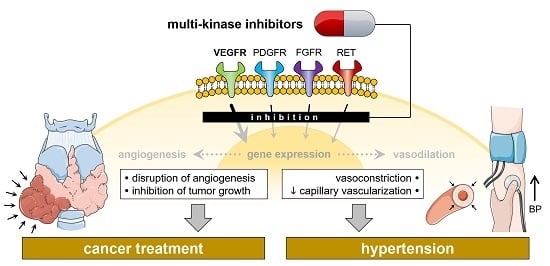
The Adverse Effect of Hypertension in the Treatment of Thyroid Cancer with Multi-Kinase Inhibitors
Abstract
:1. Introduction
2. Background
2.1. Thyroid Cancer
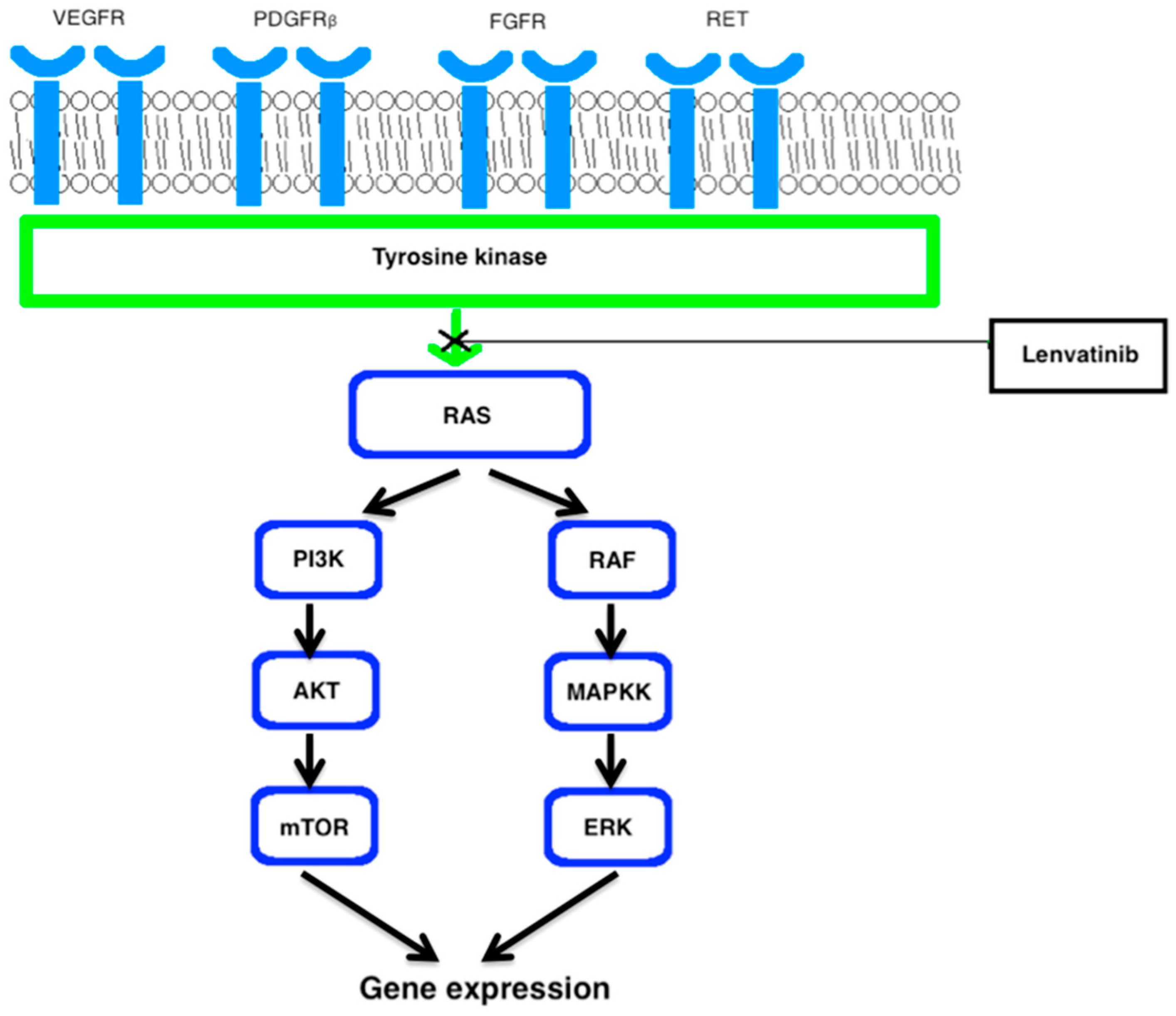
2.2. Multi-Kinase Inhibitors
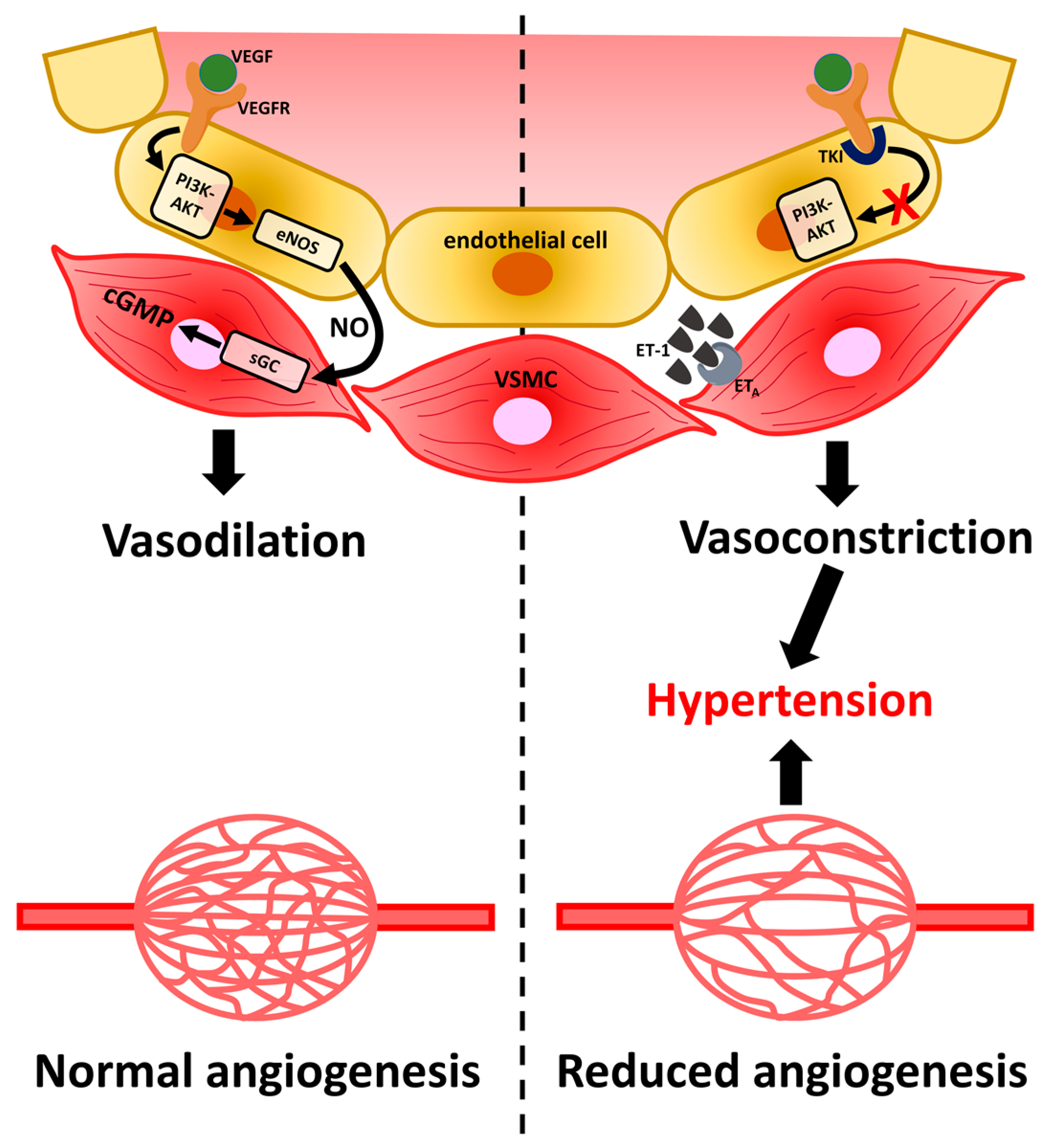
2.3. Hypertension
2.4. Efficacy of Cancer Drug Treatment
2.5. Management of Multi-Kinase Inhibitor-Caused Hypertension
2.6. Discussion
3. Conclusions
4. Outlook
5. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AIIA | angiotensin II receptor antagonists |
| ACEi | angiotensin converting enzyme inhibitors |
| AE | adverse effects |
| AKT | protein kinase B |
| ATC | anaplastic thyroid cancer |
| CCB | calcium channel blockers |
| cGMP | cyclic guanosine monophosphate |
| DTC | differentiated thyroid cancer |
| EGF | endothelial growth factor |
| eNOS | endothelial nitric oxide synthase |
| ERK | extracellular signal regulated kinase |
| ET-1 | endothelin-1 |
| ETA | endothelin receptor type A |
| FGF | fibroblast growth factor |
| FGFR | fibroblast growth factor receptor |
| FTC | follicular thyroid cancer |
| MAPKK | mitogen activated protein kinase kinase |
| MTC | medullary thyroid cancer |
| NO | nitric oxide |
| PDGF | platelet derived growth factor |
| PDGFR | platelet derived growth factor receptor |
| PI3K | phosphatidylinositol-3-kinase |
| PDTC | poorly differentiated thyroid cancer |
| PO | per os |
| PTC | papillary thyroid cancer |
| RAI | radioactive iodine |
| RAS | rat sarcoma |
| RET | ret proto-oncogene |
| sGC | soluble guanylyl cyclase |
| TKI | tyrosine kinase inhibitor |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Costa, R.; Carneiro, B.A.; Chandra, S.; Pai, S.G.; Chae, Y.K.; Kaplan, J.B.; Garrett, H.B.; Agulnik, M.; Kopp, P.A.; Giles1, F.J. Spotlight on lenvatinib in the treatment of thyroid cancer: Patient selection and perspectives. Drug Des. Dev. Ther. 2016, 10, 873–884. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, C.J.; Oucharek, J.; Learoyd, D.; Sidhu, S.B. Standard and emerging therapies for metastatic differentiated thyroid cancer. Oncologist 2010, 15, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Habra, M.A. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat. Rev. 2016, 42, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Whelton, P.K. Elevated systolic blood pressure and risk of cardiovascular and renal disease: Overview of evidence from observational epidemiologic studies and randomized controlled trials. Am. Heart J. 1999, 138, 211–219. [Google Scholar] [CrossRef]
- Thyroid Cancer 2016 Updated 31 March 2016. Available online: http://www.cancer.org/cancer/thyroidcancer/detailedguide/thyroid-cancer-what-is-thyroid-cancer (accessed on 27 December 2016).
- Tiedje, V.; Schmid, K.W.; Weber, F.; Bockisch, A.; Fuhrer, D. Differentiated thyroid cancer. Internist 2015, 56, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Shaha, A.R. Poorly differentiated thyroid cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Chen, H.; Sippel, R.S. Current understanding and management of medullary thyroid cancer. Oncologist 2013, 18, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Krook, K.A.; Fedewa, S.A.; Chen, A.Y. Prognostic indicators in well-differentiated thyroid carcinoma when controlling for stage and treatment. Laryngoscope 2015, 125, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Laursen, R.; Wehland, M.; Kopp, S.; Pietsch, J.; Infanger, M.; Grosse, J.; Grimm, D. Effects and Role of Multi-kinase Inhibitors in Thyroid Cancer. Curr. Pharm. Des. 2016, 22, 5915–5926. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed on 31 December 2016).
- Anderson, R.T.; Linnehan, J.E.; Tongbram, V.; Keating, K.; Wirth, L.J. Clinical, safety, and economic evidence in radioactive iodine-refractory differentiated thyroid cancer: A systematic literature review. Thyroid 2013, 23, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Angiogenesis: Update 2005. J. Thromb. Haemost. 2005, 3, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Infanger, M.; Shakibaei, M.; Kossmehl, P.; Hollenberg, S.M.; Grosse, J.; Faramarzi, S.; Schulze-Tanzil, G.; Paul, M.; Grimm, D. Intraluminal application of vascular endothelial growth factor enhances healing of microvascular anastomosis in a rat model. J. Vasc. Res. 2005, 42, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Baatout, S.; Witzing, A.; Grosse, J.; Bauer, J.; Cogoli, A.; Faramarzi, S.; Derradji, H.; et al. Induction of three-dimensional assembly and increase in apoptosis of human endothelial cells by simulated microgravity: Impact of vascular endothelial growth factor. Apoptosis 2006, 11, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Infanger, M.; Grosse, J.; Westphal, K.; Leder, A.; Ulbrich, C.; Paul, M.; Grimm, D. Vascular endothelial growth factor induces extracellular matrix proteins and osteopontin in the umbilical artery. Ann. Vasc. Surg. 2008, 22, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.; Kopp, S.; Wehland, M.; Pietsch, J.; Infanger, M.; Grimm, D. Latest Results for Anti-Angiogenic Drugs in Cancer Treatment. Curr. Pharm. Des. 2016, 22, 5927–5942. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Ferrara, N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin. Cancer Res. 2006, 12, 5018–5022. [Google Scholar] [CrossRef] [PubMed]
- Stjepanovic, N.; Capdevila, J. Multi-kinase inhibitors in the treatment of thyroid cancer: Specific role of lenvatinib. Biologics 2014, 8, 129–139. [Google Scholar] [PubMed]
- Hayman, S.R.; Leung, N.; Grande, J.P.; Garovic, V.D. VEGF inhibition, hypertension, and renal toxicity. Curr. Oncol. Rep. 2012, 14, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Worden, F.; Fassnacht, M.; Shi, Y.; Hadjieva, T.; Bonichon, F.; Gao, M.; Fugazzola, L.; Ando, Y.; Hasegawa, Y.; Park do, J.; et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr. Relat. Cancer 2015, 22, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Sherman, S.I.; Shong, Y.K.; Smit, J.W.; Reike, G.; Chung, J.; Kalmus, J.; Kappeler, C.; Schlumberger, M. Rationale and design of decision: A double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer 2011, 11, 349. [Google Scholar]
- Ma, X.; Wehland, M.; Schulz, H.; Saar, K.; Hübner, N.; Infanger, M.; Bauer, J.; Grimm, D. Genomic approach to identify factors that drive the formation of three-dimensional structures by EA.hy926 endothelial cells. PLoS ONE 2013, 8, e64402. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.B.; Knutsson, M.L.; Wehland, M.; Laursen, B.E.; Grimm, D.; Warnke, E.; Magnusson, N.E. Anti-vascular endothelial growth factor therapy in breast cancer. Int. J. Mol. Sci. 2014, 15, 23024–23041. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Abramson, V.; Troxel, A.B.; Nellore, A.; Puttaswamy, K.; Redlinger, M.; Ransone, K.; Mandel, S.J.; Flaherty, K.T.; Loevner, L.A.; O’Dwyer, P.J.; et al. Phase II trial of sorafenib in advanced thyroid cancer. J. Clin. Oncol. 2008, 26, 4714–4719. [Google Scholar] [CrossRef] [PubMed]
- Nexavar (Sorafenib). Tablets Prescribing Information; Bayer Health Care Pharmaceuticals, Inc.: Wayne, NJ, USA, 2012. [Google Scholar]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; Schlumberger, M.; Jarzab, B.; Martins, R.G.; Pacini, F.; Robinson, B.; McCaffrey, J.C.; Shah, M.H.; Bodenner, D.L.; Topliss, D.; et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: A clinical outcomes and biomarker assessment. Cancer 2015, 121, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Bair, S.M.; Choueiri, T.K.; Moslehi, J. Cardiovascular complications associated with novel angiogenesis inhibitors: Emerging evidence and evolving pers Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors pectives. Trends. Cardiovasc. Med. 2013, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ollero, M.; Sahali, D. Inhibition of the VEGF signalling pathway and glomerular disorders. Nephrol. Dial. Transplant. 2015, 30, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Kappers, M.H.; de Beer, V.J.; Zhou, Z.; Danser, A.H.; Sleijfer, S.; Duncker, D.J.; van den Meiracker, A.H.; Merkus, D. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension 2012, 59, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kappers, M.H.; van Esch, J.H.; Sluiter, W.; Sleijfer, S.; Danser, A.H.; van den Meiracker, A.H. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010, 56, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Steeghs, N.; Gelderblom, H.; Roodt, J.O.; Christensen, O.; Rajagopalan, P.; Hovens, M.; Putter, H.; Rabelink, T.J.; de Koning, E. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin. Cancer Res. 2008, 14, 3470–3476. [Google Scholar] [CrossRef] [PubMed]
- Eskens, F.A.; Verweij, J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors: A review. Eur. J. Cancer 2006, 42, 3127–3139. [Google Scholar] [CrossRef] [PubMed]
- De Jesus-Gonzalez, N.; Robinson, E.; Moslehi, J.; Humphreys, B.D. Management of antiangiogenic therapy-induced hypertension. Hypertension 2012, 60, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Kiyota, N.; Schlumberger, M.; Muro, K.; Ando, Y.; Takahashi, S.; Kawai, Y.; Wirth, L.; Robinson, B.; Sherman, S.; Suzuki, T.; et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 2015, 106, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Jarzab, B.; Cabanillas, M.E.; Robinson, B.; Pacini, F.; Ball, D.W.; McCaffrey, J.; Newbold, K.; Allison, R.; Martins, R.G.; et al. A Phase II Trial of the Multitargeted Tyrosine Kinase Inhibitor Lenvatinib (E7080) in Advanced Medullary Thyroid Cancer. Clin Cancer Res. 2016, 22, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; White, I.R.; Caulfield, M.J.; Deanfield, J.E.; et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014, 383, 1899–1911. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: http://www.clinicaltrials.gov (accessed on 7 March 2017).
- Yamada, K.; Yamamoto, N.; Yamada, Y.; Nokihara, H.; Fujiwara, Y.; Hirata, T.; Koizumi, F.; Nishio, K.; Koyama, N.; Tamura, T. Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors. Clin. Cancer Res. 2011, 17, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Bikas, A.; Kundra, P.; Desale, S.; Mete, M.; O’Keefe, K.; Clark, B.G.; Gandhi, R.; Barett, C.; Jelinek, J.S.; Wexler, J.A.; et al. Phase 2 clinical trial of sunitinib as adjunctive treatment in patients with advanced differentiated thyroid cancer. Eur. J. Endocrinol. 2016, 174, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Savvides, P.; Nagaiah, G.; Lavertu, P.; Fu, P.; Wright, J.J.; Chapman, R.; Wasman, J.; Dowlati, A.; Remick, S.C. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 2013, 23, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Ederhy, S.; Goldwasser, F.; Soria, J.C.; Milano, G.; Cohen, A.; Khayat, D.; Spano, J.P. Management of hypertension in angiogenesis inhibitor-treated patients. Ann. Oncol. 2009, 20, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Paglino, C.; Imarisio, I.; Bonomi, L. Uncovering Pandora’s vase: The growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin. Exp. Med. 2007, 7, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Leon-Mateos, L.; Mosquera, J.; Anton Aparicio, L. Treatment of sunitinib-induced hypertension in solid tumor by nitric oxide donors. Redox. Biol. 2015, 6, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Wasserstrum, Y.; Kornowski, R.; Raanani, P.; Leader, A.; Pasvolsky, O.; Iakobishvili, Z. Hypertension in cancer patients treated with anti-angiogenic based regimens. Cardio-Oncology 2015, 1, 6. [Google Scholar] [CrossRef]
- Kruzliak, P.; Novak, J.; Novak, M. Vascular endothelial growth factor inhibitor-induced hypertension: From pathophysiology to prevention and treatment based on long-acting nitric oxide donors. Am. J. Hypertens 2014, 27, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Laffin, L.J.; Bakris, G.L. Endothelin Antagonism and Hypertension: An Evolving Target. Semin. Nephrol. 2015, 35, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Brose, M.S.; Frenette, C.T.; Keefe, S.M.; Stein, S.M. Management of sorafenib-related adverse events: A clinician's perspective. Semin. Oncol. 2014, 41, S1–S16. [Google Scholar] [CrossRef] [PubMed]
- Walko, C.M.; Grande, C. Management of common adverse events in patients treated with sorafenib: Nurse and pharmacist perspective. Semin. Oncol. 2014, 41, S17–S28. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.J.; Bhandari, S.K.; Shi, J.; Reynolds, K.; Calhoun, D.A.; Kalantar-Zadeh, K.; Jacobsen, S.J. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015, 88, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Wehland, M.; Grosse, J.; Simonsen, U.; Infanger, M.; Bauer, J.; Grimm, D. The Effects of Newer β-Adrenoceptor Antagonists on Vascular Function in Cardiovascular Disease. Curr. Vasc. Pharmacol. 2012, 10, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Fisker, F.Y.; Grimm, D.; Wehland, M. Third-generation β-adrenoceptor antagonists in the treatment of hypertension and heart failure. Basic Clin. Pharmacol. Toxicol. 2015, 117, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.B.; Simonsen, U.; Wehland, M.; Pietsch, J.; Grimm, D. LCZ696 (Valsartan/Sacubitril)—A possible new treatment for hypertension and heart failure. Basic Clin. Pharmacol. Toxicol. 2016, 118, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk-Wojtaś, A.; Lubas, A.; Stec, R.; Szczylik, C.; Niemczyk, S. Influence of Tyrosine Kinase Inhibitors on Hypertension and Nephrotoxicity in Metastatic Renal Cell Cancer Patients. Int. J. Mol. Sci. 2016, 17, 2073. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.J.; Shi, J.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Jacobsen, S.J. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J. Am. Coll. Cardiol. 2014, 64, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Small, H.Y.; Montezano, A.C.; Rios, F.J.; Savoia, C.; Touyz, R.M. Hypertension due to antiangiogenic cancer therapy with vascular endothelial growth factor inhibitors: Understanding and managing a new syndrome. Can. J. Cardiol. 2014, 30, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Randrup Hansen, C.; Grimm, D.; Bauer, J.; Wehland, M.; Magnusson, N.E. Effects and side effects of using sorafenib and sunitinib in the treatment of metastatic renal cell carcinoma. Int. J. Mol. Sci. 2017, 18, 461. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, V.; Lheureux, S.; Welch, S.; Mackay, H.J.; Hirte, H.; Fleming, G.; Morgan, R.; Wang, L.; Blattler, C.; Ivy, P.S.; et al. A phase II trial of sunitinib in women with metastatic or recurrent endometrial carcinoma: A study of the Princess Margaret, Chicago and California Consortia. Gynecol. Oncol. 2014, 134, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Marinelli, S.; Negrini, G.; Menetti, S.; Benevento, F.; Bolondi, L. Prognostic significance of adverse events in patients with hepatocellular carcinoma treated with sorafenib. Therap. Adv. Gastroenterol. 2016, 9, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef]
- Wehland, M.; Bauer, J.; Magnusson, N.E.; Infanger, M.; Grimm, D. Biomarkers for anti-angiogenic therapy in cancer. Int. J. Mol. Sci. 2013, 14, 9338–9364. [Google Scholar] [CrossRef] [PubMed]
| Drug | Targets | Half-Life | Bioavailability | Metabolism |
|---|---|---|---|---|
| Lenvatinib | VEGF-R1-3, FGFR1-4, PDGF-RA, c-KIT, RET | 28 h | 85% | Hepatic CYP3A4 |
| Sorafenib | VEGF-R1-3, PDGF-RA-D, C-RAF, B-RAF | 25–48 h | 38%–49% | Hepatic CYP3A4 |
| Sunitinib | VEGF-R1-3, PDGF-RA-D, c-KIT, RET, CD114, CD135 | 40–60 h | 50% | Hepatic CYP3A4 |
| Title | Design | Objective | Status |
|---|---|---|---|
| A phase I trial of lenvatinib (multi-kinase inhibitor) and capecitabine (Antimetabolite) in patients with advanced malignancies. NCT02915172 | Interventional open label | This phase I study aims to find the highest tolerable dose of lenvatinib and Capecitabine that can be given to patients with advanced cancer. | Not yet recruiting |
| Post-marketing surveillance of lenvatinib mesylate in patients with unresectable thyroid cancer. NCT02430714 | Observational cohort prospective | The objective of this study is to find unknown adverse reactions, adverse drug reactions, efficacy, safety and effectiveness factors, incidence of hypertension, hemorrhagic, and thromboembolic effects and liver disorder. | Recruiting |
| A multi center, randomized, double-blind phase II trial of lenvatinib (E7080) in subjects with iodine-131 refractory differentiated thyroid cancer (RR-DTC) to evaluate whether an oral starting dose of 20 mg or 14 mg daily will provide comparable efficacy to a 24 mg starting dose, but have a better safety profile. NCT02702388 | Interventional double blind randomized | This randomized double-blinded study aims to investigate whether a lower starting dose of lenvatinib can provide comparable efficacy whilst showing a better safety profile for the patients. | Active Not recruiting |
| An open label phase I dose escalation study of E7080 administered to patients with solid tumors. NCT00280397 | Interventional open label | This study investigates the maximum tolerable dose and the related effects of E7080 (lenvatinib) given to patients with solid tumors with no successful treatment. | Completed |
| A phase II study of E7080 in subjects with advanced thyroid cancer. NCT01728623 | Interventional open label | This study was performed to evaluate the safety, efficacy and pharmacokinetics of E7080 (lenvatinib), taken orally daily in patients with advanced thyroid cancer. | Completed |
| An open label phase I dose escalation study of E7080. NCT00121719 | Interventional open label | This study aims to find the maximum tolerated dose of lenvatinib in patients with solid tumors or lymphomas. | Active Not recruiting |
| Phase II, multi-center, open-label, single arm trial to evaluate the safety and efficacy of oral E7080 in medullary and iodine-131 refractory, unresectable differentiated thyroid cancers, stratified by histology. NCT00784303 | Interventional open label non-randomized | This is a phase II study that aimed to investigate the safety and efficacy of oral E7080 (lenvatinib) in medullary and iodine-131 refractory, unresectable differentiated thyroid cancer. | Completed |
| Phase II study assessing the efficacy and safety of lenvatinib for anaplastic thyroid cancer (HOPE). NCT02726503 | Interventional open label | This phase II study aims to investigate the efficacy and safety of lenvatinib for unresectable anaplastic thyroid cancer. | Recruiting |
| A multi-center, randomised, double-blind, placebo-controlled, phase III trial of lenvatinib (E7080) in I-131-refractory differentiated thyroid cancer in China. NCT02966093 | Interventional double blind randomized | This phase III study primarily aims to compare progression-free survival of participants with radioiodine refractory differentiated thyroid cancer treated with lenvatinib or placebo, and secondarily to investigate adverse events. | Not yet recruiting |
| Post-marketing surveillance of Lenvima in Korean patients. NCT02764554 | Observational prospective | This study aims to observe the safety profile of lenvatinib (Lenvima) in normal clinical practice. | Recruiting |
| Prospective, non-interventional, post-authorization safety study that includes all patients diagnosed as unresectable differentiated thyroid carcinoma and treated with sorafenib (JPMS-DTC). NCT02185560 | Observational | Safety study that includes all patients diagnosed as unresectable differentiated thyroid carcinoma (DTC) and treated with sorafenib within a certain period. | Recruiting |
| Safety and efficacy of sorafenib in patients with advanced thyroid cancer: a Phase II clinical study. NCT02084732 | Interventional | Describe the clinical activity and safety profile of sorafenib in the treatment of patients with advanced thyroid cancer (metastatic or recurrent) among a selected group of patients refractory to or ineligible to radioactive iodine (RAI) therapy. | Recruiting |
| Prospective, non-interventional, post-authorization safety study that includes all patients diagnosed as unresectable differentiated thyroid carcinoma and treated with sorafenib (JPMS-DTC). NCT02185560 | Observational | This is a non-interventional, multi center post-authorization safety study that includes all patients diagnosed as unresectable differentiated thyroid carcinoma (DTC) and treated with sorafenib within a certain period. | Recruiting |
| Thyroid cancer and sunitinib (THYSU). NCT00510640 | Interventional | The objective of the trial is to determine the objective tumor response rate (efficacy) in patients with locally advanced or metastatic anaplastic, differentiated or medullary thyroid carcinoma treated with sunitinib; a secondary objective is to evaluate the safety of sunitinib in these patients | Completed |
| Sutent adjunctive treatment of differentiated thyroid cancer (IIT Sutent). NCT00668811 | Interventional | The primary objective is to assess clinical benefit rate, defined as complete response, partial response, or stable disease per RECIST criteria. The secondary objective will be to assess the safety of Sutent in this patient population. | Completed |
| Clinical Trial Title ID | Dose (mg/day) | # of Patients | Most Frequent Serious Adverse Effects | |
|---|---|---|---|---|
| SELECT: A multi center, randomized, double-blind, placebo-controlled, phase 3 trial of Lenvatinib (E7080) in 131I-refractory differentiated thyroid cancer. NCT01321554 [31,40] | 24 Per os (PO) | 261 | 4% | Pneumonia |
| 3% | Hypertension | |||
| 3% | Dehydration | |||
| 2% | Physical health deterioration | |||
| 2% | Renal failure | |||
| 2% | Pulmonary embolism | |||
| 2% | Sepsis | |||
| An open label phase I dose escalation study of E7080 (solid tumors). NCT00121719 | 0.1–32 PO | 93 | 5% | Abdominal pain |
| 5% | Vomiting | |||
| 4% | Hypertension | |||
| 3% | Physical health deterioration | |||
| 3% | Pyrexia | |||
| Sorafenib in treating patients with advanced anaplastic thyroid cancer. NCT00126568 [46] | 2 × 400 PO | 20 | 15% | Disease progression |
| 10% | Death | |||
| 10% | Dyspnea | |||
| 5% | Thrombosis | |||
| 5% | Pulmonary disorders | |||
| Nexavar® versus placebo in locally advanced/metastatic RAI-refractory differentiated thyroid cancer. NCT00984282 [24,25,26] | 2 × 400 PO | 207 | 5% | Secondary malignancy |
| 4% | Dyspnea | |||
| 4% | Musculoskeletal disorders | |||
| 3% | Pleural effusion | |||
| 2% | Fever | |||
| A continuation study using sunitinib malate for patients leaving treatment on a previous sunitinib study. NCT00428220 | 37.5 PO | 223 | 5% | Disease progression |
| 4% | Abdominal pain | |||
| 3% | Vomiting | |||
| 3% | Diarrhea | |||
| 2% | Physical health deterioration | |||
| 2% | Pyrexia | |||
| 2% | Anemia | |||
| Sutent adjunctive treatment of differentiated thyroid cancer (IIT Sutent). NCT00668811 [45] | 37.5 PO | 23 | 13% | Hypertension |
| 13% | Leukopenia | |||
| 9% | Hand-foot syndrome | |||
| 9% | Anorexia | |||
| 4% | Neutropenia | |||
| 4% | Lymphopenia | |||
| 4% | Thrombocytopenia | |||
| 4% | Nausea | |||
| 4% | Gastrointestinal bleeding | |||
| Class | Drug | Dose | Recommendation |
|---|---|---|---|
| CCB Dihydropyridines | Amlodipine | 2.5–10 mg/day | Great potency for reducing arterial smooth muscle cell contractility [39], effective therapy [49]. |
| ACEi | Enalapril | Start with 5–20 mg/12–24 h, then max 40 mg/12–24 h | Particularly indicated in the setting of proteinuria [39], effective [49]. |
| Ramipril | Start with 2.5 mg/day, then 5 mg/day after 2 weeks, after another 2 weeks max 10 mg/day | ||
| ARB | Losartan | 50–100 mg/day | Particularly indicated in the setting of proteinuria [39], effective [49]. |
| Valsartan | 80–320 mg/day | ||
| Irbesartan | 150–300 mg/day | ||
| BBA | Nebivolol | 2.5–5 mg/day | Indicated for DTC; begin therapy of hypertension with a BBA [53]. |
| Diuretics/Thiazides | Hydrochlorothiazide | Start with 12.5–25 mg/day, then 12.5 mg/day | Less-effective than CCB, ACEi or ARB [39], but often used [54]. |
| Nitrate derivates | Long-acting nitrates: Isosorbide dinitrate (ISDN) or Isosorbide mononitrate (ISMN) | 40–60 mg/day | Adequate response in hypertension refractory to ACEi and CCB [51]. |
| α-blockers | Prazosin | 2–20 mg/day | Used as additional therapy if BP is not sufficiently controlled. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ancker, O.V.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. The Adverse Effect of Hypertension in the Treatment of Thyroid Cancer with Multi-Kinase Inhibitors. Int. J. Mol. Sci. 2017, 18, 625. https://doi.org/10.3390/ijms18030625
Ancker OV, Wehland M, Bauer J, Infanger M, Grimm D. The Adverse Effect of Hypertension in the Treatment of Thyroid Cancer with Multi-Kinase Inhibitors. International Journal of Molecular Sciences. 2017; 18(3):625. https://doi.org/10.3390/ijms18030625
Chicago/Turabian StyleAncker, Ole Vincent, Markus Wehland, Johann Bauer, Manfred Infanger, and Daniela Grimm. 2017. "The Adverse Effect of Hypertension in the Treatment of Thyroid Cancer with Multi-Kinase Inhibitors" International Journal of Molecular Sciences 18, no. 3: 625. https://doi.org/10.3390/ijms18030625