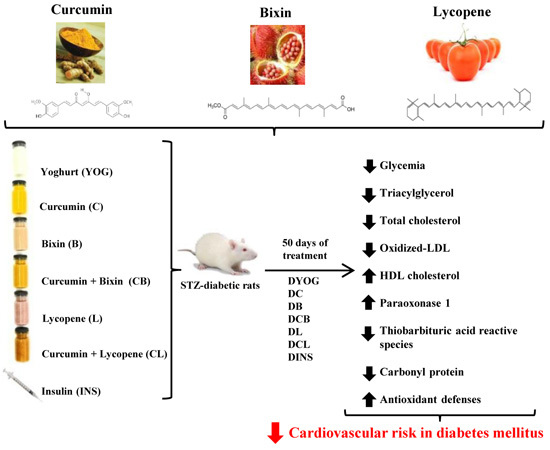
Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Experimental Model of Type 1 Diabetes Mellitus (DM)
2.2. Body Weight, Adipose Tissue and Skeletal Muscle Weights and Physiological Parameters
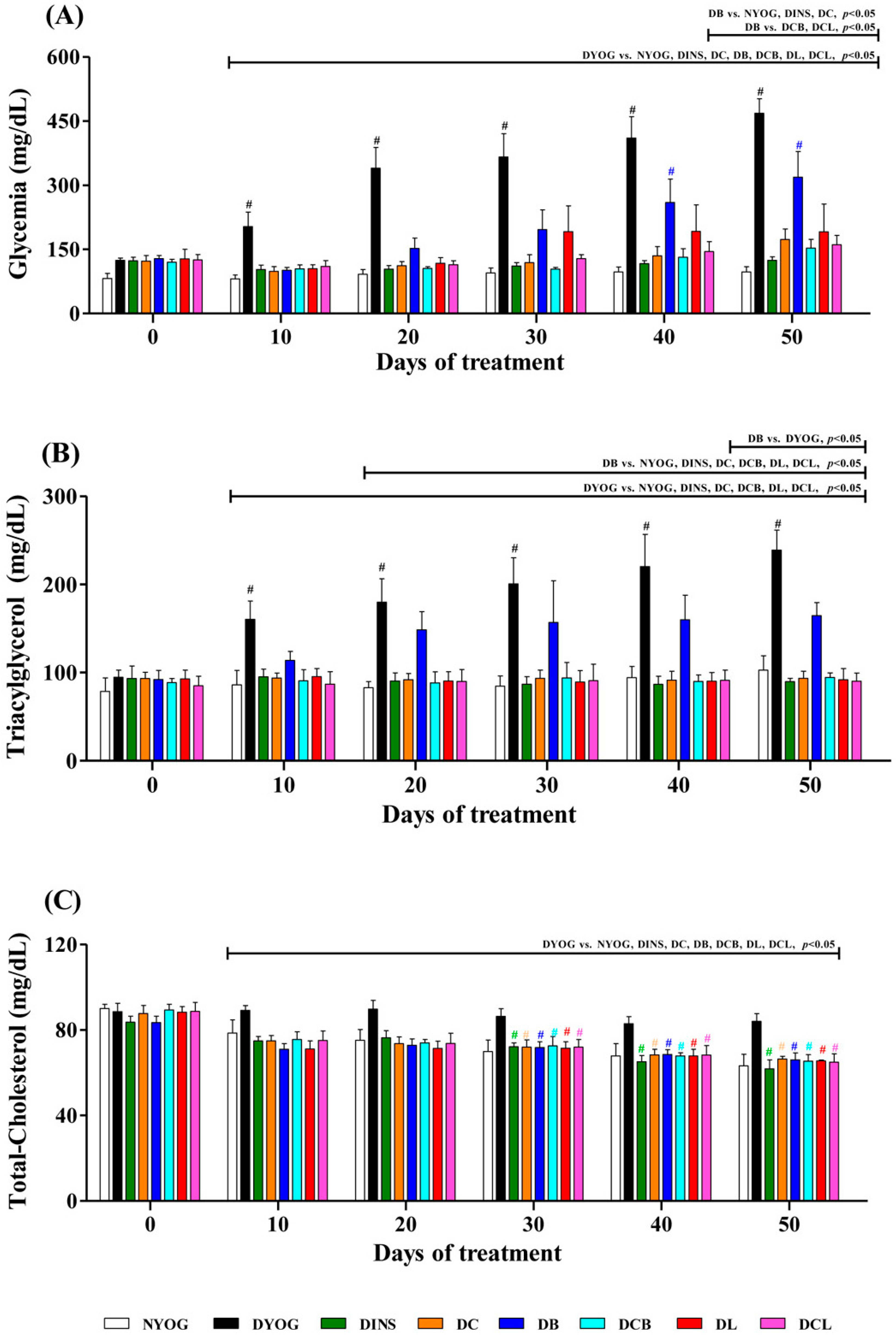
2.3. Plasma Glucose Levels
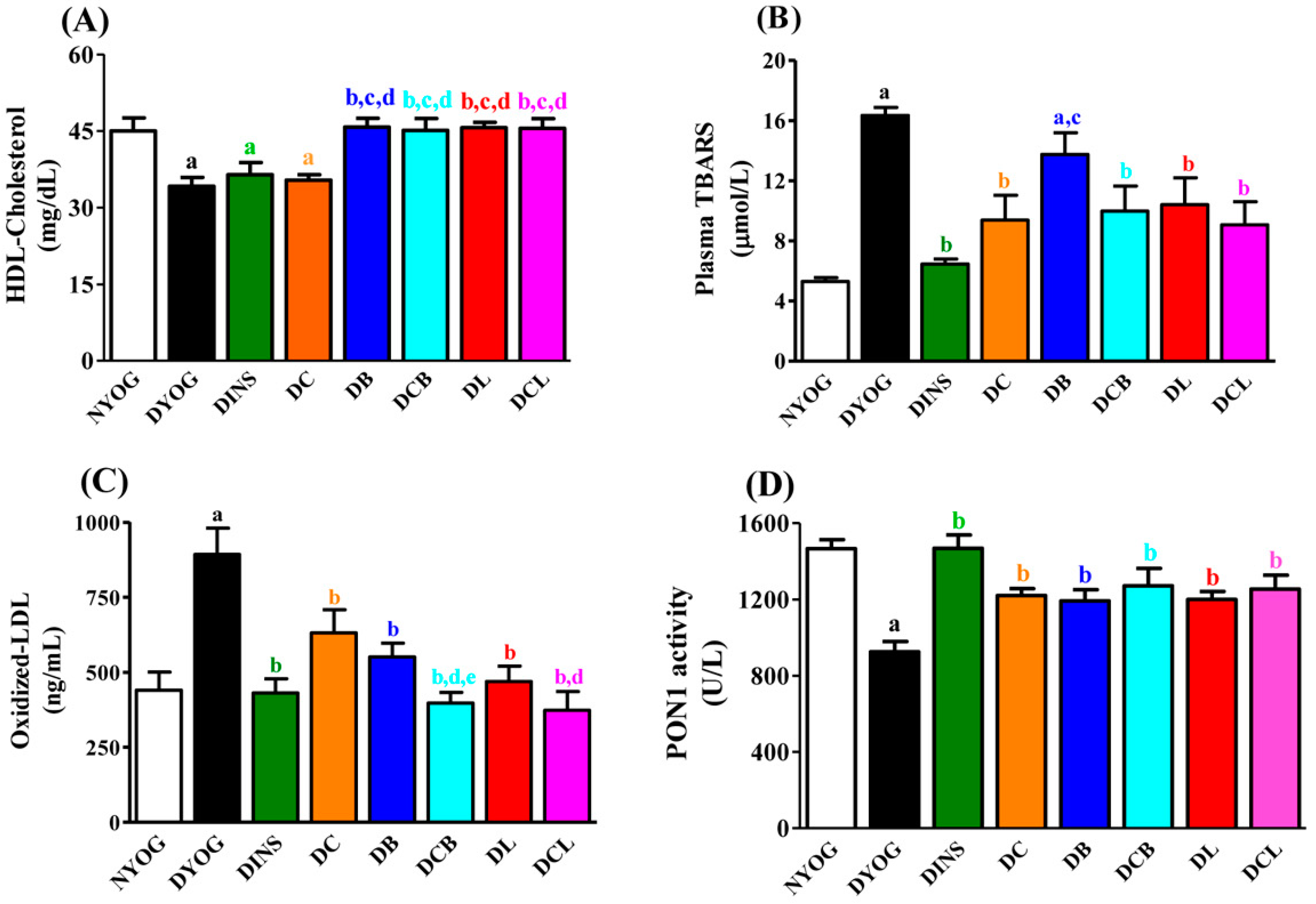
2.4. Lipid Profile
2.5. Plasma Oxidative Stress Biomarkers
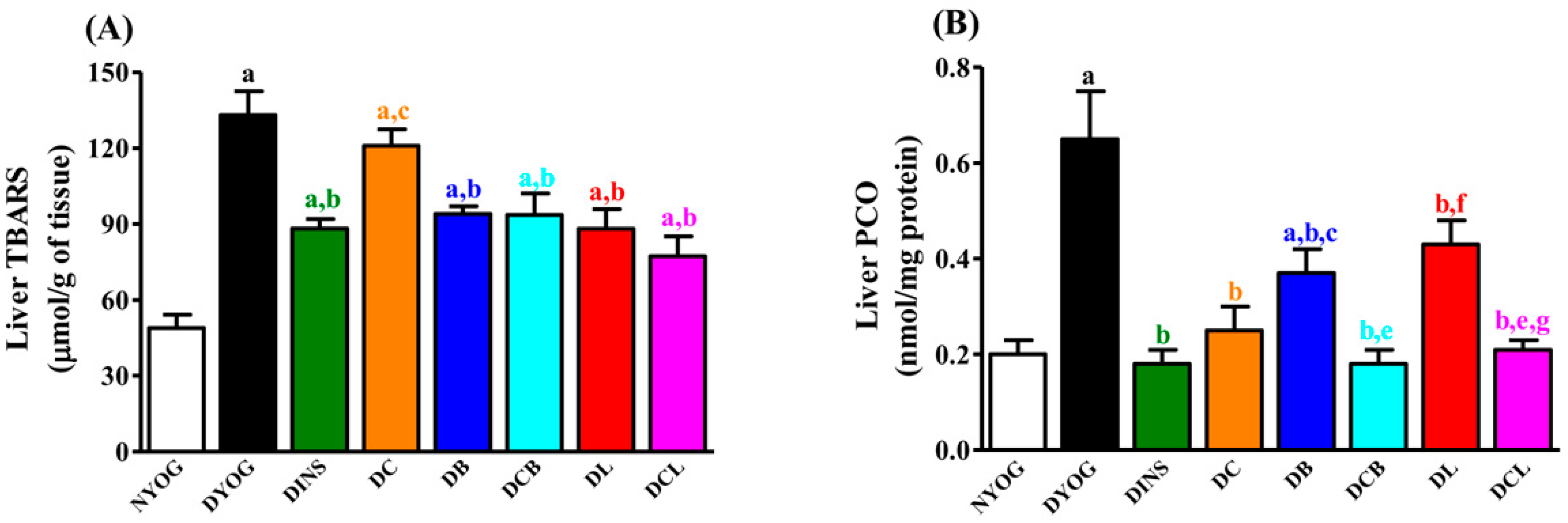
2.6. Liver Oxidative Stress Biomarkers
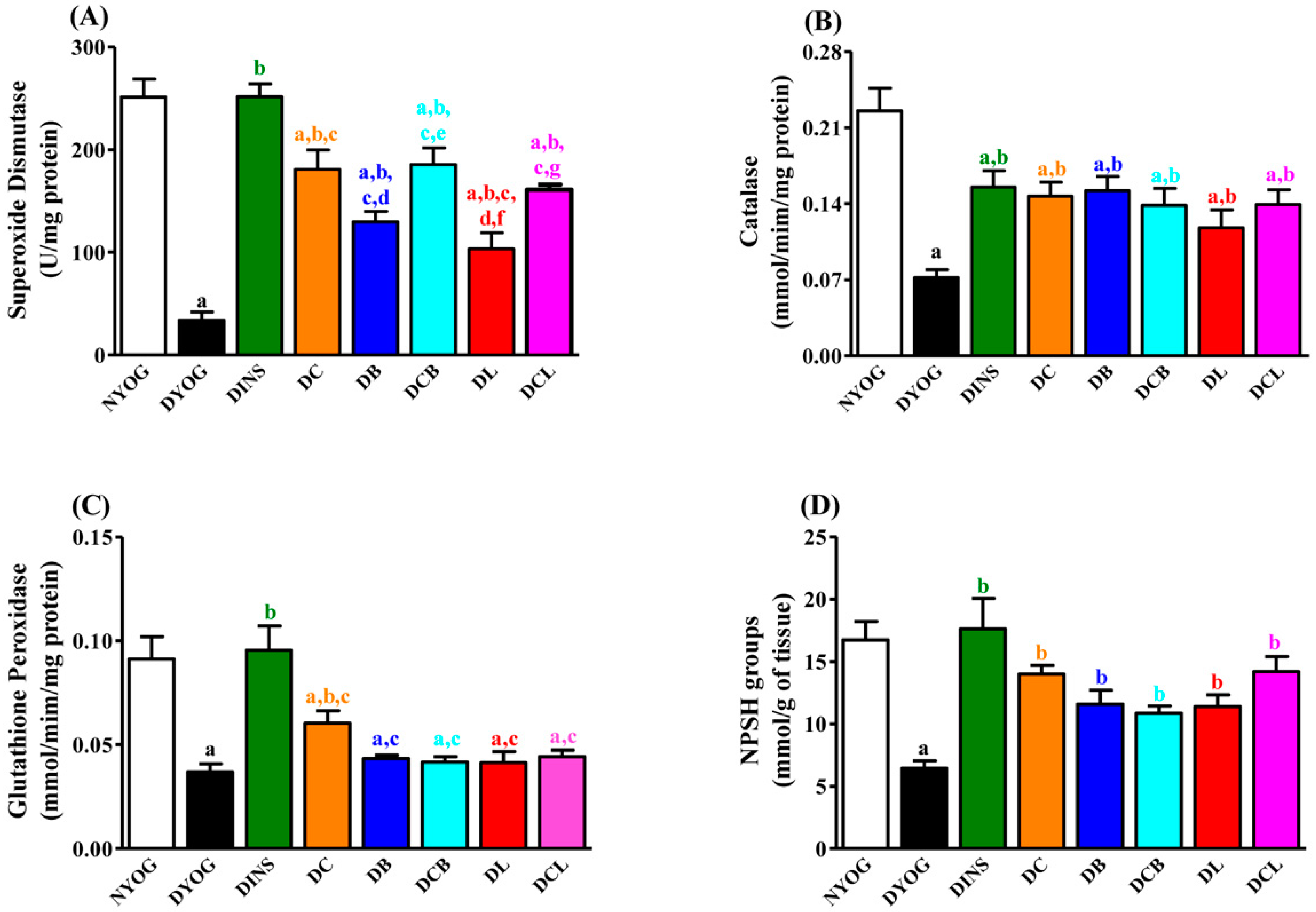
2.7. Liver Antioxidant Activity
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Induction of Experimental Diabetes Mellitus
4.3. Experimental Design and Treatment
4.4. Oxidative Stress Biomarkers
4.4.1. Lipid Peroxidation (LPO)
4.4.2. Protein Carbonyl Groups (PCO)
4.4.3. Oxidized LDL (ox-LDL)
4.4.4. Non-Protein Sulfhydryl (NPSH) Groups
4.4.5. Paraoxonase 1 (PON1) Activity
4.5. Activities of the Antioxidant Enzymes in Liver
4.5.1. Sample preparation
4.5.2. Superoxide Dismutase (SOD) Activity
4.5.3. Catalase (CAT) Activity
4.5.4. Glutathione Peroxidase (GSH-Px) Activity
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAPH | 2′,2′-azobis(2-amidinopropane) hydrochloride |
| CAT | catalase |
| CVD | cardiovascular disease |
| DM | diabetes mellitus |
| EDL | extensor digitorum longus |
| GSH-Px | glutathione peroxidase |
| H2O2 | hydrogen peroxide |
| HDL | high-density lipoprotein |
| LDL | low-density lipoprotein |
| LPO | lipid peroxidation |
| MDA | malondialdehyde |
| NADPH | nicotinamide adenine dinucleotide phosphate, reduced form |
| NBT | nitroblue tetrazolium chloride |
| NPSH | non-protein sulfhydryl groups |
| O2•− | superoxide anion radical |
| ox-LDL | oxidized LDL |
| PCO | protein carbonyl groups |
| PON1 | paraoxonase 1 |
| ROS | reactive oxygen species |
| SEM | standard error of mean |
| SOD | superoxide dismutase |
| STZ | streptozotocin |
| TBA | thiobarbituric acid |
| TBARS | thiobarbituric acid reactive substances |
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed. Available online: http://www.diabetesatlas.org/ (accessed on 12 December 2016).
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Rocha Fernandes, J.; Ogurtsova, K.; Linnenkamp, U.; Guariguata, L.; Seuring, T.; Zhang, P.; Cavan, D.; Makaroff, L.E. IDF Diabetes Atlas estimates of 2014 global health expenditure on diabetes. Diabetes Res. Clin. Pract. 2016, 117, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kalofoutis, C.; Piperi, C.; Kalofoutis, A.; Harris, F.; Phoenix, D.; Singh, J. Type II diabetes mellitus and cardiovascular risk factors: Current therapeutic approaches. Exp. Clin. Cardiol. 2010, 12, 17–28. [Google Scholar]
- Laakso, M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms. Diabetes Care 2010, 33, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.; Volkov, S.; Subbaiah, P.V. Oxidized LDL: Diversity, patterns of recognition, and pathophysiology. Antioxid. Redox Signal. 2010, 13, 39–75. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M. Modified forms of low density lipoprotein and atherosclerosis. Atherosclerosis 1993, 98, 1–9. [Google Scholar] [CrossRef]
- Steinberg, D.; Witztum, J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Lipoprotein metabolism in the macrophage: Implications for cholesterol deposition in atherosclerosis. Annu. Rev. Biochem. 1983, 52, 223–261. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.; Hansen-Hagge, T.; Wanner, C.; Seibold, S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis 2006, 185, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Volkova, N.; Rosenblat, M.; Aviram, M. Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, or garlic. Antioxid. Redox Signal. 2000, 2, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Kumar, A.; Doblec, M. Combination therapy: A new strategy to manage diabetes and its complications. Phytomedicine. 2014, 21, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.W.; Li, S.; Zhou, Y.; Li, A.; Xu, D.; Li, H. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517–520. [Google Scholar]
- Gutierres, V.O.; Pinheiro, C.M.; Assis, R.P.; Vendramini, R.C.; Pepato, M.T.; Brunetti, I.L. Curcumin-supplemented yoghurt improves physiological and biochemical markers of experimental diabetes. Br. J. Nutr. 2012, 108, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, C.A.; Gutierres, V.O.; Assis, R.P.; Moreira, T.F.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Piperine, a natural bioenhancer, nullifies the antidiabetic and antioxidant activities of curcumin in streptozotocin-diabetic rats. PLoS ONE. 2014, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, M.; Figueiredo, C.G.; Zanchi, M.M.; Bochi, G.V.; Moresco, R.N.; Quatrin, A.; Somacal, A.; Conte, L.; Emanuelli, T. Bixin and norbixin have opposite effects on glycemia, lipidemia, and oxidative stress in streptozotocin-induced diabetic rats. Int. J. Endocrinol. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, C.G.; Xu, Y.G. Lycopene attenuates endothelial dysfunction in streptozotocin-induced diabetic rats by reducing oxidative stress. Pharm. Biol. 2011, 49, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, O.; Topsakal, S.; Haligur, M.; Aydogan, A.; Dincoglu, D. Effects of caffeine and lycopene in experimentally induced diabetes mellitus. Pancreas 2016, 45, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Gahruie, H.H.; Eskandari, M.H.; Mesbahi, G.; Hanifpour, M.A. Scientific and technical aspects of yogurt fortification: A review. Food Sci. Hum. Well. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Langella, C.; Naviglio, D.; Marino, M.; Gallo, M. Study of the effects of a diet supplemented with active components on lipid and glycemic profiles. Nutrition. 2015, 31, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Modulation of gut microbiota in the management of metabolic disorders: The prospects and challenges. Int. J. Mol. Sci. 2014, 15, 4158–4188. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome. Med. 2015, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, S.S. Probiotics and Prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Catalano, A.; Simone, R.E.; Mele, M.C.; Cittadini, A. Effect of lycopene and tomato products on cholesterol metabolism. Ann. Nutr. Metab. 2012, 61, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Etoh, H.; Utsunomiya, Y.; Komori, A.; Murakami, Y.; Oshima, S.; Inakuma, T. Carotenoids in human blood plasma after ingesting paprika juice. Biosci. Biotechnol. Biochem. 2000, 64, 1096–1098. [Google Scholar] [CrossRef] [PubMed]
- Best, L.; Elliott, A.C.; Brown, P.D. Curcumin induces electrical activity in rat pancreatic beta-cells by activating the volume-regulated anion channel. Biochem. Pharmacol. 2007, 73, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Junod, A.; Lambert, A.E.; Stauffacher, W.; Renold, A.E. Diabetogenic action of streptozotocin: Relationship of dose to metabolic response. J. Clin. Investig. 1969, 48, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Gutierres, V.O.; Campos, M.L.; Arcaro, C.A.; Assis, R.P.; Baldan-Cimatti, H.M.; Peccinini, R.G.; Paula-Gomes, S.; Kettelhut, I.C.; Baviera, A.M.; Brunetti, I.L. Curcumin Pharmacokinetic and Pharmacodynamic Evidences in streptozotocin-diabetic rats support the antidiabetic activity to be via metabolite(s). Evid. Based. Complement. Altern. Med. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE. 2011, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Meghana, K.; Sanjeev, G.; Ramesh, B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: A prophylactic and protective role. Eur. J. Pharmacol. 2007, 577, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [PubMed]
- Park, J.S.; Mathison, N.D.; Chew, B.P. Uptake and immunomodulatory role of bixin in dogs. J. Anim. Sci. 2016, 94, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Lee, J.H.; Lee, S.J.; Oh, J.H.; Shin, E.; Jang, Y.P.; Lee, Y.J. The increased cellular uptake and biliary excretion of curcumin by quercetin: A possible role of albumin binding interaction. Drug Metab. Dispos. 2012, 40, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wu, Y.; Li, J.; Zhou, H.; Wang, X. Binding of curcumin with bovine serum albumin in the presence of ι-carrageenan and implications on the stability and antioxidant activity of curcumin. J. Agric. Food Chem. 2013, 61, 7150–7155. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, M.M.; Zhou, S.Q.; Kummerow, F.A. Curcumin prevents the oxidation and lipid modification of LDL and its inhibition of prostacyclin generation by endothelial cells in culture. Prostaglandins Other Lipid Mediat. 2009, 90, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Litvinov, D.; Mahini, H.; Garelnabi, M. Antioxidant and anti-inflammatory role of paraoxonase 1: Implication in arteriosclerosis diseases. N. Am. J. Med. Sci. 2012, 4, 523–532. [Google Scholar] [PubMed]
- Gugliucci, A.; Menini, T. Paraoxonase 1 and HDL maturation. Clin. Chim. Acta 2015, 439, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S.; Primo-Parmo, S.L.; La Du, B.N. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Investig. 1998, 101, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Targeting paraoxonase-1 in atherosclerosis. Expert Opin. Ther. Targets 2013, 17, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Durrington, P.N.; Abuashia, B.; Boulton, A.J.; Mackness, M.I. Low paraoxonase activity in type II diabetes mellitus complicated by retinopathy. Clin. Sci. 2000, 98, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.R.; de Abreu, I.C.M.E.; Guerra, J.F.C.; Lage, N.N.; Lopes, J.M.M.; Silva, M.; Lima, W.G.; Silva, M.E.; Pedrosa, M.L. Açai (Euterpe oleracea Mart.) Upregulates Paraoxonase 1 Gene Expression and Activity with Concomitant Reduction of Hepatic Steatosis in High-Fat Diet-Fed Rats. Oxid. Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Marchionni, C.; Caldarelli, L.; Curatola, G. Effect of glycation of high density lipoproteins on their physicochemical properties and on paraoxonase activity. Acta Diabetol. 2001, 38, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mastorikou, M.; Mackness, B.; Liu, Y.; Mackness, M. Glycation of paraoxonase-1 inhibits its activity and impairs the ability of high-density lipoprotein to metabolize membrane lipid hydroperoxides. Diabet. Med. 2008, 25, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Varatharajalu, R.; Garige, M.; Leckey, L.C.; Reyes-Gordillo, K.; Shah, R.; Lakshman, M.R. Protective Role of Dietary Curcumin in the Prevention of the Oxidative Stress Induced by Chronic Alcohol with respect to Hepatic Injury and Antiatherogenic Markers. Oxid. Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, J.; Osada, M.A. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068–4092. [Google Scholar] [CrossRef] [PubMed]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Pepato, M.T.; Keller, E.H.; Baviera, A.M.; Kettelhut, I.C.; Vendramini, R.C.; Brunetti, I.L. Anti-diabetic activity of Bauhinia forficata decoction in streptozotocin-diabetic rats. J. Ethnopharmacol. 2002, 81, 191–197. [Google Scholar] [CrossRef]
- Trinder, P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J. Clin. Pathol. 1969, 22, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Pilz, J.; Meineke, I.; Gleiter, C. Measurement of free and bound malondialdehyde in plasma by high performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J. Chromatogr. B. Biomed. Sci. Appl. 2000, 742, 315–325. [Google Scholar] [CrossRef]
- Kohn, H.I.; Liversedge, M. On a new aerobic metabolite whose production by brain is inhibited by apomorphine, emetine, ergotamine, epinephrine, and menadione. J. Pharmacol. Exp. Ther. 1944, 82, 92–300. [Google Scholar]
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1944, 233, 346–357. [Google Scholar]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissues with Ellmann’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Costa, L.G.; McDonald, B.E.; Murphy, S.D.; Omenn, G.S.; Richter, R.J.; Motulsky, A.G.; Furlong, C.E. Serum Paraoxonase and its influence on paraoxon and chlorpyritos-oxontocixity in rats. Toxicol. Appl. Pharmacol. 1990, 103, 66–76. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E.; Kinneary, J.F. The Merck Index (an Encyclopedia of Chemicals, Drugs, and Biologicals), 12th ed.; Merck: Whitehouse Station, NJ, USA, 2006; p. 1208. [Google Scholar]
- Richter, R.J.; Jarvik, G.P.; Furlong, C.E. Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ. Cardiovasc. Genet. 2008, 1, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved assays and assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Beers, R.F., Jr.; Sizer, I.W. A spectrophotometric method of measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [PubMed]
- Rush, J.W.; Sandiford, S.D. Plasma glutathione peroxidase in healthy young adults: Influence of gender and physical activity. Clin. Biochem. 2003, 36, 345–351. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, B.; Tang, L. Metabolic memory: Mechanisms and implications for diabetic retinopathy. Diabetes Res. Clin. Pract. 2012, 96, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.I.; Nakamura, N.; Matsui, T. Glycation and cardiovascular disease in diabetes: A perspective on the concept of metabolic memory. J. Diabetes 2017, 9, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O. Management of diabetes mellitus: Could simultaneous targeting of hyperglycemia and oxidative stress be a better panacea? Int. J. Mol. Sci., 2012, 13, 2965–2972. [Google Scholar] [CrossRef] [PubMed]
| Physiological Parameters | Groups | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NYOG | DYOG | DINS | DC | DB | DCB | DL | DCL | |||||||||
| Parameters | Day 0 | Day 50 | Day 0 | Day 50 | Day 0 | Day 50 | Day 0 | Day 50 | Day 0 | Day 50 | Day 0 | Day 50 | Day 0 | Day 50 | Day 0 | Day 50 |
| Body weight (g) | 134.07 ± 8.03 | 319.32 ± 20.28 (#) | 131.56 ± 2.11 | 204.10 ± 10.86 (a,#) | 126.90 ± 2.88 | 308.00 ± 10.66 (b,#) | 130.43 ± 1.72 | 281.90 ± 6.49 (a,b,#) | 130.14 ± 3.83 | 234.83 ± 17.45 (a,c,#) | 132.13 ± 3.79 | 277.50 ± 11.12 (a,b,#) | 131.00 ± 3.44 | 246.10 ± 18.56 (a,c,#) | 133.00 ± 2.10 | 279.70 ± 10.17 (a,b,#) |
| Food intake (g/12 h) | 5.75 ± 1.40 | 9.50 ± 1.59 | 7.69 ± 0.84 | 22.00 ± 1.74 (a,#) | 7.33 ± 0.85 | 8.00 ± 0.68 (b) | 7.11 ± 0.48 | 12.83 ± 2.12 (b,#) | 7.60 ± 1.89 | 17.25 ± 1.29 (a,c,#) | 7.33 ± 0.88 | 13.67 ± 0.44 (b,#) | 7.58 ± 1.29 | 15.44 ± 2.18 (a,c,#) | 7.92 ± 0.37 | 12.70 ± 1.90 (b,#) |
| Water intake (mL/24 h) | 5.14 ± 1.75 | 17.00 ± 2.60 (#) | 39.11 ± 5.30 | 91.47 ± 9.68 (a,#) | 40.40 ± 8.42 | 18.83 ± 2.93 (b,#) | 42.20 ± 4.62 | 52.20 ± 9.20 (b) | 39.00 ± 7.03 | 60.29 ± 9.80 (a,c) | 33.11 ± 7.56 | 53.20 ± 4.31 (b,#) | 42.00 ± 5.02 | 62.57 ± 9.30 (a,c) | 45.40 ± 3.86 | 56.43 ± 7.96 (b) |
| Urinary volume (mL/24 h) | 3.58 ± 2.18 | 16.17 ± 3.34 (#) | 28.33 ± 3.12 | 108.33 ± 10.76 (a,#) | 25.80 ± 6.98 | 28.00 ± 4.21 (b) | 29.13 ± 2.16 | 54.80 ± 9.65 (a,b,#) | 29.86 ± 4.90 | 67.11 ± 10.40 (a,c,#) | 25.17 ± 4.74 | 57.17 ± 9.23 (a,b,#) | 28.00 ± 3.47 | 70.00 ± 7.33 (a,c,#) | 32.40 ± 1.66 | 56.33 ± 5.06 (a,b,#) |
| Tissues Weights | Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| NYOG | DYOG | DINS | DC | DB | DCB | DL | DCL | |
| Epididymal adipose tissue (g) | 4.753 ± 0.478 | 1.226 ± 0.112 (a) | 4.348 ± 0.2588 (b) | 1.873 ± 0.119 (a,b,c) | 1.603 ± 0.262 (a,c) | 2.160 ± 0.140 (a,b,c) | 1.522 ± 0.229 (a,c) | 1.661 ± 0.033 (a,b,c) |
| Retroperitoneal adipose tissue (g) | 4.517 ± 0.304 | 0.611 ± 0.170 (a) | 3.949 ± 0.449 (b) | 1.242 ± 0.180 (a,c) | 0.768 ± 0.276 (a,c) | 1.190 ± 0.136 (a,c) | 0.808 ± 0.248 (a,c) | 1.214 ± 0.186 (a,c) |
| Soleus muscle (g) | 0.155 ± 0.003 | 0.105 ± 0.007 (a) | 0.163 ± 0.004 (b) | 0.137 ± 0.004 (a,b) | 0.109 ± 0.010 (a,c) | 0.134 ± 0.007 (a,b) | 0.107 ± 0.010 (a,c) | 0.120 ± 0.005 (a,b) |
| EDL muscle (g) | 0.151 ± 0.004 | 0.096 ± 0.006 (a) | 0.153 ± 0.005 (b) | 0.121 ± 0.004 (a,b) | 0.095 ± 0.008 (a,c) | 0.118 ± 0.004 (a,b) | 0.093 ± 0.008 (a,c) | 0.115 ± 0.008 (a,b) |
| Biochemical Parameters | DC | DB | DL | DCB | DCL |
|---|---|---|---|---|---|
| Plasma | |||||
| Glycemia | ↓ 63% | ↓ 32% | ↓ 59% | ↓ 67% | ↓ 66% |
| Triacylglycerol | ↓ 61% | ↓ 31% | ↓ 61% | ↓ 60% | ↓ 62% |
| Total-Cholesterol | ↓ 21% | ↓ 21% | ↓ 22% | ↓ 22% | ↓ 23% |
| HDL-Cholesterol | no effect | ↑ 34% | ↑ 33% | ↑ 32% | ↑ 33% |
| TBARS | ↓ 43% | no effect | ↓ 36% | ↓ 39% | ↓ 44% |
| PON1 | ↑ 32% | ↑ 29% | ↑ 30% | ↑ 38% | ↑ 36% |
| ox-LDL | ↓ 32% | ↓ 38% | ↓ 48% | ↓ 55% * | ↓ 58% |
| Liver | |||||
| TBARS | no effect | ↓ 29% | ↓ 34% | ↓ 30% | ↓ 42% |
| PCO | ↓ 61% | ↓ 43% | ↓ 38% | ↓ 72% | ↓ 68% |
| SOD | ↑ 434% | ↑ 282% | ↑ 204% | ↑ 447% | ↑ 375% |
| CAT | ↑ 104% | ↑ 111% | ↑ 66% | ↑ 92% | ↑ 93% |
| GSH-Px | ↑ 64% | no effect | no effect | no effect | no effect |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assis, R.P.; Arcaro, C.A.; Gutierres, V.O.; Oliveira, J.O.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 332. https://doi.org/10.3390/ijms18040332
Assis RP, Arcaro CA, Gutierres VO, Oliveira JO, Costa PI, Baviera AM, Brunetti IL. Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats. International Journal of Molecular Sciences. 2017; 18(4):332. https://doi.org/10.3390/ijms18040332
Chicago/Turabian StyleAssis, Renata Pires, Carlos Alberto Arcaro, Vânia Ortega Gutierres, Juliana Oriel Oliveira, Paulo Inácio Costa, Amanda Martins Baviera, and Iguatemy Lourenço Brunetti. 2017. "Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats" International Journal of Molecular Sciences 18, no. 4: 332. https://doi.org/10.3390/ijms18040332