Novel Diagnostic and Predictive Biomarkers in Pancreatic Adenocarcinoma
Abstract
:1. Introduction
2. Traditional Serum Biomarkers
2.1. CA 19-9 for Detecting Pancreatic Cancer
2.2. CA 19-9 for Determining Patient Prognosis
3. Novel Serum Biomarkers
3.1. DNA Methylation: Detection and Prognosticating
3.2. Cell-Free Nucleosomes: Detection
3.3. MicroRNAs: Detection
3.4. Cell-Free Tumor DNA: Early Response Assessment
3.5. Multimarker Panels for Detection
3.6. Multimarker Panels: Prognostication
4. Imaging Biomarkers
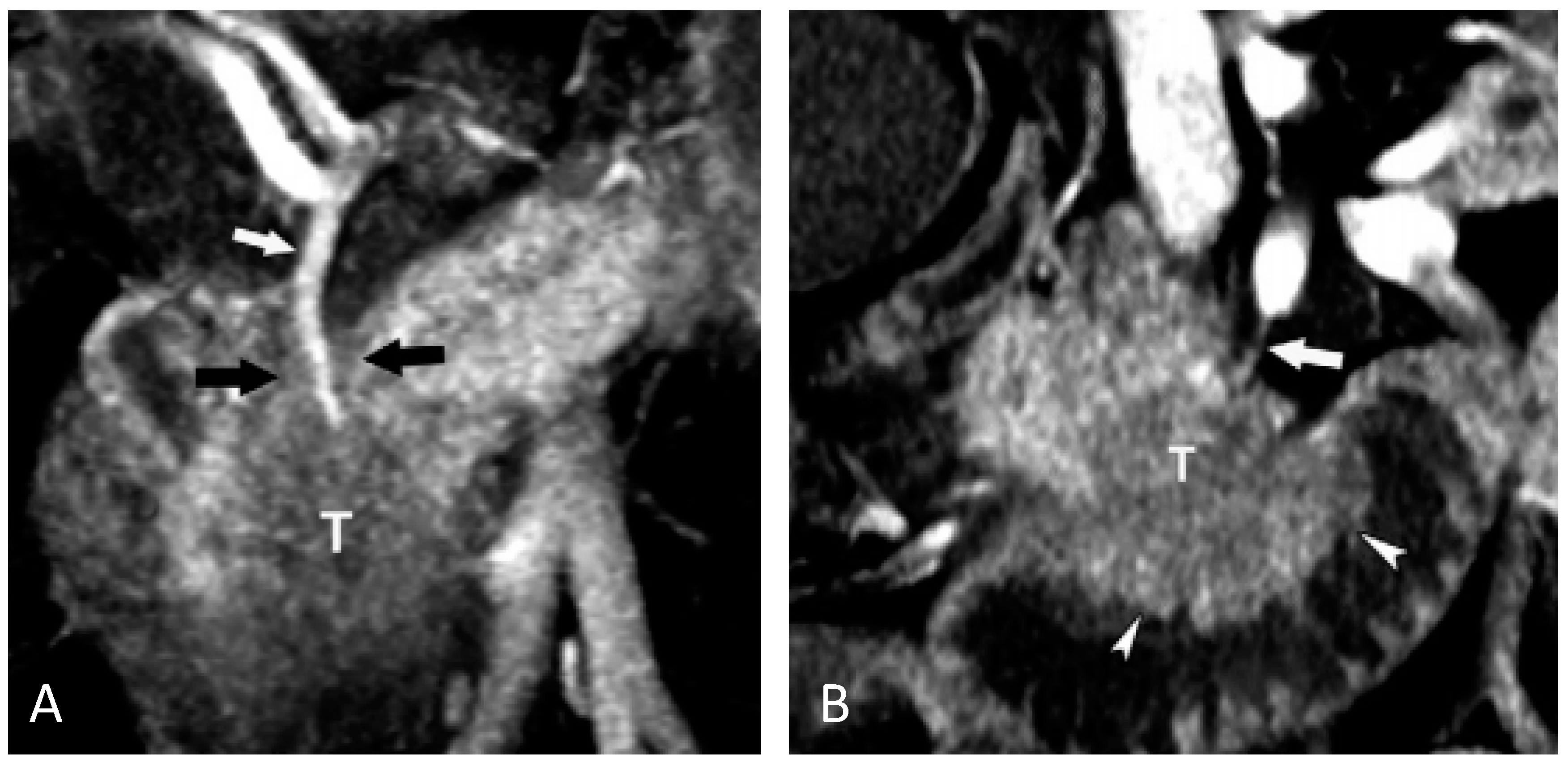
4.1. Imaging Markers for Detecting Pancreatic Cancer
4.2. Computed Tomography Imaging Markers for Predicting Patient Outcome
4.3. Positron Emission Tomography Marker for Predicting Patient Response
4.4. Functional Magnetic Resonance Parameters as Predictive Markers
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2016, 66, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Fernandez-del Castillo, C. Clinical Manifestations, Diagnosis, and Staging of Exocrine Pancreatic Cancer; Tanabe, K.K., Howell, D.A., Eds.; UpToDate: Waltham, MA, USA, 2016. [Google Scholar]
- Esposito, I.; Konukiewitz, B.; Schlitter, A.; Kloeppel, G. Pathology of pancreatic ductal adenocarcinoma: Facts, challenges, and future developments. World J. Gastroenterol. 2014, 20, 13833–13841. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 326, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Matthaei, H.; Wu, J.; Hong, S.M.; Yu, J.; Borges, M.; Hruban, R.H.; Maitra, A.; Kinzler, K.; Vogelstein, B.; et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012, 142, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010.
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Ritchey, J.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S. Validation of the 6th edition AJCC pancreatic cancer staging system: Report from the national cancer database. Cancer 2007, 110, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Schomer, D.; Dragovich, T. Anatomical, physiological, and molecular imaging of pancreatic cancer: Current clinical use and future implications. BioMed Res. Int. 2015, 2015, 269641. [Google Scholar] [CrossRef] [PubMed]
- Goggins, M. Molecular markers of early pancreatic cancer. J. Clin. Oncol. 2005, 23, 4524–4531. [Google Scholar] [CrossRef] [PubMed]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [PubMed]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. EJSO 2007, 33, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Tessler, D.A.; Catanzaro, A.; Velanovich, V.; Havstad, S.; Goel, S. Predictors of cancer in patients with suspected pancreatic malignancy without a tissue diagnosis. Am. J. Surg. 2006, 191, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, J.R.; Puig, C.A.; Schubert, C.R.; Groeschl, R.T.; Habermann, E.B.; Kendrick, M.L.; Nagorney, D.M.; Smoot, R.L.; Farnell, M.B.; Truty, M.J. Carbohydrate antigen 19-9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: A national cancer database study. J. Am. Coll. Surg. 2016, 223, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.H.; Kim, S.Y. Elevated serum CA 19-9 at screening tests: Underlying conditions and role of abdominopelvic CT. Eur. Radiol. 2014, 24, 2435–2448. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, K.T.; Lee, J.K.; Paik, S.W.; Rhee, J.C.; Choi, K.W. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J. Gastroenterol. Hepatol. 2004, 19, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Satake, K.; Takeuchi, T.; Hommas, T; Ozaki, H. CA 19-9 as a screening and diagnostic tool in symptomatic patients: The Japanese experience. Pancreas 1994, 9, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.G.; Canter, R.J.; Bold, R.J. Preoperative CA 19-9 kinetics as a prognostic variable in radiographically resectable pancreatic adenocarcinoma. J. Surg. Oncol. 2015, 111, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Distler, M.; Pilarksy, E.; Kersting, S.; Grutzmann, R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas—A retrospective tumor marker prognostic study. Int. J. Surg. 2013, 11, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, P.; Wang, F.; Li, D.; Xie, E.; Zhang, Y.; Pan, S. Relationship between serum CA19-9 and CEA levels and prognosis of pancreatic cancer. Ann. Transl. Med. 2015, 3, 328–331. [Google Scholar] [PubMed]
- Imaoka, H.; Mizuno, N.; Hara, K.; Hijioka, S.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Hieda, N.; Yoshida, T.; et al. Prognostic impact of carcinoembryonic antigen (CEA) on patients with metastatic pancreatic cancer: A retrospective cohort study. Pancreatology 2016, 16, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Heinemann, V.; Kullmann, F.; Laubender, R.; Klose, C.; Bruns, C.J.; Holdenreider, S.; Modest, D.P.; Schulz, C.; Boeck, S. Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: Results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J. Cancer Res. Clin. Oncol. 2013, 139, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Karabulut, S.; Ciftci, R.; Sen, F.; Sakar, B.; Disci, R.; Duranyildiz, D. Serum levels of LDH, CEA, and CA 19–09 have prognostic roles on survival in patients with metastatic pancreatic cancer receiving gemcitabine-based chemotherapy. Cancer Chemother. Pharacol. 2014, 73, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Mroczko, B.; Groblewska, M.; Gryko, M.; Kedra, B.; Szmitkowski, M. Diagnostic usefulness of serum interleukin 6 (IL-6) and C-reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J. Clin. Lab. Anal. 2010, 24, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, O.; Tryndyak, V.P.; Montgomery, B.; Boyko, A.; Kutanzi, K.; Zemp, F.; Warbritton, A.R.; Latendresse, J.R.; Kovalchuk, I.; Beland, F.A.; et al. Estrogen-induced rat breast carcinogenesis is characterized by alterations in DNA methylation, histone modifications, and aberrant microRNA expression. Cell Cycle 2007, 6, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S; Bamlet, W.R.; Oberg, A.L.; de Andrade, M.; Matsumoto, M.E.; Tang, H.; Thibodeau, S.N.; Wang, L. Leukocyte DNA methylation signature differentiates pancreatic cancer patients from healthy controls. PLoS ONE 2011, 6, e18223. [Google Scholar] [CrossRef] [PubMed]
- Dauksa, A.; Gulbinas, A.; Barauskas, G.; Pundzius, J.; Oldenburg, J.; El-Maarri, O. Whole blood DNA aberrant methylation in pancreatic adenocarcinoma shows association with the course of the disease: A pilot study. PLoS ONE 2012, 7, e37509. [Google Scholar] [CrossRef] [PubMed]
- Bauden, M.; Pamart, D.; Ansari, D.; Herzog, M.; Eccleston, M.; Micalleft, J.; Andersson, B.; Andersson, R. Circulating nucleosomes as epigenetic biomarkers in pancreatic cancer. Clin. Epigenet. 2015, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Ichikawa, D.; Miyamae, M.; Morimura, R.; Ikoma, H.; Konishi, H.; Shiozaki, A.; Taniguchi, H.; Otsuji, E. Malignant potential in pancreatic neoplasm: New insights provided by circulating miR0223 in plasma. Expert Opin. Biol. Ther. 2015, 15, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, H.; Nouso, K.; Miyahara, K.; Morimoto, Y.; Dohi, C.; Tsutsumi, K.; Kato, H.; Matsubara, T.; Okada, H.; Yamamoto, K. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer 2015, 121, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.E.; Nolen, B.M.; Zeh, H.J.; Allen, P.J.; Eloubeidi, M.A.; Goldberg, M.; Elton, E.; Arnoletti, J.P.; Christein, J.D.; Vickers, S.M.; et al. Serum biomarker panels for the detection of pancreatic cancer. Clin. Cancer Res. 2011, 17, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Zahn, J.M.; Horecka, J.; Kunz, P.L.; Ford, J.M.; Fisher, G.A.; Le, Q.T.; Chang, D.T.; Ji, H.; Koong, A.C. Identification of a biomarker panel using a multiplex proximity ligation assay improves accuracy of pancreatic cancer diagnosis. J. Transl. Med. 2009, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Nolen, B.M.; Brand, R.E.; Prosser, D.; Velikokhatnaya, L.; Allen, P.J.; Zeh, H.J.; Grizzle, W.E.; Lomakin, A.; Lokshin, A.E. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS ONE 2014, 9, e94928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.-L.; Lan, C.; Pei, H.; Yang, S.-N.; Liu, Y.-F.; Xiao, L.-L. Applicative value of serum CA 19-9, CEA, CA 125 and CA 242 in diagnosis and prognosis for patients with pancreatic cancer treated by concurrent chemoradiotherapy. Asian Pac. J. Cancer Prev. 2015, 16, 6569–6573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, J.; Li, L.; Li, Z.; Du, Y.; Gong, Y. The neuronal pentraxin II gene (NPTX2) inhibit proliferation and invasion of pancreatic cancer cells in vitro. Mol. Biol. Rep. 2011, 38, 4903–4911. [Google Scholar] [CrossRef]
- Kremer, A.; Wilkowski, R.; Holdenrieder, S.; Nagel, D.; Stieber, P.; Seidel, D. Nucleosomes in pancreatic cancer patients during radiochemotherapy. Tumor Biol. 2005, 26, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittwer, C.; Boeck, S.; Heinemann, V.; Haas, M.; Stieber, P.; Nagel, D.; Holdenreider, S. Circulating nucleosomes and immunogenic cell death markers HMGB1, sRAGE and DNAse in patients with advanced pancreatic cancer undergoing chemotherapy. Int. J. Cancer 2013, 133, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Croce, C.M. MicroRNAs in cancer: Small molecules with a huge impact. J. Clin. Oncol. 2009, 27, 5848–5856. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.A.; Pai, J.; Chiu, W.; Ng, K.; Hellendag, M.G.; Heestand, G.; Chang, D.T.; Tu, D.; Moore, M.J.; Parulekar, W.R.; et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA 2014, 311, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Kundranda, M.N.; Beck, J.; Braun, D.P.; Urnovitz, H.B.; Mitchell, W.M.; Schutz, E. Genomic change index (GCI) and liquid biopsies in predicting and monitoring reponse to therapy in advanced pancreatic ductal adenocarcinoma (PDAC). J. Clin. Oncol. 2015, 33 (Suppl. S3), 309. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Li, H.; Wu, Y.; Zhang, H.; Chen, W. Tumor markers CA19-9, CA 242 and CEA in the diagnosis of pancretic cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 11683–11691. [Google Scholar] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.-C.; Liang, P.-C.; Jan, S.; Yang, C.-Y.; Tien, Y.-W.; Wei, S.-C.; Wong, J.-M.; Chang, Y.-T. Increase diagnostic accuracy in differentiating focal type autoimmune pancreatitis from pancreatic cancer with combined serum IgG4 and CA 19-9 levels. Pancreatology 2014, 14, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.S.; Hwang, I.K.; Kang, B.K.; Cho, J.Y.; Yoon, Y.-S.; Hang, H.-S.; Hwang, J.-H. Postoperative carcinoembryonic antigen as a complementary tumor marker of carbohydrate antigen 19-9 in pancreatic ductal adenocarcinoma. J. Korean Med. Sci. 2015, 30, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Reitz, D.; Gerger, A.; Seidel, J.; Kornprat, P.; Samonigg, H.; Stotz, M.; Szkandera, J.; Pichler, M. Combination of tumour marker CEA and CA 19-9 improves the prognostic prediction in patients with pancreatic cancer. J. Clin. Path. 2015, 68, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, H.; Wang, W.; Wu, C.; Chen, Y.; Yang, J.; Cen, P.; Xu, J.; Liu, C.; Long, J.; et al. A preoperative serum signature of CEA+/CA125+/CA19-9>=1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int. J. Cancer 2015, 136, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Tsavaris, N.; Kosmas, C.; Papadoniou, N.; Kopteridis, P.; Tsigritis, K.; Dokou, A.; Sarantonis, J.; Skopelitis, H.; Tzivras, M.; Gennatas, K.; et al. CEA and CA-19.9 serum tumor markers as prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: A retrospective analysis. J. Chemother. 2009, 21, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Gangi, S.; Fletcher, J.G.; Christensen, J.A.; Harmsen, W.S.; Crownhart, B.S.; Chari, S.T. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: Retrospective review of CT scans obtained before diagnosis. Am. J. Roentgenol. 2004, 182, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Kim, M.-J.; Choi, J.-Y.; Hong, H.-S.; Chung, Y.E.; Lim, J.S. Indicative findings of pancreatic cacner in prediagnostic CT. Eur. Radiol. 2009, 19, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Krishna, N.B.; Mehra, M.; Reddy, A.V.; Agarwal, B. EUS/EUSFNA for suspected pancreatic cancer: Influence of chronic pancreatitis and clinical presentation with or without obstructive jaundice on performance characteristics. Gastrointest. Endosc. 2009, 70, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Krishna, N.B.; Tummala, P.; Mehan, C.D.; Reddy, A.V.; Harman, J.A.; Agarwal, B. Small and potentially resectable focal pancreatic lesions noted on CT/MRI scans in nonjuaundiced patients: Likelihood of neoplasia and utility of EUS. J. Gastrointest. Surg. 2012, 16, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Laeseke, P.F.; Chen, R.; Jeffrey, R.B.; Brentnall, T.A.; Willmann, J.K. Combining in vitro diagnostics with in vivo imaging for earlier detection of pancreatic ductal adenocarcinoma: Challenges and solutions. Radiology 2015, 277, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Jeffrey, R.B.; Patel, B.N.; Dimaio, M.A.; Rosenberg, J.; Willmann, J.K.; Olcott, E.W. Preoperative multidetector CT diagnosis of extrapancreatic perineural or duodenal invasion is associated with reduced postoperative survival after pancreaticoduodenectomy for pancreatic adenocarcinoma: Preliminary experience and implications for patient care. Radiology 2016, 283, 816–825. [Google Scholar]
- Koay, E.J.; Truty, M.J.; Cristini, V.; Thomas, R.B.; Chen, R.; Chatterjee, D.; Kang, Y.; Bhosale, P.R.; Tamm, E.P.; Crane, C.H.; et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J. Clin. Investig. 2014, 124, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Gabata, T.; Matsui, O.; Mochizuki, K.; Kitagawa, H.; Kayahara, M.; Ohta, T.; Nakanuma, Y. Enhancement patterns of pancreatic adenocarcinoma on conventional dynamic multi-detector row CT: Correlation with angiogenesis and fibrosis. World J. Gastroenterol. 2009, 15, 3114–3121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Li, J.-S.; Lu, G.-M.; Zhang, X.-H.; Chen, Z.-Q.; Meng, K. Correlation of CT enhancement, tumor angiogenesis and pathologic grading of pancreatic carcinoma. World J. Gastroenterol. 2003, 9, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Fukukura, Y.; Takumi, K.; Higashi, M.; Shinchi, H.; Kamimura, K.; Yoneyama, T.; Tateyama, A. Contrast-enhanced CT and diffusion-weighted MR imaging: Performance as a prognostic factor in patients with pancreatic ductal adenocarcinoma. Eur. J. Radiol. 2014, 83, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Shin, J.Y.; Park, J.-S.; Jeong, S.; Jeon, Y.S.; Choi, M.H.; Choi, H.J.; Moon, J.H.; Hwang, J.C.; Yang, M.J.; et al. Vascular enhancement pattern of mass in computed tomography may predict chemo-responsiveness in advanced pancreatic cancer. Pancreatitis 2017, 17, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, X.; Xue, H.; Wu, H.; Chen, G.; Sun, H.; He, Y.; Jin, Z.; Liang, Z.; Zhang, Z. CT imaging biomarkers predict clinical outcomes after pancreatic cancer surgery. Medicine 2016, 95, e2664. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Ji, S.; Qin, Y.; Xu, J.; Zhang, B.; Xu, W.; Liu, J.; Long, J.; Liu, C.; Liu, L.; et al. Metabolic tumor burden is associated with major oncogenomic alterations and serum tumor markers in patients with resected pancreatic cancer. Cancer Lett. 2015, 360, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Davis-Yadley, A.H.; Abbott, A.M.; Pimiento, J.M.; Chen, D.-T.; Malafa, M.P. Increased Expression of the Glucose Transporter Type 1 Gene Is Associated With Worse Overall Survival in Resected Pancreatic Adenocarcinoma. Pancreas 2016, 45, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Chikamoto, A.; Inoue, R.; Komohara, Y.; Sakamaki, K.; Hashimoto, D.; Shiraishi, S.; Takamori, H.; Yamashita, Y.-I.; Yoshida, N.; Yamanaka, T.; et al. Preoperative High Maximum Standardized Uptake Value in Association with Glucose Transporter 1 Predicts Poor Prognosis in Pancreatic Cancer. Ann. Surg. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pimiento, J.M.; Davis-Yadley, A.H.; Kim, R.D.; Chen, D.-T.; Eikman, E.A.; Berman, C.G.; Malafa, M.P. Metabolic activity by 18F-FDG-PET/CT is prognostic for stage I and II pancreatic cancer. Clin. Nucl. Med. 2016, 41, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Cao, S.; Sun, Y.-N.; Wu, R.; Chi, F.; Tang, M.-Y.; Jin, X.-Y.; Chen, X.-D. Standardized uptake value on positron emission tomography/computed tomography predicts prognosis in patients with locally advanced pancreatic cancer. Abdom. Radiol. 2015, 40, 3117–3121. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Chung, H.W.; Park, S.W.; Chung, J.B.; Yun, M.; Lee, J.D.; Song, S.Y. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J. Clin. Gastroenterol. 2006, 40, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Heilbrun, L.K.; Venkatramanamoorthy, R.; Lawhorn-Crews, J.M.; Zalupski, M.M.; Shields, A.F. Using 18F Fluorodeoxyglucose positron emission tomography (FDG PET) to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am. J. Clin. Oncol. 2010, 33, 257–261. [Google Scholar]
- Rose, D.M.; Delbeke, D.; Beauchamp, R.D.; Chapman, W.C.; Sandler, M.P.; Sharp, K.W.; Richards, W.O.; Wright, J.K.; Frexes, M.E.; Pinson, C.W.; et al. 18Fluorodeoxyglucose-positron emission tomography in the management of patients with suspected pancreatic cancer. Ann. Surg. 1999, 229, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Ueno, M.; Ohkawa, S.; Yoshida, T.; Doiuchi, T.; Ito, K.; Inoue, T. Advanced pancreatic cancer: The use of the apparent diffusion coefficient to predict response to chemotherapy. Br. J. Radiol. 2009, 82, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, L.; Yan, X.; Sun, Y.; Zhao, D.; Ji, Y.; Wang, K.; Chen, X.-D.; Shen, B. DCE-MRI-derived parameters in evaluating abraxane-induced early vascular response and the effectiveness of its synergistic interaction with cisplatin. PLoS ONE 2016, 11, e162601. [Google Scholar] [CrossRef] [PubMed]
| Patient Groups | Sensitivity (%) | Specificity (%) | PPV | NPV |
|---|---|---|---|---|
| Symptomatic | 79–81 | 82–90 | 72 | 81–96 |
| Asymptomatic | 100 | 98.5 | 0.03–0.9 | - |
| CA 19-9; CEA | Mean Survival (months) |
|---|---|
| ≤75 U/mL; ≤3 ng/mL | 33.3 |
| >75 U/mL; ≤3 ng/mL | 28.5 |
| or | |
| ≤75 U/mL; >3 ng/mL | |
| >75 U/mL; >3 ng/mL | 23.9 |
| References | Marker Class | Markers | Comments |
|---|---|---|---|
| [26] | DNA Methylation | IL-10_ P348, LCN2_P86, ZAP70_P220, AIM2_P624 and TAL1_P817 | Sen: 72%; Spec: 70% for detecting PDAC. Never-smoked population |
| [27] | DNA Methylation | TNFRSCF10C, ACIN1 | Hypermethylation indicates shorter survival |
| DNA Methylation | Line-1 and ALU repeats | PDAC patients have decreased methylation in ALU and Line-1 CpG repeats | |
| [28] | Cell Free Nucleosomes | 5MC, H2AZ, H2A1.1, H3K4Me2, CA 19-9 | AUC: 0.98; Sen: 92%; Spec: 90% for detecting PDAC |
| [29] | MicroRNA | miR-223 | Elevated miR-223 increased risk for PDAC |
| [30] | Cell Free DNA | KRAS mutation | 77% concordant between actual biopsy and liquid biopsy in detecting mutation |
| References | Markers (Protein) | Comments |
|---|---|---|
| [31] | CA 19-9, ICAM-1, OPG | Sen: 78% and Spec: 94% in detecting pancreatic cancer |
| [32] | CA 19-9, OPN, CHI3L1 | Sen: 93% in detecting pancreatic cancer Studied in stage II/III patients |
| [33] | CA 19-9, CEA, Cyfra 21-1 | Increased sensitivity of detection at high level of specificity in asymptomatic subjects Studied in prostate, lung, colorectal, and ovarian screening study population |
| [34] | CA 19-1, CA 242, CA 125, CEA | Sen: 90% and Spec: 94% Studied in patients undergoing chemoradiation |
| Imaging Finding | Sensitivity (%) | Specificity (%) | Accuracy |
|---|---|---|---|
| Focal mass | 75 | 84 | 0.81 |
| Pancreatic duct dilation | 50 | 78 | 0.7 |
| Duct interruption | 45 | 82 | 0.71 |
| Upstream atrophy | 45 | 96 | 0.81 |
| Contour abnormality | 15 | 92 | 0.7 |
| CBD dilation | 5 | 92 | 0.67 |
| Reference | Patient Population | Contrast Phase: ∆HU from Unenhanced CT | Survival |
|---|---|---|---|
| [57] | Unresectable | Arterial: ≥28 | 20.8 vs. 10.9 months |
| Unresectable | Portovenous: ≥34 | 20.8 vs. 10.9 months | |
| Unresectable | Delayed: ≥36 | 20.8 vs. 11.8 months | |
| [57] | Resectable | Arterial: ≥48 | 60.8 vs. 18.3 months |
| Resectable | Portovenous: ≥56 | 60.8 vs. 18.3 months | |
| Resectable | Delayed: ≥57 | 60.8 vs. 16.4 months | |
| [58] | Unresectable (Pancreatic mass enhancement) | Arterial: ≥31.5 | Sen: 62.8%; Spec: 91.3% for predicting response to chemotherapy |
| Unresectable (Liver mass enhancement) | Arterial: ≥18 | Sen: 76%; Spec: 85.7% for predicting response to chemotherapy |
| Reference | Patient Population/Therapy | SUV Threshold | Overall Survival |
|---|---|---|---|
| [63] | Stage I/II:Curative Resection | <5 | 28 vs. 16 months |
| [64] | Locally Advanced/Chemoradiation | ≤5.5 | 16.6 vs. 12.6 months |
| [66] | Locally Advanced/Chemoradiation | ≥50% decrease following therapy | 1 year survival of 87% vs. 28% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.C.; Kundranda, M. Novel Diagnostic and Predictive Biomarkers in Pancreatic Adenocarcinoma. Int. J. Mol. Sci. 2017, 18, 667. https://doi.org/10.3390/ijms18030667
Chang JC, Kundranda M. Novel Diagnostic and Predictive Biomarkers in Pancreatic Adenocarcinoma. International Journal of Molecular Sciences. 2017; 18(3):667. https://doi.org/10.3390/ijms18030667
Chicago/Turabian StyleChang, John C., and Madappa Kundranda. 2017. "Novel Diagnostic and Predictive Biomarkers in Pancreatic Adenocarcinoma" International Journal of Molecular Sciences 18, no. 3: 667. https://doi.org/10.3390/ijms18030667





