Biological Effect of Licochalcone C on the Regulation of PI3K/Akt/eNOS and NF-κB/iNOS/NO Signaling Pathways in H9c2 Cells in Response to LPS Stimulation
Abstract
:1. Introduction
2. Results
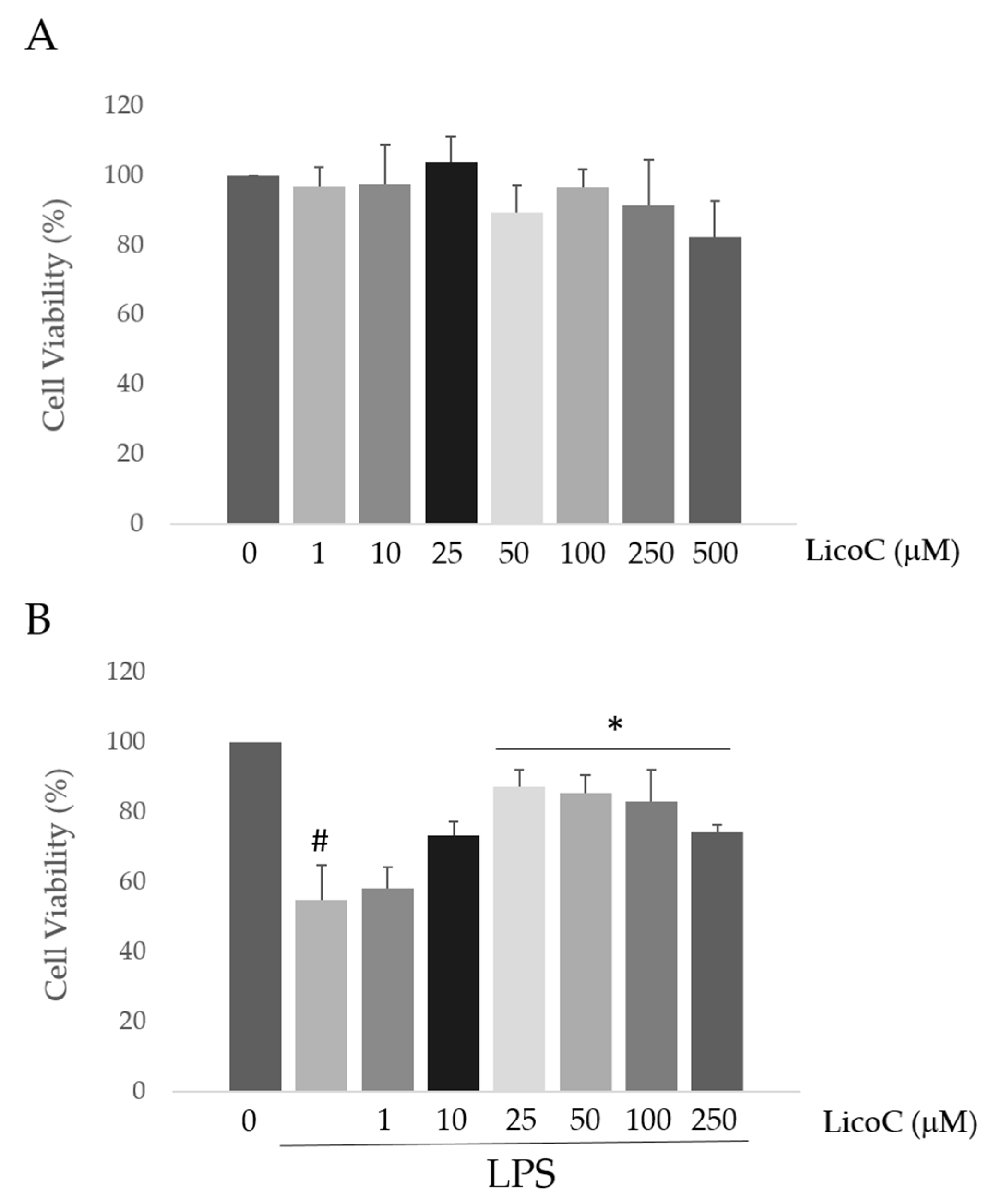
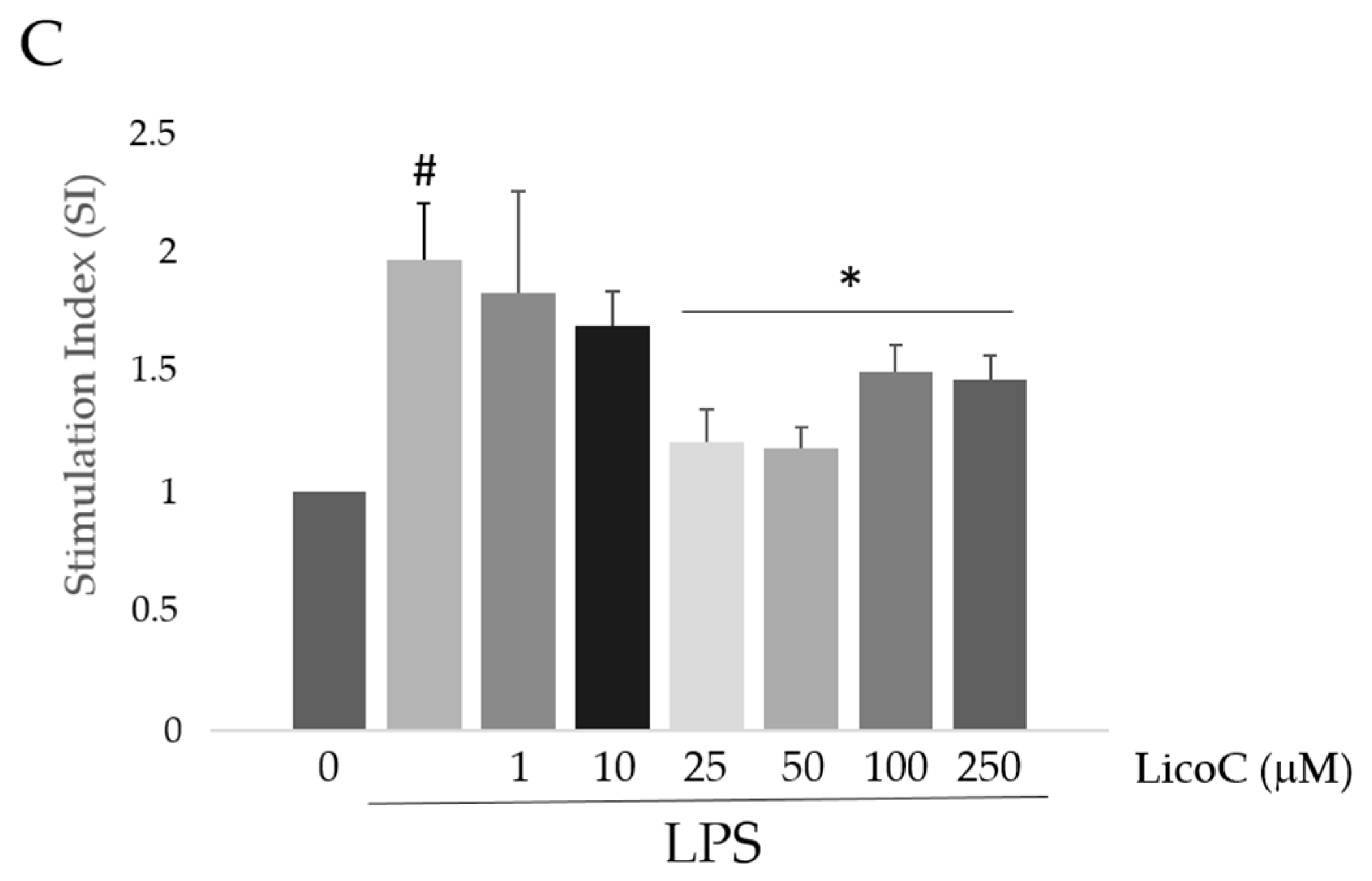
2.1. Licochalcone C (LicoC) Does Not Affect the Viability of and Alleviated Lipopolysaccharide (LPS)-Induced Cytotoxicity in H9c2 Cells
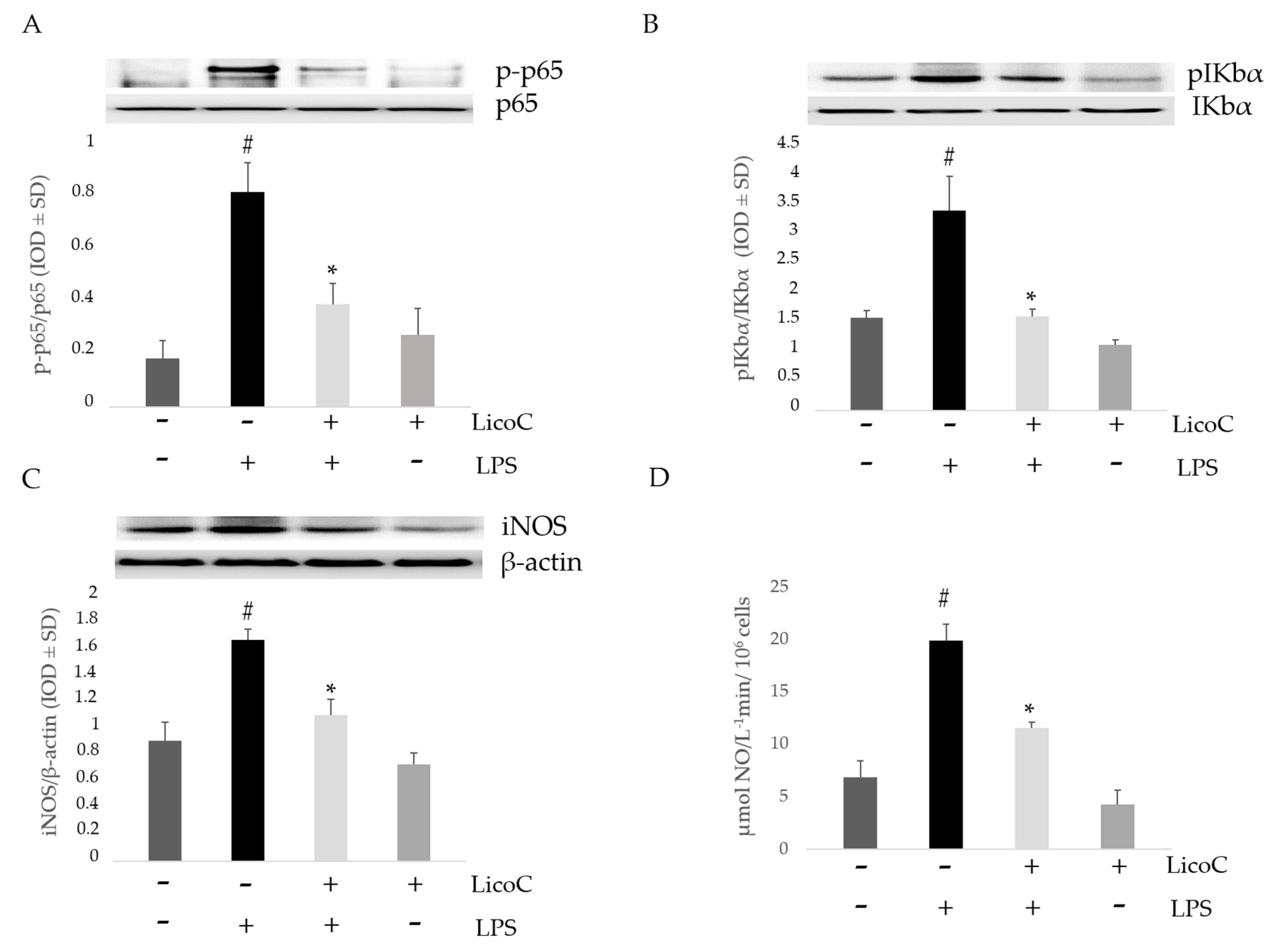
2.2. Effect of LicoC on Nuclear Factor Kappa B (NF-κB)/Inducible Nitric Oxide Sinthase (iNOS)/Nitric Oxide (NO) Pathway
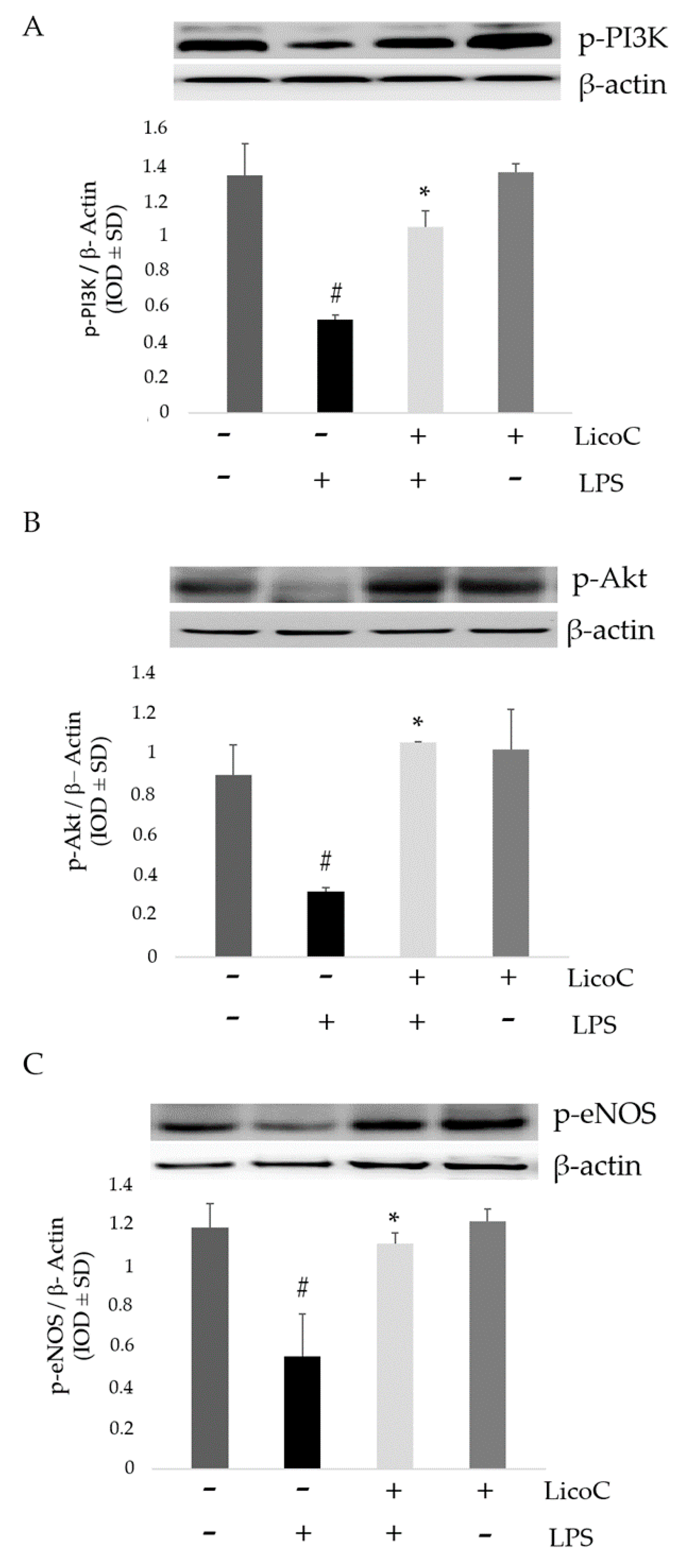
2.3. LicoC Elevated PI3-K/Akt/eNOS Pathways
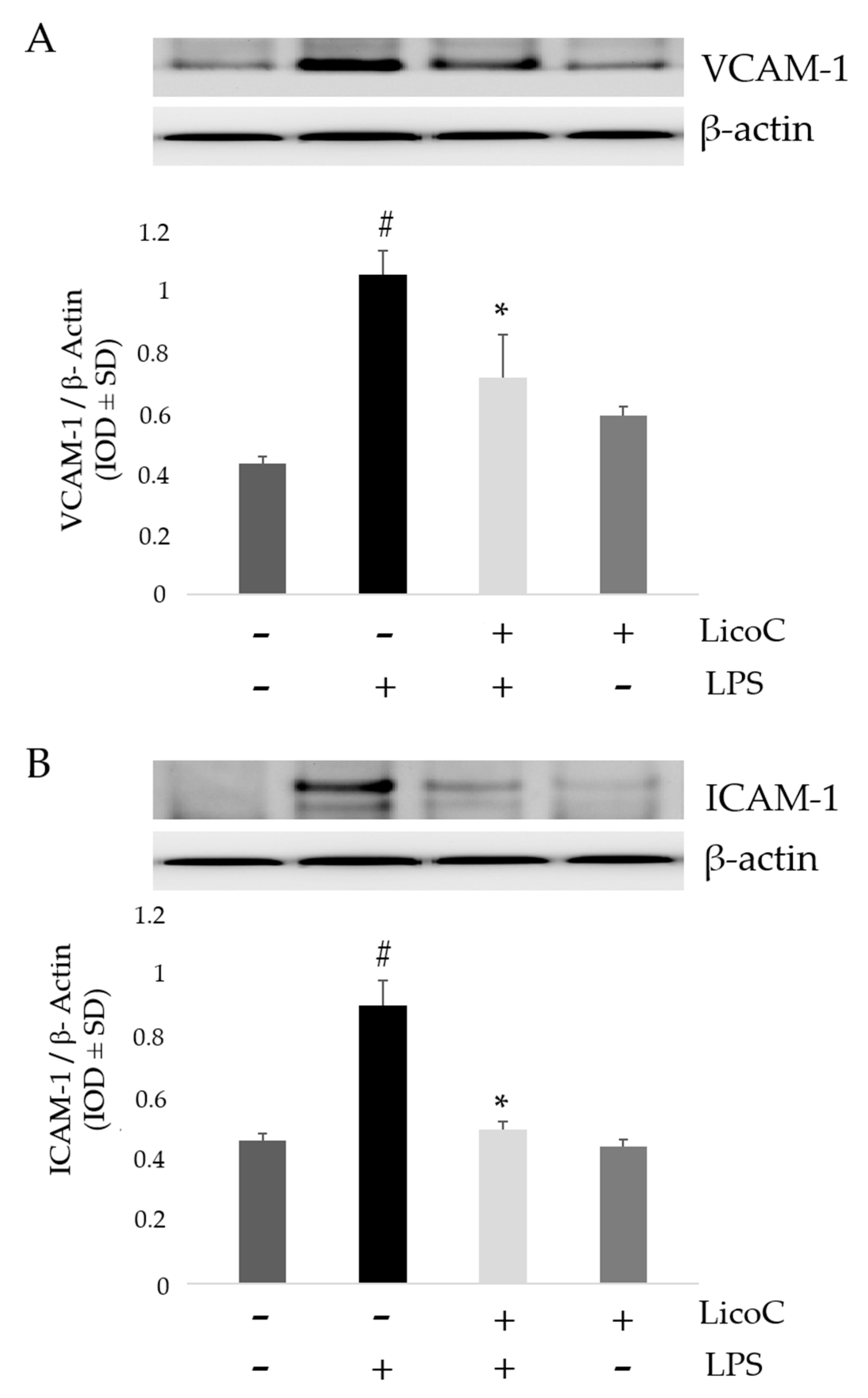
2.4. LicoC Inhibits Intercellular Adhesion Molecule 1 (ICAM-1) and Vascular Cell Adhesion Protein 1 (VCAM-1)
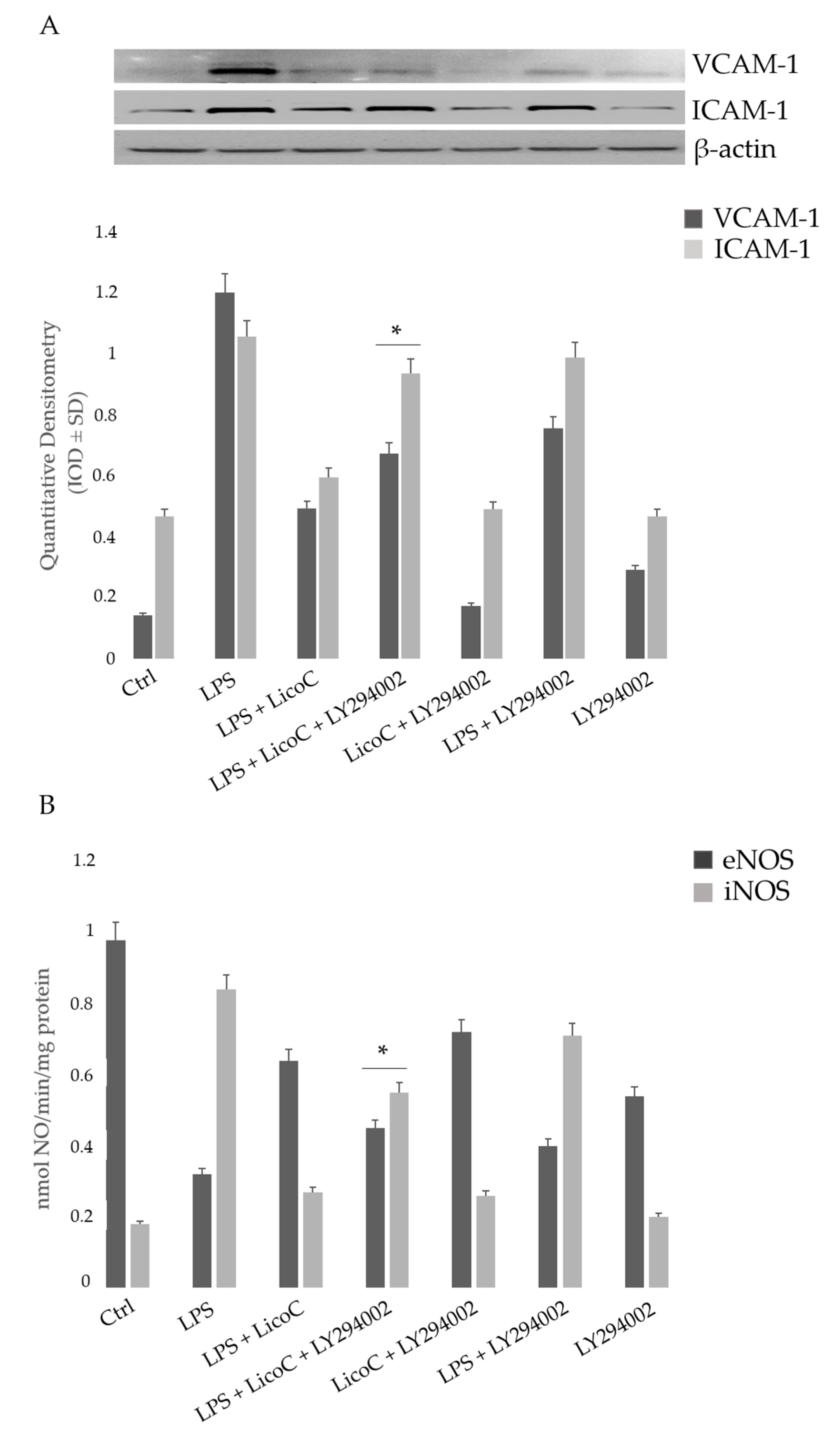
2.5. Involvement of PI3K on the Protective Effect of LicoC on LPS-Induced H9c2 Inflammation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. 3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium Bromide (MTT) Assay for Cell Viability and Cytotoxicity
4.3. Nitro Blue-Tetrazolium Assay
4.4. Western Blot Analysis
4.5. Griess Assay
4.6. Nitric Oxide Sinthase (NOS) Activity
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LPS | lipopolysaccharide |
| iNOS | inducible nitric oxide synthase |
| ICAM-1 | intercellular adhesion molecule-1 |
| eNOS | endothelial nitric oxide synthase |
| ROS | reactive oxygen species |
| PI3K | phosphoinositide 3-kinase |
| VCAM-1 | vascular cell adhesion molecule-1 |
References
- Perner, A.; Rhodes, A.; Venkatesh, B.; Angus, D.C.; Martin-Loeches, I.; Preiser, J.C.; Vincent, J.L.; Marshall, J.; Reinhart, K.; Joannidis, M.; et al. Sepsis: Frontiers in supportive care, organi-sation and research. Intensive Care Med. 2017, 43, 496–508. [Google Scholar] [PubMed]
- Speranza, L.; Franceschelli, S.; Riccioni, G. The biological effects of ivabradine in cardiovascular disease. Molecules 2012, 17, 4924–4235. [Google Scholar] [PubMed]
- Patruno, A.; Franceschelli, S.; Pesce, M.; Maccallini, C.; Fantacuzzi, M.; Speranza, L.; Ferrone, A.; de Lutiis, M.A.; Ricciotti, E.; Amoroso, R.; et al. Novel aminobenzyl-acetamidine deriva-tive modulate the differential regulation of NOSs in LPS induced inflammatory response: Role of PI3K/Akt pathway. Biochim. Biophys. Acta 2012, 1820, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, X.; Zhang, X.; Ha, T.; Ma, H.; Liu, L.; Kalbfleisch, J.H.; Gao, X.; Kao, R.L.; Wil-liams, D.L.; et al. Attenuation of Cardiac Dysfunction in polymicrobial sepsis by microRNA-146a is mediated via targeting of IRAK1 and TRAF6 expression. J. Immunol. 2015, 195, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Fenton, K.E.; Parker, M.M. Cardiac function and dysfunction in sepsis. Clin. Chest Med. 2016, 37, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Ferrone, A.; Rizzuto, A.; Tatangelo, R.; Iezzi, I.; Ladu, S.; Franceschelli, S.; Speranza, L.; Patruno, A.; Felaco, M.; et al. The SHP-1 expression is associated with cytokines and psy-chopathological status in unmedicated first episode schizophrenia patients. Brain Behav. Immun. 2014, 41, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Baek, S.H.; Lee, S.H.; Kim, T.H.; Kim, S.Y. Fucoidan, a sulfated polysaccharide, inhibits osteoclast differentiation and function by modulating RANKL signaling. Int. J. Mol. Sci. 2014, 15, 18840–18855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, G.; Liang, G.; Li, J.; Chiu, I.; Barrington, R.A.; Liu, D. Suppression of complement regulatory protein C1 inhibitor in vascular endothelial activation by inhibiting vascular cell adhesion molecule-1 action. Biochem. Biophys. Res. Commun. 2007, 358, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, H. Pathophysiology of sepsis-induced myocardial dysfunction. Mil. Med. Res. 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.P. Nitric oxide synthase inhibition as therapy for sepsis: A decade of promise. Surg. Infect. 2001, 2, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Prauchner, C.A. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns 2016. [Google Scholar] [CrossRef] [PubMed]
- Amalakuhan, B.; Habib, S.A.; Mangat, M.; Reyes, L.F.; Rodriguez, A.H.; Hinojosa, C.A.; Soni, N.J.; Gilley, R.P.; Bustamante, C.A.; Anzueto, A.; et al. Endothelial adhesion molecules and multiple organ failure in patients with severe sepsis. Cytokine 2016, 88, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.; Hua, F.; Grant, D.; Xia, Y.; Ma, J.; Gao, X.; Kelley, J.; Williams, D.L.; Kalbfleisch, J.; Browder, I.W.; et al. Glucan phosphate attenuates cardiac dysfunction and inhibits car-diac MIF expression and apoptosis in septic mice. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1910–H1918. [Google Scholar] [CrossRef]
- You, W.; Min, X.; Zhang, X.; Qian, B.; Pang, S.; Ding, Z.; Li, C.; Gao, X.; Di, R.; Cheng, Y.; et al. Cardiac-specific expression of heat shock protein 27 attenuated endotoxin-induced cardiac dysfunction and mortality in mice through a PI3K/Akt-dependent mechanism. Shock 2009, 32, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.W.; Monick, M.M.; Stabler, J.M.; Yarovinsky, T.; Carter, A. B.; Hunninghake, G. W. Respiratory syncytial virus inhibits apoptosis and induces NF-κB activity through a phos-phatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 2002, 277, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Arbibe, L.; Mira, J.P.; Teusch, N.; Kline, L.; Guha, M.; Mackman, N.; Godowski, P.J.; Ulevitch, R.J.; Knaus, U.G. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat. Immunol. 2000, 1, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Madrid, L.V.; Mayo, M.W.; Reuther, J.Y.; Baldwin, A.S., Jr. Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-κB through utilization of the IκB kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 2001, 276, 18934–18940. [Google Scholar] [CrossRef] [PubMed]
- Pahan, K.; Raymond, J.R.; Singh, I. Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide synthase in lipopolysaccharide- or cytokine-stimulated C6 glial cells. J. Biol. Chem. 1999, 274, 7528–7536. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Menghini, L.; Patruno, A.; Vinciguerra, I.; de Lutiis, M.A.; Felaco, M.; Felaco, P.; Grilli, A. Anti-inflammatory properties of the plant Verbascum mallophorum. J. Biol. Regul. Homeost. Agents 2009, 23, 189–195. [Google Scholar] [PubMed]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.A.; Ahmad, S.A.; Shukor, M.Y. Antiartherosclerotic effects of plant flavonoids. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [PubMed]
- Song, N.R.; Kim, J.E.; Park, J.S.; Kim, J.R.; Kang, H.; Lee, E.; Kang, Y.G.; Son, J.E.; Seo, S.G.; Heo, Y.S.; et al. Licochalcone A, a polyphenol present in licorice, suppresses UV-induced COX-2 expression by targeting PI3K, MEK1, and B-Raf. Int. J. Mol. Sci. 2015, 16, 4453–4470. [Google Scholar] [CrossRef] [PubMed]
- Katsori, A.M.; Hadjipavlou-Litina, D. Recent progress in therapeutic applications of chalcones. Expert Opin. Ther. Pat. 2011, 21, 1575–1596. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Pesce, M.; Vinciguerra, I.; Ferrone, A.; Riccioni, G.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Licocalchone-C extracted from Glycyrrhiza glabra inhibits lipopolysaccharide-interferon-γ inflammation by improving antioxidant conditions and regulating inducible nitric oxide synthase expression. Molecules 2011, 16, 5720–5734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, L.; Wang, W.; Han, J.; Ren, H.; Zheng, Q.; Wang, D. Role of Licochalcone C in cardioprotection against ischemia/reperfusion injury of isolated rat heart via antioxidant, anti-inflammatory, and anti-apoptotic activities. Life Sci. 2015, 132, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Egom, E.E.; Mohamed, T.M.; Mamas, M.A.; Shi, Y.; Liu, W.; Chirico, D.; Stringer, S.E.; Ke, Y.; Shaheen, M.; Wang, T.; et al. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1487–H1495. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.J.; Xue, J.; Luce, W.A.; Liu, Y. MAPK signaling drives inflammation in LPS-stimulated cardiomyocytes: The route of crosstalk to G-protein-coupled receptors. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Ferrone, A.; Pesce, M.; Riccioni, G.; Speranza, L. Biological functional relevance of asymmetric dimethylarginine (ADMA) in cardiovascular disease. Int. J. Mol. Sci. 2013, 14, 24412–24421. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; de Lutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Astaxanthin treatment confers protection against oxidative stress in U937 cells stimulated with lipopolysaccharide reducing O2− production. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lee, C.H.; Chang, Y.W.; Wang, H.M.; Chen, C.Y.; Chen, Y.H. Pheophytin a inhibits inflammation via suppression of LPS-induced nitric oxide synthase-2, prostaglandin E2, and inter-leukin-1β of macrophages. Int. J. Mol. Sci. 2014, 15, 22819–22834. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Patruno, A.; Pasqualone, L.; Carlucci, G.; Ferrone, V.; Carlucci, M.; de Lutiis, M.A.; Grilli, A.; et al. A novel biological role of α-mangostin in modulating inflammatory response through the activation of SIRT-1 signaling pathway. J. Cell. Physiol. 2016, 231, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; de Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Xu, Y.; Xie, H.; Wang, W.; Chen, X.H. Astragaloside IV prevents lipopolysaccha-ride-induced injury in H9C2 cardiomyocytes. Chin. J. Nat. Med. 2015, 13, 127–132. [Google Scholar] [PubMed]
- Ha, T.; Hu, Y.; Liu, L.; Lu, C.; McMullen, J.R.; Kelley, J.; Kao, R.L.; Williams, D.L.; Gao, X.; Li, C. TLR2 ligands induce cardioprotection against ischaemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovasc. Res. 2010, 87, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Wei, H.; Hagen, T.; Frei, B. α-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Pesce, M.; Grilli, A.; Speranza, L.; Franceschelli, S.; de Lutiis, M.A.; Vianale, G.; Costantini, E.; Amerio, P.; Muraro, R.; et al. mTOR Activation by PI3K/Akt and ERK Signaling in Short ELF-EMF Exposed Human Keratinocytes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shen, L.; Wang, Z.; Jiang, H.P.; Liu, L.X. Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 2016, 84, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xia, Z.Y.; Meng, Q.T.; Zhu, J.; Lei, S.; Xu, J.; Dou, J. Shen-Fu injection preconditioning inhibits myocardial ischemia-reperfusion injury in diabetic rats: Activation of eNOS via the PI3K/Akt pathway. J. Biomed. Biotechnol. 2010, 2011. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Li, T.; Liu, B.; Song, X.; Yang, G.; Wang, L.; Miao, S.; Liu, C. The Protective Effect of Bcl-xl Overexpression against Oxidative Stress-Induced Vascular Endothelial Cell Injury and the Role of the Akt/eNOS Pathway. Int. J. Mol. Sci. 2013, 14, 22149–22162. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, K.; Jalkanen, S.; Kaunismäki, K.; Vänttinen, E.; Saukko, P.; Alanen, K.; Kallajoki, M.; Voipio-Pulkki, L.M.; Salmi, M. Vascular adhesion protein-1, intercellular adhesion molecule-1 and P-selectin mediate leukocyte binding to ischemic heart in humans. J. Am. Coll. Cardiol. 2000, 36, 122–129. [Google Scholar] [CrossRef]
- Franceschelli, S.; Gatta, D.M.; Pesce, M.; Ferrone, A.; Patruno, A.; de Lutiis, M.A.; Grilli, A.; Felaco, M.; Croce, F.; Speranza, L. New approach in translational medicine: Effects of electro-lyzed reduced water (ERW) on NF-κB/iNOS pathway in U937 cell line under altered redox state. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Fornasari, E.; di Stefano, A.; Cerasa, L.S.; Marinelli, L.; Baldassarre, L.; Sozio, P.; Turkez, H.; Franceschelli, S.; Ferrone, A.; et al. Synthesis of a novel cyclic prodrug of S-allyl-glutathione able to attenuate LPS-induced ROS production through the inhibition of MAPK pathways in U937 cells. Mol. Pharm. 2015, 12, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Pesce, M.; Franceschelli, S.; Bucciarelli, T.; Gallina, S.; Riccioni, G.; Patruno, A.; Felaco, M. The biological evaluation of ADMA/SDMA and eNOS in patients with ACHF. Front. Biosci. 2013, 5, 551–557. [Google Scholar] [CrossRef]
- Conti, P.; Reale, M.; Barbacane, R.C.; Felaco, M.; Grilli, A.; Theoharides, T.C. Mast cell recruitment after subcutaneous injection of RANTES in the sole of the rat paw. Br. J. Haematol. 1998, 103, 798–803. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Vinciguerra, I.; de Lutiis, M.A.; Grilli, A.; Felaco, M.; Patruno, A. Phosphodiesterase type-5 inhibitor and oxidative stress. Int. J. Immunopathol. Pharmacol. 2008, 21, 879–889. [Google Scholar] [CrossRef]
- Hevel, J.M.; Marletta, M.A. Nitric oxide synthase assays. Methods Enzymol. 1994, 233, 250–258. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschelli, S.; Pesce, M.; Ferrone, A.; Gatta, D.M.P.; Patruno, A.; Lutiis, M.A.D.; Quiles, J.L.; Grilli, A.; Felaco, M.; Speranza, L. Biological Effect of Licochalcone C on the Regulation of PI3K/Akt/eNOS and NF-κB/iNOS/NO Signaling Pathways in H9c2 Cells in Response to LPS Stimulation. Int. J. Mol. Sci. 2017, 18, 690. https://doi.org/10.3390/ijms18040690
Franceschelli S, Pesce M, Ferrone A, Gatta DMP, Patruno A, Lutiis MAD, Quiles JL, Grilli A, Felaco M, Speranza L. Biological Effect of Licochalcone C on the Regulation of PI3K/Akt/eNOS and NF-κB/iNOS/NO Signaling Pathways in H9c2 Cells in Response to LPS Stimulation. International Journal of Molecular Sciences. 2017; 18(4):690. https://doi.org/10.3390/ijms18040690
Chicago/Turabian StyleFranceschelli, Sara, Mirko Pesce, Alessio Ferrone, Daniela Maria Pia Gatta, Antonia Patruno, Maria Anna De Lutiis, José Luis Quiles, Alfredo Grilli, Mario Felaco, and Lorenza Speranza. 2017. "Biological Effect of Licochalcone C on the Regulation of PI3K/Akt/eNOS and NF-κB/iNOS/NO Signaling Pathways in H9c2 Cells in Response to LPS Stimulation" International Journal of Molecular Sciences 18, no. 4: 690. https://doi.org/10.3390/ijms18040690






