1. Introduction
Pain is the most persistent and common symptom in cancer patients, especially in the end-of-life setting of palliative care. Hence, international guidelines, such as those emitted during the latest American Society of Clinical Oncology (ASCO) meeting, health care professionals, as well as researchers have highlighted the importance of pain control while preserving the quality-of-life (QOL) of patients, the latter being recognized as one of the most important parameters to measure while assessing medical therapies. The guidelines also discussed the “importance of translating patient preferences for end-of-life care into specific physician medical orders” in regards to personalized medicine. In that context, opioids, and in particular morphine, remains the mainstay of analgesic therapy [
1].
Unfortunately, pain management remains unsatisfactory and it is clear that different factors, including genetic and environmental factors, are important in identifying, designing, and targeting relevant interventions in different pain settings [
2]. Traditionally, this variability has been explained by differences in bioavailability, metabolism, differences in pain perception, neurophysiological mechanisms, socio-cultural factors, as well as pharmacogenetic factors. Indeed, many studies have investigated the association between non-genetic/genetic factors and the variability of response to opioids in cancer patients [
3]. Among genetic factors, polymorphisms, in particular single nucleotide polymorphisms (SNPs) in
OPRM1, the gene encoding the mu opioid receptor and the most important target for morphine, are primary candidates for genetic influences on the efficacy of opioids. Numerous SNPs in the
OPRM1 have been identified, but only a few have been explored for possible relevance in opioid analgesia, including the c.118A>G SNP (rs1799971); patients carrying the GG genotype required higher morphine doses compared to AA patients [
4,
5]. Another relevant gene that has been identified as an important modulator of opioid efficacy and toxicity is the
COMT gene encoding the catechol-
O-methlytransferase. This enzyme metabolizes the catecholamines and is a key modulator of dopaminergic and adrenergic neurotransmission [
6]. A common functional genetic variation in the
COMT gene has been particularly studied: the c.472G>A polymorphism (rs4680; p.Val158Met) causes a valine (Val) to methionine (Met) substitution at codon 158 in the membrane-bound isoform enzyme, leading to a three- to four-fold reduced activity of the enzyme [
7,
8]. Patients with the Val allele have an enzyme up to four times more active than in patients with the Met allele and studies have shown that patients with the Met/Met genotype required lower morphine doses to achieve pain relief compared to Val/Met and Val/Val patients [
6,
9]. Finally, the c.3435C>T SNP (rs1045642) of
ABCB1 is an interesting factor affecting the pharmacokinetics of morphine. In fact, this gene encodes for P-glycoprotein (P-gp), a transmembrane efflux transporter that belongs to the family of ATP binding cassette (ABC) transporters and the variant results in a C-to-T substitution at nucleotide 3435 that has been associated with a reduced expression of duodenal P-gp in homozygous TT patients [
10]. It has also been associated with a 1.5- to 2-fold reduction in mRNA levels and/or a reduction in protein expression in some tissues [
11]. Publications have shown that
ABCB1 TT patients were “good responders” compared to CC patients [
12] and required fewer morphine doses for pain relief [
9,
13,
14].
Among all published studies, some heterogeneity in the inclusions could be noted as most of these studies included patients treated by various oral opioids (not a single molecule and rarely given intravenously) and from different pain settings (palliative and non-palliative settings). In our study, we decided to include only palliative care patients, especially patients in an end-of-life setting treated with intravenous morphine given via an infusion regimen. High pain scores and reduced QOL are two important dimensions that characterize these patients in the remaining days of their lives.
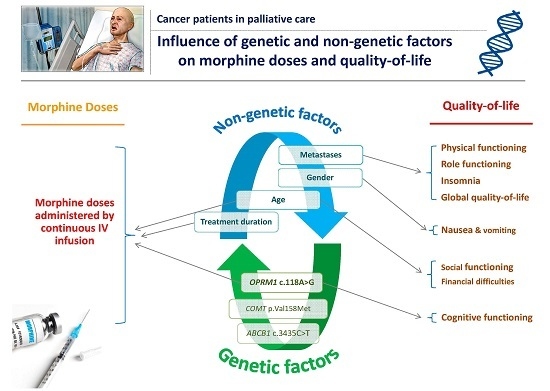
The main objective of this study was to investigate the association between genetic or non-genetic factors and morphine requirements in end-of-life setting cancer patients. The secondary objectives were to assess the relationship between these genetic and demographic/clinical factors on one side, and pain intensity and QOL on the other side. The ideal goal would be to increase the effectiveness of the available treatments, in particular morphine, and enhance the QOL of these patients.
3. Discussion
Pain management in patients with cancer, in particular in end-of-life settings, remains a challenge for clinicians due to unpredictable responses to opioid therapy. Pain has devastating consequences if unrelieved [
21], causing numerous psychosocial responses [
22,
23] as well as severe and detrimental impacts on patients’ QOL [
22,
24]. A recent review of the literature determined the importance of overcoming barriers toward effective pain treatment and the need to develop and implement interventions to optimally manage pain in patients with cancer [
22]. One of the interventions could be understanding the different factors affecting inter-individual variability in pain perception as well as drug efficacy and reported adverse drug reactions. Pharmacogenetics, also referred to as genotype-guided prescribing, is a new concept that aims to adapt medical treatments to patients’ genetic status [
25]. It allows us to understand how the genetic variations could be used to tailor pain management therapies while improving the QOL of cancer patients. Recent guidelines highlighted the importance of assessing the risk of adverse effects of opioids used in pain treatment and outlined the precautions that help ensure that cancer patients with persistent pain use opioids safely and effectively [
26].
In end-of-life pain settings, the use of infusion regimens for morphine delivery is indicated in patients in whom traditional administration routes are poorly effective or in those who cannot tolerate high doses because of systemic side effects [
27,
28,
29]. However, the administrated morphine doses are highly variable and unpredictable among patients, who continue to experience intractable pain [
27,
28]. We therefore conducted this study to evaluate the added value of determining some genetic and non-genetic factors in optimizing cancer pain treatment, in particular in end-of-life settings.
In this study, we first evaluated the allelic frequency of
OPRM1 c.118A>G,
COMT p.Val158Met, and
ABCB1 c.3435C>T. Allelic frequencies in our population were similar either to those described in Caucasian populations for
OPRM1 and
COMT SNPs [
16,
17], or to those of Asian populations for
ABCB1 c.3435C>T [
18]. This is not surprising since the Lebanese population is known for its admixture and numerous lineages [
30]. As expected, the allelic distribution was similar to the one we previously reported in post-operative Lebanese patients (
n = 95, [
15]).
We then investigated the determinants of morphine doses, pain intensity, morphine side effects and the QOL of patients.
In our study, patient age and the duration of morphine therapy were significant predictors of morphine consumption. Elderly patients required significantly less morphine than younger patients. This could be due to altered pharmacokinetics of morphine in this population (distribution, metabolism, and elimination), which can explain their reduced need for morphine to achieve pain relief [
31,
32,
33]. In addition, morphine doses increased with the duration of treatment as previously reported [
9]. Two hypotheses could be put forward to explain this result. First, it is possible that this time interval is reflective of the progression of the disease; hence, they required higher morphine doses with the progression of their disease. Second, it could be due to the desensitization or down regulation of mu opioid receptors related to the repeated administration of morphine (tolerance) [
34,
35,
36]. Moreover, patients with at least one 118G allele for
OPRM1 (AG, as none of the patients exhibited the GG genotype) received significantly higher doses of morphine than AA patients. Indeed, as previously described, the c.118A>G SNP in
OPRM1 induced a change from an asparagine to an aspartic acid residue at amino acid position 40 (p.N40D). This polymorphism is responsible for the loss of a putative N-linked glycosylation site in the N-terminal domain of the receptor, affecting the activation of the transduction pathway. In addition, the c.118A>G is associated with the μ opioid receptor (μOR) expression, the variant associated with a decrease in both mRNA expression and translation into a functional protein [
37]. Studies have shown that the variant protein exhibits three times greater binding affinity for the endopeptide β-endorphin in vitro [
38] and a reduced potency of morphine-6-glucuronide (M6G) [
39], which explains the modified response to opiates [
38]. This is probably why a homozygous carrier of the mutant 118G allele of
OPRM1 needs larger doses of morphine for pain control. Numerous studies have evaluated the association of
OPRM1 c.118A>G and the doses required for pain relief [
3]. Results, such as those reported in the present study, have shown that patients carrying at least one 118G allele required higher morphine doses for cancer pain relief [
4,
5,
9,
12]. Nevertheless, the European Pharmacogenetic Opioid Study (EPOS), which included 2294 cancer patients, has not confirmed these results. The patients were however diagnosed by different types of cancer and were treated with various opioids (morphine, oxycodone, fentanyl, and other opioids) via numerous routes (oral, subcutaneous, intravenous, intrathecal, etc.) [
40].
We did not detect any association between genetic or non-genetic factors with pain scores assessed by the VAS scores or the EORTC-QLQ-C30. Our results are in line with previously reported studies [
9,
41].
Concerning the QOL of our cancer patients, we identified different interesting factors significantly associated with various items of the EORTC-QLQ-C30 questionnaire. This latter is a very well recognized and validated assessment tool that has been formally translated into many languages. The main four factors were metastases, age, OPRM1 SNP, and gender.
Hence, our results have shown that cancer patients with metastasis have lower physical and role functioning as well as a lower GQOL and a significantly higher incidence of insomnia. All these factors seem to be interrelated as patients with metastasis have many physical and psychosocial problems associated with the disease, its progression, and treatment that compromises their QOL [
42]. In particular, patients with metastasis express more pain, anxiety, and depression [
43,
44] and many studies have suggested a bidirectional relationship between anxiety/depression and insomnia [
45,
46]. Moreover, insomnia is a major public health issue affecting the QOL of a large number of people all over the world with deleterious consequences such as long-term physical and mental exhaustion with altered mood, concentration, and memory. Therefore, people with insomnia have a deterioration of their general condition with a decrease in intellectual abilities and cognitive behavior [
47,
48].
Age was associated with social functioning and financial difficulties: the social functioning scores increased with age but the financial difficulties scores decreased with age. These results are accurate, as with age, the economic impact on the disease is less important than in younger patients and consequently, the financial difficulties are less pronounced. In addition, the impact of cancer, especially in end-of-life settings, on the social role of patients would be more problematic when the patient is young because of the increased prevalence of depression and anxiety [
43].
Cognitive alterations in cancer patients are well described. They may be attributed to the cancer disease itself, comorbidities, and treatments including opioid therapy [
49,
50]. However, the effects of SNPs on the cognitive function of opioid-treated patients with cancer have rarely been explored. A recently published study aimed at identifying associations between 113 SNPs of 41 candidate genes, high opioid dose, and cognitive dysfunction [
49]. Unfortunately, they did not explore SNPs in
OPRM1. The association of the opioid system with cognitive function has been studied in particular with the dynorphin/κ-opioid receptor (κOR) but not with the mu-opioid receptor. Thus, studies have shown that the κOR system is implicated in emotion and cognition [
51,
52]. In addition, the pharmacological blockade of κOR prevented impairments in memory performance, whereas its activation induced cognitive deficits in mice [
51,
53]. In our study, AA patients for
OPRM1 SNP had significantly lower cognitive function than AG patients. To the best of our knowledge, this is the first study to report a significant association of the opioid system with the cognitive functioning of patients treated by morphine for pain. We could stipulate that patients with the AA genotype, exhibiting a better activation of the mu-receptor, would have cognitive deficits as described with the activation of the KOR, which can explain their lower cognitive function. In the future, it would be interesting to conduct more studies to better explore these patterns using validated tools for cognitive assessment such as the Mini-Mental State Examination (MMSE).
Furthermore, our study showed that women had significantly more nausea/vomiting than men, as previously described in a large sample of European patients treated by different opioids [
54]. These results could be explained by the increased sensitivity of women treated by morphine due to hormonal variations.
Finally, our study showed that QOL was not significantly associated with morphine doses or with the type of cancer. However, the relatively small sample size in each category does not allow us to draw more conclusions.
Strengths and Limitations
We recognize the strengths and limitations of this study. Our study included patients with a plethora of different cancers. We acknowledge that some types of cancers are related to more severe pain than others as the type of pain varies across cancer types; however, the VAS scores did not vary with the type of cancer as previously discussed. In addition, to the best of our knowledge, this is first study including a homogenous population of palliative care patients especially in an end-of-life setting, receiving a stable IV daily dose of morphine for a minimum of three consecutive days. These patients did not require any other opioid or intermittent morphine doses. We acknowledge as well that it is a relatively small study for genetic associations, but this study was conducted in a homogeneous sample of Lebanese patients and numerous previous studies had been conducted with a similar number of patients [
55,
56,
57]. Further multi-centric studies with the same rigorous inclusion criteria are consequently needed on a larger sample to confirm and generalize our results on Lebanese patients, as well as for other populations. It would be interesting to include patients treated by a single molecule (morphine, oxycodone, fentanyl, etc.) and diagnosed with a single type of cancer to reduce the heterogeneity of the included population. As for the clinical assessment (for cancer pain and quality of life), researchers should consider using modular approaches for disease-specific treatment measurements such as the European Organization for Research and Treatment-QOL questionnaire EORTC-QLQ-BR23 or the functional assessment of cancer-therapy breast QOL (FACT-B) for breast cancer, the EORTC-QLQ-BN20 or FACT-Br for primary brain tumors, the EORTC-QLQ-CR29 FACT-C for colorectal cancer, etc. [
58,
59,
60]. In fact, even if the EORTC-QLQ-C30 is a well-validated assessment tool, it remains a general tool assessing the generic aspects of QOL and does not take into account the symptoms related to a specific tumour site or the treatment modality (side effects associated with a given treatment) or additional QOL domains affected by the disease or treatment (e.g., sexuality, body-image, fear of disease recurrence, etc.) [
58,
59,
60]. A final point that should be raised within this study is the chance of false positives in multiple testing. In fact, when different factors are assessed with specific endpoints (such as morphine doses or QOL items in our study), repeated statistical tests on the same data will cause α inflation and lead the researcher to a higher probability of making a Type I error. Therefore, it would be much more likely to report significant differences between some of the pairs that have no real difference. To overcome this problem, some corrections for multiple testing could be performed. In our study, although we performed multivariate analysis, we included the results of the Bonferroni correction for its information. The only factor that remained significant after the correction was the duration since the beginning of the treatment with morphine, which drives us to be more cautious when interpreting the results.
4. Materials and Methods
4.1. Study Design and Patients
Patients admitted to the Department of Hemato-oncology at Hôtel-Dieu de France Hospital (Saint-Joseph University of Beirut, Beirut, Lebanon) were enrolled in this prospective study from 14 January 2011 until 30 November 2016. The study was approved by the hospital ethical committee (Protocol No. 336, 2013) and all patients gave their written informed consent.
All included patients were above 18 years old, were diagnosed as suffering from malignant diseases as described in the results section, and had received scheduled morphine treatment corresponding to step III at the analgesic ladder of the World Health Organization (WHO) [
61]. Patients were treated with a stable morphine dose for at least three days before inclusion (no changes in the morphine dose occurred during the last three days).
Patients received an initial morphine dose via infusion pump followed by a titration until VAS < 4 (morphine diluted in normal saline serum; infusion rate 0.5 mL/h over 24 h). If the VAS ≥ 4, rescue doses of morphine (corresponding to 1/6 of the baseline daily dose) were administered. The total 24 h dose as well as pain scores were reevaluated every 24 or 48 h: if the patient required four or more rescue doses of morphine during 24 h, a new baseline dose was calculated. The new baseline dose was the sum of the old baseline and the total rescue doses needed. This baseline dose was maintained until the VAS score became ≥4, and then consequently new rescue doses were recalculated.
Other opioids from the step III analgesic ladder were not allowed. Only oral tramadol or codeine were permitted on demand if the patient still felt pain. It is noteworthy to add that none of the patients received or had received chemo or radiotherapy in the previous two weeks before enrollment.
Kidney function was assessed prior to enrollment by estimated creatinine clearance e-Clcr (using Cockcroft-Gault formula) and estimated Glomerular Filtration Rate e-GFR (using Modification of Diet in Renal Disease MDRD formula). Patients with e-Clcr less than 25 mL/min or e-GFR less than 25 mL/min/1.73 m2 were not included in the study.
Clinical and demographic information including age, gender, weight, height for the body mass index (BMI) calculation, ethnicity, time since the start of morphine treatment, cancer diagnosis and localization of metastases, and co-medication were registered by a health care provider (physician or pharmacist). Patients’ quality of life was evaluated and reported by answering the EORTC-QLQ-C30. This questionnaire is a very well recognized and validated assessment tool that has been formally translated into many languages. Moreover, all evaluated items of the EORTC-QLQ-C30 are independent [
20].
Hence, the functional state of the patients, including “Physical functioning” and “Role functioning” during the previous week, was assessed by answering a “yes” or “no” for the asked questions.
For the rest of the items in the functional state (social functioning, emotional functioning, cognitive functioning) and for all the items evaluating symptom intensity during the previous week, a four-point verbal rating scale with the notations “not at all, a little, quite a bit, and very much” was used. Social, emotional, and cognitive functioning were assessed using scores calculated by the answers of combined items of the EORTC questionnaire (items 26–27, items 21–24, items 20–25 respectively;
Table S6) as recommended [
20].
Reported symptoms were: fatigue, pain, nausea/vomiting, dyspnea, insomnia, loss of appetite, constipation, and diarrhea. In addition, and apart from the EORTC-QLQ-C30 pain score, pain was self-rated by the patients using the item of “average pain” by the VAS during a 24 h period. Briefly, patients rated pain on a numeric scale, where 0 represents “no pain” and 10 represents “pain as bad as you can imagine”, as recommended for use in clinical studies of pain. The pain scores were reported in integral numbers. In order to avoid confusion in the interpretation of results, the pain rated by the EORTC-30 score will be noted as the “normalized pain score” and the one evaluated by the VAS score will be mentioned as the “VAS score”.
Finally, patients were able to relate their global health status/quality of life. This dimension employed the 7-point response scale, from 1 to 7, with “1” corresponding to a very poor quality of life and “7” to an excellent quality of life.
4.2. Genotyping
DNA was extracted from blood cells using the QIAamp DNA Mini® Blood (Qiamp DNA Mini kit cat nb: 51304, QIAGEN®, Hilden, Germany), as recommended by the manufacturer.
Genotyping for the three SNPs was performed using the Lightcycler® 2.0 (Roche Diagnostics GmbH, Mannheim, Germany).
In summary, the reaction was carried out using 25 ng of DNA (10 ng/μL solution or 2.5 µL) in a final volume of 10 µL. The reaction mixture (10 μL) contained Fast Start Taq polymerase (10×), buffer and dNTPs, MgCl2 (10 mM); Lightcycler Fast Start DNA Master Hybridization Probes Kit® (catalogue no. 03 003 248 001, Roche Diagnostics GmbH), and 0.2 μL of each primer (20 mM) and fluorescent probes (anchor and sensor, 20 mM) (TIB Molbiol®, TIBMOLBIOL, Berlin, Germany). The samples were then loaded into composite plastic/glass capillaries (20 μL LC capillaries, Roche Diagnosis, catalogue no. 04 929 292 001, Roche Diagnostics GmbH), centrifuged, and were placed in the LightCycler sample carousel.
Genotyping of
OPRM1 (rs1799971) and
ABCB1 (rs1045642) were performed according to previously published methods [
62,
63]. Primers and probes were synthesized using TIB MOLBIOL Syntheselabor GmbH, Berlin, Germany. The sequences were as follows: for
OPRM1 c.118A>G, primer forward 5′-GCTTGGAACCCGAAAAGT-3′, primer reverse 5′-GTAGAGGGCCATGATCGTGA-3′, probes CCCGGTTCCTGGGTCAACTTGTCC-FL and 640-CTTAGATGGCAACCTGTCCGACC-PH and for ABCB1 c.3435C>T, primer forward 5′-TGTTTTCAGCTGCTTGATGG-3′, primer reverse 5′-AAGGCATGTATGTTGGCCTC-3′, probes 640-GACAACAGCCGGGTGGTGTCA and GGAAGAGATCGTGAGGGCAG-PH. For
COMT, primers were selected using the Primer 3 software [
64] and were synthesized by TIB MOLBIOL Syntheselabor GmbH, Berlin, Germany.
The PCR protocol and conditions are presented in the
Table S7. Positive heterozygous and homozygous controls (defined by direct sequencing) and negative controls (water) were systematically included in experiments.
The genotyping was conducted on patients following their evaluation. The genotyping was performed in the laboratory and none of the investigators, clinical care providers, or observers of this study were aware of the genotyping results. Therefore, the genetic testing could not have biased the pain assessment process.
4.3. Data and Statistical Analysis
Clinical data are presented as mean ± standard deviation (SD). The statistical analysis was performed using a software program (Statistical Package Software for Social Science—SPSS—for Windows version 16.0, SPSS Inc., Chicago, IL, USA). The α error was set at 0.05.
The formula for calculating the sample size requirements was the one published by Tabachnick and Fidell [
65] that takes into account the number of independent variables included in the model:
n = 50 + 8
m (
m is the number of independent variables); given that
m = 5, at least 90 subjects have to be included in the present study.
Deviation from the Hardy–Weinberg equilibrium was tested using χ2 analysis with one degree of freedom.
To determine the genetic and non-genetic factors associated with the morphine doses at 24 h, univariate analyses of categorical and continuous variables were carried out using the Mann–Whitney test or Kruskal–Wallis test and the Spearman correlation coefficient, respectively. Multiple regression analysis was used with the dose of morphine as the dependent continuous variable. Variables that showed associations with
p-values < 0.25 in univariate analyses were candidates for the multivariate model, according to the Enter method [
19]. Collinearity among the independent variables was also tested. Independent variables that highly correlated were excluded. It has already been suggested not to include two independent variables where there is a correlation of 0.7 or more. Multiple regression analyses were also used for each of the quality of life domain as the dependent variable.
Finally, we included the Bonferroni Correction for univariate analysis (pairwise comparisons using the Dunn-Bonferroni approach) that provides more confidence in the results because it reduces the probability of a Type I error by its limits on α inflation in multiple testing. Considering that morphine doses were assessed with 14 factors, we needed a p-value < 0.003 for statistical significance. Moreover, each item of the QOL was assessed with 9 factors, and the significant p-value was therefore set to a value <0.005.