Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action
Abstract
:1. Introduction
2. Treatment of Cancer through Targeting Apoptosis
3. Natural Compounds as Anti-Cancer Agents
4. Traditional Chinese Medicines (TCMs) as Anti-Cancer Agents
4.1. Natural Compounds from TCM as Cancer Therapeutics
4.2. Alkaloids
4.3. Flavonoids
4.4. Saponins
4.5. Drugs Based on Mixtures of Compounds
5. Alternative Approach of Triggering Apoptosis Using Metal-Derivatized Natural Compounds
6. Chemoresistance in Cancer and Chemosensitization by Natural Compounds
7. Apoptosis in MDR Cells through Modulation of MicroRNA (miRNA) Networks by Natural Compounds
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CKI | Compound Kushen Injection |
| MDR | Multi-drug Resistance |
| ATB | Antitumor B |
| NPACT | Naturally Occurring Plant-based Anti-cancer Compound-Activity-Target Database |
| TCM | Traditional Chinese Medicine |
| QoL | Quality of Life |
| MT | Matrine |
| GO | Gene Ontology |
| IRO | Indirubin-3′-monoxime |
| CS-IVa-Be | Chikusetsusaponin IV a Butyl Ester |
| VEGF | Vascular Endothelial Growth Factor |
| Akt | Protein Kinase B |
| NF-κB | Nuclear Factor κ-light-chain-enhancer of activated B cells |
| ER | Endoplasmic Reticulum |
| MAPK | Mitogen Activated Protein Kinase |
References
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Programmed Cell Death (Apoptosis). In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Abrams, J.; Alnemri, E.; Baehrecke, E.; Blagosklonny, M.; Dawson, T.M.; Dawson, V.; El-Deiry, W.; Fulda, S. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell. Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Bredesen, D.E. Dependence receptors: From basic research to drug development. Sci. Signal. 2011, 4, mr2. [Google Scholar] [CrossRef] [PubMed]
- Wong, R. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, H.; Liang, B.; Liu, G.; Tang, M.; Jia, R.; Fan, X.; Jing, W.; Zhou, X.; Wang, H. Downregulation of ASPP2 improves hepatocellular carcinoma cells survival via promoting BECN1-dependent autophagy initiation. Cell Death Dis. 2016, 7, e2512. [Google Scholar] [CrossRef] [PubMed]
- Codogno, P.; Meijer, A.J. Atg5: More than an autophagy factor. Nat. Cell Biol. 2006, 8, 1045–1046. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; McCurrach, M.E.; de Stanchina, E.; Wallace-Brodeur, R.R.; Lowe, S.W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999, 13, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Dive, C.; Hickman, J. Drug-target interactions: Only the first step in the commitment to a programmed cell death? Br. J. Cancer 1991, 64, 192. [Google Scholar] [PubMed]
- Chai, S.; To, K.; Lin, G. Circumvention of multi-drug resistance of cancer cells by Chinese herbal medicines. Chin. Med. 2010, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Yu-Jen, C. Potential role of tetrandrine in cancer therapy. Acta Pharmacol. Sin. 2002, 23, 1102–1106. [Google Scholar]
- Tian, H.; Pan, Q. A comparative study on effect of two bisbenzylisoquinolines, tetrandrine and berbamine, on reversal of multidrug resistance. Acta Pharm. Sin. 1997, 32, 245–250. [Google Scholar]
- Choi, S.-U.; Park, S.-H.; Kim, K.-H.; Choi, E.-J.; Kim, S.; Park, W.-K.; Zhang, Y.-H.; Kim, H.-S.; Jung, N.-P.; Lee, C.-O. The bis benzylisoquinoline alkaloids, tetrandine and fangchinoline, enhance the cytotoxicity of multidrug resistance-related drugs via modulation of P-glycoprotein. Anti Cancer Drugs 1998, 9, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, E.; Kim, W.; Seong, K.M.; Youn, H.; Kim, J.W.; Kim, J.; Youn, B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. J. Biol. Chem. 2013, 288, 27343–27357. [Google Scholar] [CrossRef] [PubMed]
- Graw, S.; Meier, R.; Minn, K.; Bloomer, C.; Godwin, A.K.; Fridley, B.; Vlad, A.; Beyerlein, P.; Chien, J. Robust gene expression and mutation analyses of RNA-sequencing of formalin-fixed diagnostic tumor samples. Sci. Rep. 2015, 5, 12335. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Earnshaw, W.C. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000, 256, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Zamora, P.; Feliu, J.; Barón, M.G. Classification of anticancer drugs—A new system based on therapeutic targets. Cancer Treat. Rev. 2003, 29, 515–523. [Google Scholar] [CrossRef]
- Manson, M.M. Cancer prevention–the potential for diet to modulate molecular signalling. Trends Mol. Med. 2003, 9, 11–18. [Google Scholar] [CrossRef]
- Prakash, O.; Kumar, A.; Kumar, P. Anticancer potential of plants and natural products: A review. Am. J. Pharmacol. Sci. 2013, 1, 104–115. [Google Scholar] [CrossRef]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from Mother Nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Evasion of apoptosis as a cellular stress response in cancer. Int. J. Cell Biol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; Kucuk, O.; Sarkar, F.H. Genistein inhibits NF-κB activation in prostate cancer cells. Nutr. Cancer 1999, 35, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Stephens, F.O. The rising incidence of breast cancer in women and prostate cancer in men. Dietary influences: A possible preventive role for nature’s sex hormone modifiers-the phytoestrogens (review). Oncol. Rep. 1999, 6, 865–935. [Google Scholar] [CrossRef] [PubMed]
- Mangal, M.; Sagar, P.; Singh, H.; Raghava, G.P.; Agarwal, S.M. NPACT: Naturally occurring plant-based anti-cancer compound-activity-target database. Nucleic Acids Res. 2013, 41, D1124–D1129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Seto, S.W.; Chang, D.; Kiat, H.; Razmovski-Naumovski, V.; Chan, K.; Bensoussan, A. Synergistic effects of Chinese herbal medicine: A comprehensive review of methodology and current research. Front. Pharmacol. 2016, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Zhang, Y.; Fan, H.T.; Lin, H.S. Traditional Chinese medicine and cancer: History, present situation, and development. Thorac. Cancer 2015, 6, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Chang, R. Bioactive polysaccharides from traditional Chinese medicine herbs as anticancer adjuvants. J. Altern. Complement. Med. 2002, 8, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.H.; Li, X.; Xu, Y.; Liu, J.P. Chinese herbal medicine for advanced pancreatic cancer. Cochrane Libr. 2012. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Li, J.; He, L.; Tripathy, D. Chinese medicinal herbs to treat the side-effects of chemotherapy in breast cancer patients. Cochrane Libr. 2007. [Google Scholar] [CrossRef]
- Guo, Z.; Jia, X.; Liu, J.P.; Liao, J.; Yang, Y. Herbal medicines for advanced colorectal cancer. Cochrane Libr. 2012. [Google Scholar] [CrossRef]
- Shoemaker, M.; Hamilton, B.; Dairkee, S.H.; Cohen, I.; Campbell, M.J. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother. Res. 2005, 19, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Zhou, H.-J.; Fang, X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharmacol. Res. 2003, 48, 231–236. [Google Scholar] [CrossRef]
- Sun, M.; Cao, H.; Sun, L.; Dong, S.; Bian, Y.; Han, J.; Zhang, L.; Ren, S.; Hu, Y.; Liu, C. Antitumor activities of kushen: Literature review. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Henkel, T. Traditional Chinese medicine (TCM): Are polyphenols and saponins the key ingredients triggering biological activities? Curr. Med. Chem. 2002, 9, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, J. Types of anticancer agents isolated from plants. Cancer Treat. Rep. 1976, 60, 1031–1067. [Google Scholar] [PubMed]
- Lu, J.-J.; Bao, J.-L.; Chen, X.-P.; Huang, M.; Wang, Y.-T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-H.; Kim, K.-J.; Cha, J.-D.; Kim, H.-K.; Lee, Y.-E.; Choi, N.-Y.; You, Y.-O. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J. Med. Food 2005, 8, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lin, H.; Huang, W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med. Sci. Monit. Basic Res. 2011, 17, RA164–RA167. [Google Scholar] [CrossRef]
- Ji, Y. Active Ingredients of Traditional Chinese Medicine: Pharmacology and Application; People’s Medical Publishing House Cp., LTD: Shelton, CT, USA, 2011. [Google Scholar]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Liu, Y.; Xu, Y.; Ji, W.; Li, X.; Sun, B.; Gao, Q.; Su, C. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumor Biol. 2014, 35, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Z.; Zhang, J.K.; Shi, Z.; Liu, B.; Shen, C.Q.; Tao, H.M. Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother. Pharmacol. 2012, 69, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Q.; Liu, K.; Yagasaki, K.; Wu, E.; Zhang, G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κB signaling. Cytotechnology 2009, 59, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Ji, H.; Mao, T.; Bai, Z. Effects of matrine on the proliferation and apoptosis of human medulloblastoma cell line D341. Int. J. Clin. Exp. Med. 2014, 7, 911–918. [Google Scholar] [PubMed]
- Xie, S.-B.; He, X.-X.; Yao, S.-K. Matrine-induced autophagy regulated by p53 through AMP-activated protein kinase in human hepatoma cells. Int. J. Oncol. 2015, 47, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 2015, 32, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Ran, Z.H.; Xu, Q.; Xiao, S.D. Experimental study of the killing effects of oxymatrine on human colon cancer cell line SW1116. Chin. J. Dig. Dis. 2005, 6, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-S.; Yang, T.; Gu, J.; Li, M.-L.; Wu, W.-G.; Weng, H.; Ding, Q.; Mu, J.-S.; Bao, R.-F.; Shu, Y.-J. Effects of oxymatrine on the apoptosis and proliferation of gallbladder cancer cells. Anti Cancer drugs 2014, 25, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Zhao, Y.-X.; Deng, S.-H.; Sun, Q. Preparation and in vitro anticancer activity of oxymatrine mixed micellar nanoparticles. Die Pharm. Int. J. Pharm. Sci. 2011, 66, 506–510. [Google Scholar]
- Wang, B.; Han, Q.; Zhu, Y. Oxymatrine inhibited cell proliferation by inducing apoptosis in human lung cancer A549 cells. Bio Med. Mater. Eng. 2015, 26, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Su, B.-S.; Chang, L.-H.; Gao, Q.; Chen, K.-L.; An, P.; Huang, C.; Yang, J.; Li, Z.-F. Oxymatrine induces apoptosis in human cervical cancer cells through guanine nucleotide depletion. Anti Cancer Drugs 2014, 25, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-M.; Chen, Y.; Chen, J.-C.; Lin, T.-Y.; Tseng, S.-H. Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice. Cancer Lett. 2010, 287, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Shen, H.; Cao, Y.; Fang, Y.; Li, H.; Chen, Q.; Xu, W. Tetrandrine induces mitochondria-mediated apoptosis in human gastric cancer BGC-823 cells. PLoS ONE 2013, 8, e76486. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Jordan, M.A.; Chu, K.C.; Wilson, L. Differential effects of vinblastine on polymerization and dynamics at opposite microtubule ends. J. Biol. Chem. 1996, 271, 29807–29812. [Google Scholar] [CrossRef] [PubMed]
- Brugières, L.; Pacquement, H.; Le Deley, M.-C.; Leverger, G.; Lutz, P.; Paillard, C.; Baruchel, A.; Frappaz, D.; Nelken, B.; Lamant, L. Single-drug vinblastine as salvage treatment for refractory or relapsed anaplastic large-cell lymphoma: A report from the French Society of Pediatric Oncology. J. Clin. Oncol. 2009, 27, 5056–5061. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.; Shi, J.; Tomas-Barberan, F.; Datta, N.; Singanusong, R.; Chen, S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1. [Google Scholar] [PubMed]
- Zhou, H.; Lutterodt, H.; Cheng, Z.; Yu, L. Anti-inflammatory and antiproliferative activities of trifolirhizin, a flavonoid from Sophora flavescens roots. J. Agric. Food Chem. 2009, 57, 4580–4585. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yang, G.; Ma, Y.; Xu, B.; Hu, M.; You, M.; Gao, S. Developing an activity and absorption-based quality control platform for Chinese traditional medicine: Application to Zeng-Sheng-Ping (Antitumor B). J. Ethnopharmacol. 2015, 172, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Aratanechemuge, Y.; Hibasami, H.; Katsuzaki, H.; Imai, K.; Komiya, T. Induction of apoptosis by maackiain and trifolirhizin (maackiain glycoside) isolated from sanzukon (Sophora Subprostrate Chen et T. Chen) in human promyelotic leukemia HL-60 cells. Oncol. Rep. 2004, 12, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Chen, S.; Guo, D.; Pan, S.; Yu, Z. Proteomic identification of differentially expressed proteins in curcumin-treated MCF-7 cells. Phytomedicine 2011, 18, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.C.; Mock, C.D.; Thilagavathi, R.; Selvam, C. Molecular mechanisms of curcumin and its semisynthetic analogues in prostate cancer prevention and treatment. Life Sci. 2016, 152, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017, 541, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer 2014, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-H.; Song, H.-Y.; Zhou, Y.-F.; Yuan, G.-Y.; Zheng, F.-J. Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Exp. Ther. Med. 2013, 6, 1155–1158. [Google Scholar] [PubMed]
- Zhou, J.; Liang, S.; Fang, L.; Chen, L.; Tang, M.; Xu, Y.; Fu, A.; Yang, J.; Wei, Y. Quantitative proteomic analysis of HepG2 cells treated with quercetin suggests IQGAP1 involved in quercetin-induced regulation of cell proliferation and migration. OMICS J. Integr. Biol. 2009, 13, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.E.; Zeki, K.; Sugiura, T.; Yoshida, Y.; Yamashita, U. Chinese medicinal herb, Acanthopanax gracilistylus, extract induces cell cycle arrest of human tumor cells in vitro. Jpn. J. Cancer Res. 2000, 91, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qian, S.; Cai, X.; Lu, W.; Hu, C.; Sun, X.; Yang, Y.; Yu, Q.; Gao, S.P.; Cao, P. Chikusetsusaponin IVa butyl ester (CS-IVa-Be), a novel IL-6R antagonist, inhibits IL-6/STAT3 signaling pathway and induces cancer cell apoptosis. Mol. Cancer Ther. 2016, 15, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Chan, J.Y.-W.; Kong, S.-K.; Yu, B.; Eng-Choon, V.O.; Nai-Ching, H.W.; Mak Chung-Wai, T.; Fung, K.-P. Effects of polyphyllin D, a steroidal saponin in Paris polyphylla, in growth inhibition of human breast cancer cells and in xenograft. Cancer Biol. Ther. 2005, 4, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Raju, J.; Bird, R.P. Diosgenin, a naturally occurring furostanol saponin suppresses 3-hydroxy-3-methylglutaryl CoA reductase expression and induces apoptosis in HCT-116 human colon carcinoma cells. Cancer Lett. 2007, 255, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-T.; Way, T.-D.; Tsai, S.-J.; Lin, J.-K. Diosgenin, a naturally occurring steroid, suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett. 2007, 581, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.-Z.; Qi, Q.; Tao, L.; Zhao, Q.; Mu, R.; Gu, H.-Y.; Wang, M.; Feng, X.; Guo, Q.-L. Macranthoside B, a hederagenin saponin extracted from Lonicera macranthoides and its anti-tumor activities in vitro and in vivo. Food Chem. Toxicol. 2009, 47, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Guan, F.; Zhao, X.; Wang, M.; Chen, Y.; Wang, Q.; Feng, X. Macranthoside B Induces Apoptosis and Autophagy Via Reactive Oxygen Species Accumulation in Human Ovarian Cancer A2780 Cells. Nutr. Cancer 2016, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-J.; Zhao, Y.; Goto, M.; Hsieh, K.-Y.; Yang, X.-M.; Morris-Natschke, S.L.; Liu, L.-N.; Zhao, B.-Y.; Lee, K.-H. Alkaloids from Oxytropis ochrocephala and Antiproliferative Activity of Sophoridine Derivatives Against Cancer Cell Lines. Bioorganic Med. Chem. Lett. 2015, 26, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, H.; Liu, J.; Chen, L.; Zhang, Q.; Wang, Z. Identification and determination of the chemical constituents in a herbal preparation, Compound Kushen injection, by HPLC and LC-DAD-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 207–220. [Google Scholar] [CrossRef]
- Wang, W.; You, R.-L.; Qin, W.-J.; Hai, L.-N.; Fang, M.-J.; Huang, G.-H.; Kang, R.-X.; Li, M.-H.; Qiao, Y.-F.; Li, J.-W. Anti-tumor activities of active ingredients in Compound Kushen Injection. Acta Pharmacol. Sin. 2015, 36, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Polakis, P. Wnt signaling and cancer. Genes Dev. 2000, 14, 1837–1851. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lin, H.; Zhang, Y.; Chen, X.; Hua, B.; Hou, W.; Qi, X.; Pei, Y.; Zhu, X.; Zhao, Z. Compound Kushen Injection suppresses human breast cancer stem-like cells by down-regulating the canonical Wnt/b-catenin pathway. J. Exp. Clin. Cancer Res. 2011, 30, 103. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-M.; Huang, Y.-X.; Shen, H.-H.; Sang, X.-X.; Ma, X.; Zhao, Y.-L.; Xiao, X.-H. Efficacy of Compound Kushen Injection in Relieving Cancer-Related Pain: A Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Cui, J.; Harata-Lee, Y.; Aung, T.N.; Feng, Q.; Raison, J.; Kortschak, R.D.; Adelson, D.L. Identification Of Candidate Anti-Cancer Molecular Mechanisms Of Compound Kushen Injection Using Functional Genomics. Oncotarget 2016, 7, 66003–66019. [Google Scholar] [CrossRef] [PubMed]
- Xudong, L.; Yan, L.; Xi, Y.; Xiaoguang, W.; Xiaoguang, H. Long noncoding RNA H19 regulates EZH2 expression by interacting with miR-630 and promotes cell invasion in nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2016, 473, 913–919. [Google Scholar]
- Zhang, Z.; Wang, Y.; Yao, R.; Li, J.; Yan, Y.; La Regina, M.; Lemon, W.L.; Grubbs, C.J.; Lubet, R.A.; You, M. Cancer chemopreventive activity of a mixture of Chinese herbs (antitumor B) in mouse lung tumor models. Oncogene 2004, 23, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Sun, Z.; Chen, X.; Wu, H.; Zhang, X. Inhibitory effects of Zengshengping fractions on DMBA-induced buccal pouch carcinogenesis in hamsters. Chin. Med. J. 2012, 125, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Rajan, K.; Eberhart, C.G. Effects of Zeng Sheng Ping/ACAPHA on Malignant Brain Tumor Growth and Notch Signaling. Anticancer Res. 2012, 32, 2689–2696. [Google Scholar] [PubMed]
- Sun, Z.; Guan, X.; Li, N.; Liu, X.; Chen, X. Chemoprevention of oral cancer in animal models, and effect on leukoplakias in human patients with ZengShengPing, a mixture of medicinal herbs. Oral Oncol. 2010, 46, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Voyno-Yasenetskaya, T.A.; Pace, A.M.; Bourne, H.R. Mutant alpha subunits of G12 and G13 proteins induce neoplastic transformation of Rat-1 fibroblasts. Oncogene 1994, 9, 2559–2565. [Google Scholar] [PubMed]
- Berestetskaya, Y.V.; Faure, M.P.; Ichijo, H.; Voyno-Yasenetskaya, T.A. Regulation of apoptosis by α-subunits of G12 and G13 proteins via apoptosis signal-regulating kinase-1. J. Biol. Chem. 1998, 273, 27816–27823. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.H.; Li, Z.F.; Zhou, S.B.; Liang, Y.Y.; He, D.C.; Wang, D.S. Differentially expressed proteins of MCF-7 human breast cancer cells affected by Zilongjin, a complementary Chinese herbal medicine. Proteom. Clin. Appl. 2010, 4, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, X.; Xiong, S.; Wen, S.; Gao, S.; Wang, L.; Cao, B. Genome wide expression analysis of the effect of the Chinese patent medicine zilongjin tablet on four human lung carcinoma cell lines. Phytother. Res. 2011, 25, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-C.; Clarkin, K.C.; Wahl, G.M. Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc. Natl. Acad. Sci. USA 1996, 93, 4827–4832. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Yao, M.; Zhao, X.; Fritsche, J.; Schmitt-Kopplin, P.; Cai, Z.; Wan, D.; Lu, X.; Yang, S. Metabonomics study on the effects of the ginsenoside Rg3 in a β-cyclodextrin-based formulation on tumor-bearing rats by a fully automatic hydrophilic interaction/reversed-phase column-switching HPLC-ESI-MS approach. Anal. Chem. 2008, 80, 4680–4688. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Morvan, D. Metabolomics reveals metabolic targets and biphasic responses in breast cancer cells treated by curcumin alone and in association with docetaxel. PLoS ONE 2013, 8, e57971. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, L.; Shi, J.; Zhang, G.; Lu, L.; Zhu, L.; Zhang, J.; Liu, Z. Toxic Markers of Matrine Determined Using 1H-NMR-Based Metabolomics in Cultured Cells In Vitro and Rats In Vivo. Evid. Based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef]
- Yue, Q.-X.; Song, X.-Y.; Ma, C.; Feng, L.-X.; Guan, S.-H.; Wu, W.-Y.; Yang, M.; Jiang, B.-H.; Liu, X.; Cui, Y.-J. Effects of triterpenes from Ganoderma lucidum on protein expression profile of HeLa cells. Phytomedicine 2010, 17, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.-F.; Wei, Y.-X.; Cheng, W.-D.; Yan, M.-F.; Su, G.; Hu, Y.; Ma, Y.-Q.; Han, C.; Lu, Y.; Hui-Ming, C. Proteomic analysis of the effect of triterpenes from Patrinia heterophylla on leukemia K562 cells. J. Ethnopharmacol. 2012, 144, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Kaur, S.; George, J.; Nihal, M.; Hahn, M.C.P.; Scarlett, C.O.; Ahmad, N. Molecular signatures of sanguinarine in human pancreatic cancer cells: A large scale label-free comparative proteomics approach. Oncotarget 2015, 6, 10335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Q.-M.; Lu, Y.-Y.; Jia, D.; Su, S.-B. Aidi Injection () Alters the Expression Profiles of MicroRNAs in Human Breast Cancer Cells. J. Tradit. Chin. Med. 2011, 31, 10–16. [Google Scholar] [CrossRef]
- Dyson, P.J.; Sava, G. Metal-based antitumour drugs in the post genomic era. Dalton Trans. 2006, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-F.; Liang, H.; Liu, Y.-C. Traditional Chinese Medicine Active Ingredient-Metal Based Anticancer Agents; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Dholwani, K.; Saluja, A.; Gupta, A.; Shah, D. A review on plant-derived natural products and their analogs with anti-tumor activity. Indian J. Pharmacol. 2008, 40, 49–58. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Mao, L.; Liu, L.-M.; Liu, Y.-C.; Peng, Y.; Hong, X.; Wang, H.-H.; Liu, H.-G.; Liang, H. Potential new inorganic antitumour agents from combining the anticancer traditional Chinese medicine (TCM) matrine with Ga (III), Au (III), Sn (IV) ions, and DNA binding studies. J. Inorg. Biochem. 2011, 105, 171–180. [Google Scholar] [CrossRef]
- Tan, C.; Wu, S.; Lai, S.; Wang, M.; Chen, Y.; Zhou, L.; Zhu, Y.; Lian, W.; Peng, W.; Ji, L. Synthesis, structures, cellular uptake and apoptosis-inducing properties of highly cytotoxic ruthenium-Norharman complexes. Dalton Trans. 2011, 40, 8611–8621. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-F.; Liu, Y.-C.; Huang, K.-B.; Liang, H. Alkaloid-metal based anticancer agents. Curr. Top. Med. Chem. 2013, 13, 2104–2115. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, J.; Pan, Y.; Chen, Z.; Liang, H.; Liu, H.; Wang, H. Synthesis, cytotoxic activity, and DNA binding properties of copper (II) complexes with hesperetin, naringenin, and apigenin. Bioinorg. Chem. Appl. 2009. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, B.; Zhu, L. DNA binding and oxidative DNA damage induced by a quercetin copper (II) complex: Potential mechanism of its antitumor properties. JBIC J. Biol. Inorg. Chem. 2009, 14, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Glavinas, H.; Krajcsi, P.; Cserepes, J.; Sarkadi, B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 2004, 1, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, H.; Zhang, J.-T. Expression profiling of ABC transporters in a drug-resistant breast cancer cell line using AmpArray. Mol. Pharmacol. 2005, 68, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shimizu, C.; Shimoyama, T.; Takeda, M.; Ando, M.; Kohno, T.; Katsumata, N.; Kang, Y.-K.; Nishio, K.; Fujiwara, Y. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res. Treat. 2006, 99, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Ishii, T.; Kamimura, R.; Kajiwara, M.; Machimoto, T.; Nakatsuji, N.; Suemori, H.; Ikai, I.; Yasuchika, K.; Uemoto, S. Alpha-fetoprotein-producing pancreatic cancer cells possess cancer stem cell characteristics. Cancer Lett. 2011, 308, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kioka, N.; Hosokawa, N.; Komano, T.; Hirayoshi, K.; Nagate, K.; Ueda, K. Quercetin, a bioflavonoid, inhibits the increase of human multidrug resistance gene (MDR1) expression caused by arsenite. FEBS Lett. 1992, 301, 307–309. [Google Scholar] [CrossRef]
- Cai, X.; Chen, F.; Han, J.; Gu, C.; Zhong, H.; Ouyang, R. Restorative effect of quercetin on subcellular distribution of daunorubicin in multidrug resistant leukemia cell lines K562/ADM and HL-60/ADM. Chin. J. Cancer 2004, 23, 1611–1615. [Google Scholar]
- Cai, X.; Chen, F.; Han, J.; Gu, C.; Zhong, H.; Teng, Y.; Ouyang, R. Reversal of multidrug resistance of HL-60 adriamycin resistant leukemia cell line by quercetin and its mechanisms. Chin. J. Oncol. 2005, 27, 326–329. [Google Scholar]
- Tang, X.-Q.; Bi, H.; Feng, J.-Q.; Cao, J.-G. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol. Sin. 2005, 26, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Amiji, M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm. 2009, 6, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Fix, L.N.; Shah, M.; Efferth, T.; Farwell, M.A.; Zhang, B. MicroRNA expression profile of MCF-7 human breast cancer cells and the effect of green tea polyphenon-60. Cancer Genom. Proteom. 2010, 7, 261–277. [Google Scholar]
- Kasinski, A.L.; Slack, F.J. MicroRNAs en route to the clinic: Progress in validating and targeting microRNAs for cancer therapy. Nat. Rev. Cancer 2011, 11, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.E.; Ghoshal, K.; Ramaswamy, B.; Roy, S.; Datta, J.; Shapiro, C.L.; Jacob, S.; Majumder, S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008, 283, 29897–29903. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, O.; Filkowski, J.; Meservy, J.; Ilnytskyy, Y.; Tryndyak, V.P.; Vasyl’F, C.; Pogribny, I.P. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol. Cancer Ther. 2008, 7, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Angst, B.D.; Marcozzi, C.; Magee, A.I. The cadherin superfamily. J. Cell Sci. 2001, 114, 625–626. [Google Scholar] [PubMed]
- Hurteau, G.J.; Carlson, J.A.; Spivack, S.D.; Brock, G.J. Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res. 2007, 67, 7972–7976. [Google Scholar] [CrossRef] [PubMed]
- Adam, L.; Zhong, M.; Choi, W.; Qi, W.; Nicoloso, M.; Arora, A.; Calin, G.; Wang, H.; Siefker-Radtke, A.; McConkey, D. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin. Cancer Res. 2009, 15, 5060–5072. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, L.; Jin, Y.; Zhang, Y.; Wang, D.; Tan, Y.; Zheng, C. Combination of Matrine and Sorafenib Decreases the Aggressive Phenotypes of Hepatocellular Carcinoma Cells. Chemotherapy 2014, 60, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, S.; Liu, X.; Wu, H.; Lin, X.; Gu, J.; Wang, H.; Duan, Y. Matrine alters microRNA expression profiles in SGC-7901 human gastric cancer cells. Oncol. Rep. 2014, 32, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, K.; Zhao, F. Oxymatrine suppresses proliferation and facilitates apoptosis of human ovarian cancer cells through upregulating microRNA‑29b and downregulating matrix metalloproteinase‑2 expression. Mol. Med. Rep. 2015, 12, 5369–5374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Liu, P.; Chen, Q.; Situ, H.; Xie, T.; Zhang, J.; Peng, C.; Lin, Y.; Chen, J. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014, 5, 7013–7026. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Deng, W.; Wang, X.; Lü, H.; Han, L.; Chen, B.; Chen, X.; Li, N. Molecular mechanism of indirubin-3′-monoxime and Matrine in the reversal of paclitaxel resistance in NCI-H520/TAX25 cell line. Chin. Med. J. 2013, 126, 925–929. [Google Scholar] [PubMed]
- Schmitt, C.A.; Lowe, S.W. Apoptosis and chemoresistance in transgenic cancer models. J. Mol. Med. 2002, 80, 137–146. [Google Scholar] [CrossRef] [PubMed]
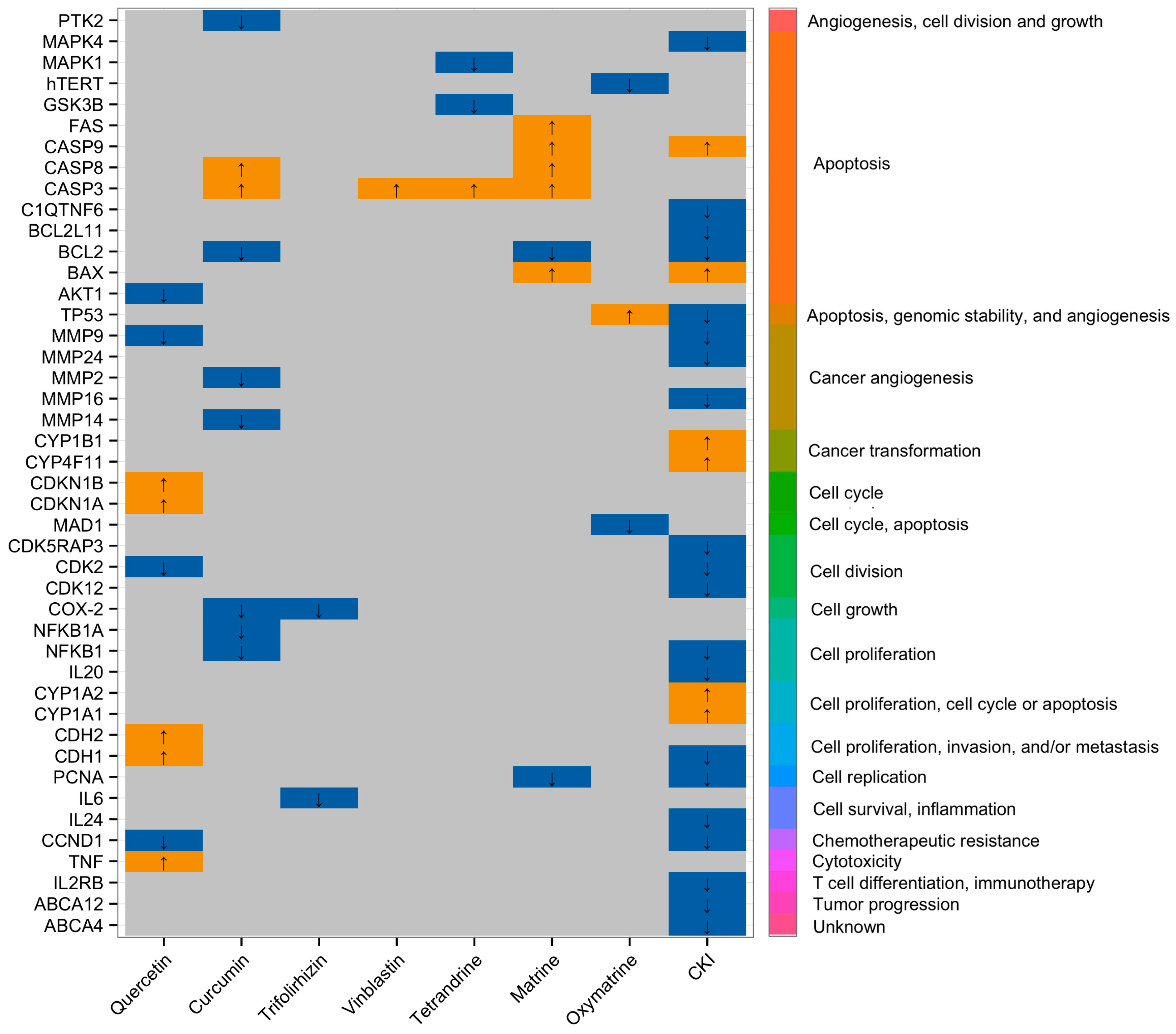

| Ref. | Herbal Medicines | Types of Cancer | Cell Lines/Model | Mechanisms of Actions |
|---|---|---|---|---|
| [97] | Curcumin | Colorectal | Colorectal cancer stem cells (CCSCs) | Apoptosis |
| [98] | Ginsenoside Rg3 | Liver | Tumor bearing rats | Apoptosis, Immune responses |
| [99] | Curcumin | Breast | MCF-7 | Anti-inflammation |
| [100] | Matrine | Lung | HepG2 | Proliferation and metastasis chemosensitization |
| [72] | Quercetin | Lung | HepG2 | Apoptosis |
| [95] | Zilongjin | Breast | MCF-7 | Inhibits malignant proliferation, apoptosis |
| [101] | Triterpenes from Ganoderma lucidum | Cervical | HeLa | Cell death, oxidative stress, calcium signaling, and ER stress |
| [64] | Curcumin | Breast | MCF-7 | Apoptosis |
| [102] | Triterpenes from Patrinia heterophylla | Leukemia | K562 | Energy metabolism, oxidative stress, signal transduction, differential induction, protein biosynthesis, and apoptosis |
| [54] | Oxymatrine | Cervical | HeLa | Inhibits proliferation, apoptosis |
| [103] | Sanguinarine from Papaveraceae family | Pancreatic | BxPC-3, MIA PaCa-2 | Decreases cellular hypoxia and cell proliferation, induces apoptosis leading to cancer cells inhibition |
| [89] | Zeng Sheng Ping (Antitumor B) | Lung | Mouse lung | Ubiquitin-proteasome, Notch, Ras-MAPK, G13 pathway, cell proliferation, differentiation, and apoptosis |
| [104] | Aidi injection | Breast | MCF-7 | Inhibits proliferation, apoptosis |
| [96] | Zilongjin | Lung | A549, H446, H460, and H520 | Cell cycle regulation, MAPK cascade, and apoptosis |
| [87] | Compound Kushen Injection | Breast | MCF-7 | Cell cycle regulation, cell growth related pathway |
| Groups of Natural Compounds | Metal Derivatized Natural Compounds | Source | Cell Lines/Model | Mechanisms of Actions | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Alkaloids | GL331 | Compound: Podophyllotoxin Plant: Podophyllum species | KB/VCR, MCF-7/ADR, and HL60/VCR | TOPO II inhibitor, cell cycle arrest at G2, cause DNA breakage and apoptosis via inhibiting protein tyrosine kinase | GL331 shows greater cytotoxicity in vitro and in vivo, and overcomes multi-drug resistance (MDR) compared to etoposide. GL331 is now in phase II clinical trial | [107] |
| [H-MT][GaCl4] [H-MT][AuCl4] [Sn(H-MT)Cl5] | Compound: MT (Matrine) Plant: Sophora flavescens | SW480, HeLa, HepG2, and MCF-7 | Cell cycle arrest at the G2/M phase | MT + Gallium (GaCl4) and MT + Gold (AuCl4) enhanced the cytotoxicity better than MT alone and cisplatin | [108] | |
| [Ru(N–N)2 (Norharman)2] (SO3CF3)2 | Compound: Norharman Plant: Peganum harmala L. | HepG2, HeLa, MCF-7, and MCF-10A | Cell cycle arrest at G0/G1, apoptosis via mitochondrial dysfunction and ROS accumulation | IC50 value of the complex is much lower and the anti-proliferative activity is much higher than those of Norharman and cisplatin | [109] | |
| [L+H][AuCl4] [AuCl3 L] | Compound: Liriodenine (L) Plant: Zanthoxylum nitidum | MCF-7 | TOPO I inhibitor, cell cycle arrest at S phase | Higher anti-proliferative activity than cisplatin, Adriamycin, liriodenine alone, and NaAuCl4 | [110] | |
| Flavonoids | hesperetin [CuL2(H2O)2]nH2O | Compound: hesperetin, Plant: Stilbella fimetaria | HepG2, and SGC-7901 | Growth inhibition | DNA binding affinity of hesperetin-Cu(II) complex is stronger than that of free hesperetin | [111] |
| Zn(morin)2.3H2O Cu(morin)2.2H2O | Compound: Morin Plant: Maclura pomifera | Hep-2, BBHK-2, BHK21, and HL-60 | In vitro antitumor activity | Higher anti-proliferative activity than morin alone | [106] | |
| Cu(Que)2(H2O)2 | Compound: quercetin, Plant: various fruits and vegetables | A549 | DNA breakage, apoptosis via generation of ROS and intercalation into DNA | Higher cytotoxic activity than that of quercetin alone | [112] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int. J. Mol. Sci. 2017, 18, 656. https://doi.org/10.3390/ijms18030656
Aung TN, Qu Z, Kortschak RD, Adelson DL. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. International Journal of Molecular Sciences. 2017; 18(3):656. https://doi.org/10.3390/ijms18030656
Chicago/Turabian StyleAung, Thazin Nwe, Zhipeng Qu, R. Daniel Kortschak, and David L. Adelson. 2017. "Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action" International Journal of Molecular Sciences 18, no. 3: 656. https://doi.org/10.3390/ijms18030656








