood 2014, 124, 10. [Google Scholar]
- Seymour, J.F.; Döhner, H.; Schuh, A.C.; Stone, R.M.; Minden, M.; Weaver, J.; Songer, S.; Beach, C.L.; Dombret, H. Azacitidine (AZA) vs. Conventional Care Regimens (CCR) in Patients with Acute Myeloid Leukemia (AML) with Myelodyspasia-Related Changes (MRC) in AZA-AML-001 per Central Review. Available online: http://learningcenter.ehaweb.org/eha/2016/21st/133461/john.f.seymour.azacitidine.28aza29.vs.conventional.care.regimens.28ccr29.in.html?f=p16m3l9759 (accessed on 9 February 2017).
- Schuh, A.; Dombret, H.; Sandhu, I.; Seymour, J.F.; Stone, R.M.; Kathrin Al-Ali, H.; Alimena, G.; Lewis, I.; Kyun, S.S.; Geddes, M.; et al. Overall Survival (OS) without Complete Remission (CR) in Older Patients with Acute Myeloid Leukemia (AML): Azacitidine (aza) vs. Conventional Care Regimens (CCR) in the AZAAML001 Study. Available online: http://learningcenter.ehaweb.org/eha/2015/20th/100716/%5B%5B$item.link%5D%5D (accessed on 9 February 2017).
- Ramos, F.; Thepot, S.; Pleyer, L.; Maurillo, L.; Itzykson, R.; Bargay, J.; Stauder, R.; Venditti, A.; Seegers, V.; Martinez-Robles, V.; et al. Azacitidine frontline therapy for unfit acute myeloid leukemia patients: Clinical use and outcome prediction. Leuk. Res. 2015, 39, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Tombak, A.; Ucar, M.A.; Akdeniz, A.; Tiftik, E.N.; Şahin, D.G.; Akay, O.M.; Yıldırım, M.; Nevruz, O.; Kış, C.; Gürkan, E.; et al. The role of azacitidine in the treatment of elderly patients with AML—Results of a retrospective multicenter study. Turk. J. Haematol. 2016, 33, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sudan, N.; Rossetti, J.M.; Shadduck, R.K.; Latsko, J.; Lech, J.A.; Kaplan, R.B.; Kennedy, M.; Gryn, J.F.; Faroun, Y.; Lister, J. Treatment of acute myelogenous leukemia with outpatient azacitidine. Cancer 2006, 107, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.J.; Seftel, M.D.; Richardson, C.; Barbaric, D.; Barnett, M.J.; Bruyere, H.; Forrest, D.L.; Horsman, D.E.; Smith, C.; Song, K.; et al. Leukocyte count as a predictor of death during remission induction in acute myeloid leukemia. Leuk. Lymph. 2006, 47, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mazzola, E.; Neuberg, D.; Allen, S.L.; Pigneux, A.; Stuart, R.K.; Wetzler, M.; Rizzieri, D.; Erba, H.P.; Damon, L.; et al. Phase III open-label randomized study of cytarabine in combination with amonafide L-malate or daunorubicin as induction therapy for patients with secondary acute myeloid leukemia. J. Clin. Oncol. 2015, 33, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, H.T.; Lash, T.L.; Rothman, K.J. Beyond randomized controlled trials: A critical comparison of trials with nonrandomized studies. Hepatology 2006, 44, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Mollgard, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Hoglund, M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Hulegardh, E.; Nilsson, C.; Lazarevic, V.; Garelius, H.; Antunovic, P.; Rangert, D.A.; Mollgard, L.; Uggla, B.; Wennstrom, L.; Wahlin, A.; et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: A report from the Swedish acute leukemia registry. Am. J. Hematol. 2015, 90, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gross, C.P.; Maggiore, R.J.; Halene, S.; Soulos, P.R.; Raza, A.; Galili, N.; Ma, X. Pattern of hypomethylating agents use among elderly patients with myelodysplastic syndromes. Leuk. Res. 2011, 35, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Wang, R.; Davidoff, A.J.; Ma, S.; Zhao, Y.; Gore, S.D.; Gross, C.P.; Ma, X. Disease-related costs of care and survival among medicare-enrolled patients with myelodysplastic syndromes. Cancer 2016, 122, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- NCI. Seer Cancer Statistics Review 1975–2013. Available from: http://seer.Cancer.Gov/csr/1975_2013/browse_csr.Php?Sectionsel=13&pagesel=sect_13_table.13.html (accessed on 27 July 2016).
- Pleyer, L.; Stauder, R.; Thaler, J.; Ludwig, H.; Pfeilstöcker, M.; Steinkirchner, T.; Melchardt, T.; Weltermann, A.; Lang, A.; Linkesch, W.; et al. Overall survival data of patients with MDS, AML and CMML from the Austrian Azacitidine Registry of 184 consecutive patients. Leuk. Res. 2011, 35, 100. [Google Scholar] [CrossRef]
- Pleyer, L.; Stauder, R.; Thaler, J.; Ludwig, H.; Pfeilstocker, M.; Steinkirchner, S.; Melchardt, T.; Weltermann, A.; Lang, A.; Linkesch, W.; et al. Age- and comorbidity-specific evaluation of azacitidine treatment, response and overall survival in 184 patients in the Austrian Azacitidine Registry. Leuk. Res. 2011, 35, 101. [Google Scholar] [CrossRef]
- Gliklich, R.E.; Dreyer, N.A.; Leavy, M.B. Registries for Evaluating Patient Outcomes: A User’s Guide. Agency for Healthcare research and Quality (AHRQ): Rockville, MD, USA, 2014. [Google Scholar]
- Wandt, H.; Schakel, U.; Kroschinsky, F.; Prange-Krex, G.; Mohr, B.; Thiede, C.; Pascheberg, U.; Soucek, S.; Schaich, M.; Ehninger, G. MLD according to the WHO classification in AML has no correlation with age and no independent prognostic relevance as analyzed in 1766 patients. Blood 2008, 111, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology for Acute Myeloid Leukemia. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed on 10 February 2017).
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Buchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- NCI Common Terminology Criteria for Adverse Events (CTCAE). Available online: http://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed on 9 February 2017).
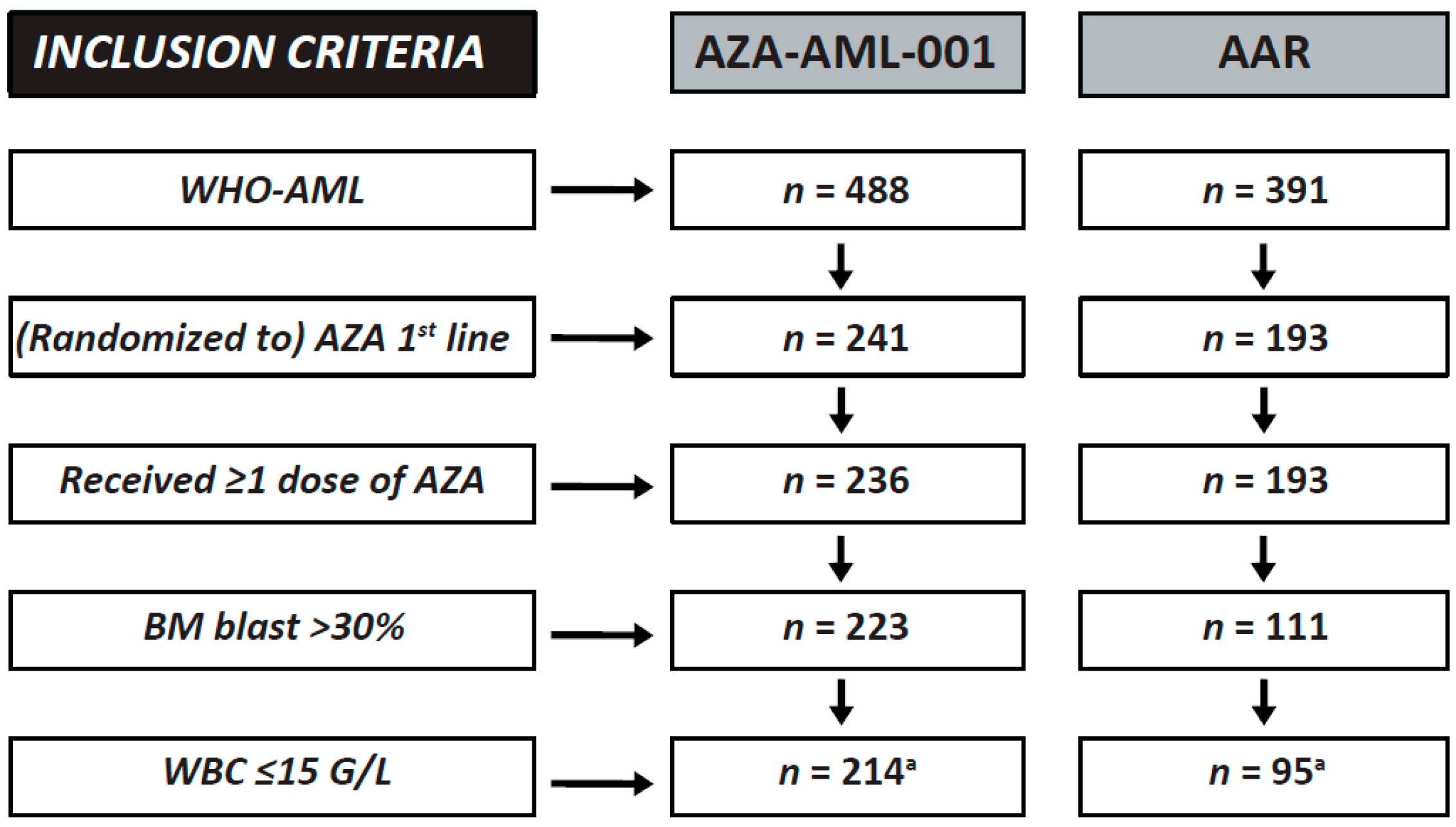
| Baseline Characteristics | AML-001 Trial | AAR (001-Like) | AAR (WHO-AML) |
|---|---|---|---|
| Subset | Subset | Subset | |
| (n = 214) | (n = 95) | (n = 193) | |
| Age, median (mean) [SD], years | 76 (75.5) [5.6] | 77 (75.2) [11.5] | 77 (75.6) [10.2] |
| Age ≥75 years, % | 58.4 | 56.8 | 58.5 |
| Male, % | 57.5 | 54.7 | 58.6 |
| ECOG-PS, % | |||
| 0–1 | 76.6 | 67.3 | 67.9 |
| 2 | 23.4 | 23.2 | 24.4 |
| 3–4 a | 0 | 9.5 | 1.6 |
| AML classification, % | |||
| AML-MRC b | 57.0 | 66.3 | 70.8 |
| AML-NOS | 37.3 | 24.2 | 18.8 |
| AML-RCA | 2.3 | 4.2 | 3.7 |
| t-AML | 3.3 | 5.3 | 6.8 |
| Antecedent haematological disease, % | 19.6 | 25.3 | 31.6 |
| Prior MDS, % | 19.6 | 21.1 | 24.9 |
| NCCN cytogenetic risk, % | |||
| Good c | 0 | 2.1 | 2.4 |
| Intermediate | 65.0 | 58.9 | 66.9 |
| Normal | 47.7 | 42.1 | 47.3 |
| Poor | 34.6 | 27.4 | 30.8 |
| BM blasts, median (range), % | 73.0 (31–100) | 55.5 (31–96) | 40.0 (20–100) |
| BM blasts ≥50%, % | 78.0 | 58.9 | 38.9 |
| Number of cytopenias, % | |||
| 0–1 | 13.1 | 14.7 | 16.6 |
| 2–3 | 86.9 | 85.3 | 83.4 |
| RBC-TD, % | 70.1 | 60.0 | 52.9 |
| PLT-TD, % | 40.2 | 30.5 | 24.9 |
| WBC, median (range), G/L | 3.0 (0.3–14.7) | 2.1 (0.6–14.4) | 2.5 (0.6–74.1) |
| Hb, median (range), g/dL | 9.5 (5.0–13.4) | 9.1 (5.8–13.6) | 9.1 (5.8–14.2) |
| ANC, median (range), G/L | 0.3 (0–5.3) | 0.5 (0–7.7) | 0.6 (0–37.2) |
| PLT, median (range), G/L | 54.0 (3–585) | 49.0 (7–1.270) | 52.0 (6–1,270) |
| Treatment Characteristics | AML-001 | AAR | AAR |
|---|---|---|---|
| Trial | (001-Like) | (WHO-AML) | |
| (n = 214) | (n = 95) | (n = 193) | |
| AZA cycles, median, n | 6 | 5 | 6 |
| (Mean) [SD] | (8.4) [7.1] | (8.5) [9.1] | (8.4) [6.0] |
| AZA cycles ≥6, % | 50.0 | 46.3 | 51.3 |
| AZA cycles ≥12, % | 28.5 | 24.2 | 24.9 |
| Days of AZA application, median, days | 42 | 34 | 39 |
| (Mean) [SD] | (58.0) [49.8] | (55.8) [61.1] | (57.1) [57.3] |
| Daily of AZA dose, median, mg | 130.1 | 131.6 | 132.0 |
| (Mean) [SD] | (129.4) [17.8] | (128.7) [26.5] | (126.4) [33.3] |
| Reasons for AZA discontinuation, % | |||
| AE/no response/relapse/PD/death | 66.8 | 74.8 | 73.1 |
| Withdrew consent/patient’s wish | 11.7 | 9.5 | 7.3 |
| Others | 11.7 | 9.5 | 11.4 |
| Still on AZA at study closure | 9.8 | 6.3 | 8.3 |
| Outcome | AML-001 Trial (n = 214) | AAR (001-Like) (n = 95) | p-Value | AAR (001-Like) (n = 95) | AAR (WHO-AML) (n = 193) | p-Value |
|---|---|---|---|---|---|---|
| Median OS, mo | 9.9 | 10.7 | 0.9553 a | 10.7 | 11.8 | 0.599 a |
| Median RFS (CR/CRi), mo | 16.3 | 13.8 | 0.6817 a | 13.8 | 13.3 | 0.621 a |
| Median EFS (all pts), mo | 6.9 | 8.3 | 0.2909 a | 8.3 | 8.1 | 0.941 a |
| Median CR/CRi duration, mo | 8.6 | 11.1 | 0.1740 a | 11.1 | 11.5 | 0.818 a |
| 1-Year survival, % | 54.2 | 53.7 | 0.843 b | 53.7 | 50.8 | 0.476 b |
| 30-Day mortality, % | 7.0 | 8.4 | 0.924 b | 8.4 | 7.8 | 0.848 b |
| ORR (CR, CRi, PR), % | 30.4 | 18.9 | 0.0379 b | 18.9 | 23.1 | 0.685 b |
| RBC-TI, % | 39.3 | 42.1 | 0.7522 b | 42.1 | 42.2 | 0.517 b |
| PLT-TI, % | 37.2 | 35.7 | 1.0000 b | 35.7 | 41.7 | 0.688 b |
| Univariate Analysis | ||
| Baseline Parameter | HR (95% CI) | p-Value |
| Study group (AML-001 vs. AAR) | 1.02 (0.78, 1.32) | 0.8998 |
| Age (as a continuous variable) | 1.02 (1.00, 1.04) | 0.0182 |
| Age (<75 vs. ≥75 years) | 0.70 (0.54, 0.90) | 0.0053 |
| Gender (female vs. male) | 0.82 (0.64, 1.05) | 0.1243 |
| RBC-TD (No vs. Yes) | 0.89 (0.69, 1.16) | 0.3857 |
| PLT-TD (No vs. Yes) | 0.68 (0.53, 0.88) | 0.0028 |
| ECOG-PS (0–1 vs. ≥2) | 0.54 (0.41, 0.71) | <0.001 |
| MDS-related changes present (Yes vs. No) | 0.90 (0.70, 1.16) | 0.4326 |
| Prior MDS (No vs. Yes) | 1.01 (0.74, 1.38) | 0.9366 |
| No. of cytopenias at baseline (0–1 vs. 2–3) | 0.83 (0.58, 1.19) | 0.3082 |
| NCCN cytogenetic risk (Intermediate vs. Poor) | 0.51 (0.39, 0.66) | <0.001 |
| BM blasts (30%–49% vs. ≥50%) | 0.90 (0.69, 1.18) | 0.4511 |
| WBC (as a continuous variable) | 1.00 (0.97, 1.04) | 0.8400 |
| ANC (as a continuous variable) | 1.05 (0.93, 1.17) | 0.4514 |
| PLT count (as a continuous variable) | 1.00 (1.00, 1.00) | 0.1487 |
| Hb (as a continuous variable) | 0.96 (0.88, 1.05) | 0.3344 |
| Multivariate Analysis | ||
| Baseline Covariate | HR (95% CI) | p-Value |
| Age (<75 vs. ≥75 years) | 0.76 (0.58, 0.98) | 0.0366 |
| PLT-TD (No vs. Yes) | 0.69 (0.53, 0.90) | 0.0057 |
| ECOG-PS (0–1 vs. ≥2) | 0.65 (0.48, 0.87) | 0.0041 |
| NCCN cytogenetic risk (Intermediate vs. Poor) | 0.51 (0.39, 0.67) | <0.001 |
| AML-001 vs. AAR a | 1.11 (0.84, 1.47) | 0.4509 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleyer, L.; Döhner, H.; Dombret, H.; Seymour, J.F.; Schuh, A.C.; Beach, C.; Swern, A.S.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; et al. Azacitidine for Front-Line Therapy of Patients with AML: Reproducible Efficacy Established by Direct Comparison of International Phase 3 Trial Data with Registry Data from the Austrian Azacitidine Registry of the AGMT Study Group. Int. J. Mol. Sci. 2017, 18, 415. https://doi.org/10.3390/ijms18020415
Pleyer L, Döhner H, Dombret H, Seymour JF, Schuh AC, Beach C, Swern AS, Burgstaller S, Stauder R, Girschikofsky M, et al. Azacitidine for Front-Line Therapy of Patients with AML: Reproducible Efficacy Established by Direct Comparison of International Phase 3 Trial Data with Registry Data from the Austrian Azacitidine Registry of the AGMT Study Group. International Journal of Molecular Sciences. 2017; 18(2):415. https://doi.org/10.3390/ijms18020415
Chicago/Turabian StylePleyer, Lisa, Hartmut Döhner, Hervé Dombret, John F. Seymour, Andre C. Schuh, CL Beach, Arlene S. Swern, Sonja Burgstaller, Reinhard Stauder, Michael Girschikofsky, and et al. 2017. "Azacitidine for Front-Line Therapy of Patients with AML: Reproducible Efficacy Established by Direct Comparison of International Phase 3 Trial Data with Registry Data from the Austrian Azacitidine Registry of the AGMT Study Group" International Journal of Molecular Sciences 18, no. 2: 415. https://doi.org/10.3390/ijms18020415






