Triazole Fungicides Inhibit Zebrafish Hatching by Blocking the Secretory Function of Hatching Gland Cells
Abstract
:1. Introduction
1.1. Environmental Disruption of Hatching
1.2. Hatching Onset Regulation in Fish
1.3. Triazole Fungicide Toxicity and Environmental Relevance
1.4. Some Advantages of Zebrafish in Toxicology Research
2. Results
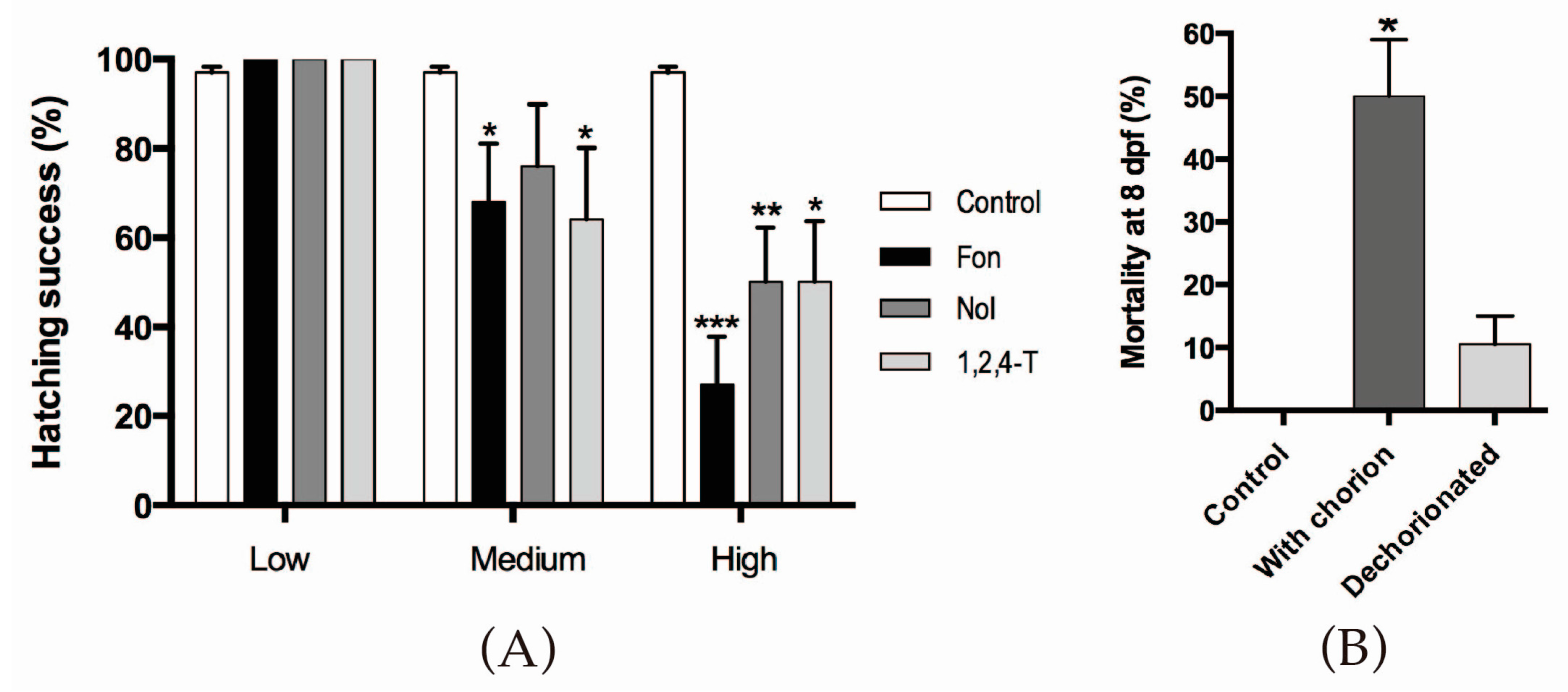
2.1. Triazoles Reduce Hatching Success in Zebrafish Embryos
2.2. Triazoles Do Not Have a Cytotoxic Effect on the HGCs
2.3. Triazoles Do Not Reduce HGC Number but Modify Their Morphology
2.4. Triazole Exposed Pre-Hatching Embryos Present Reduced Secreted Proteolytic Activity
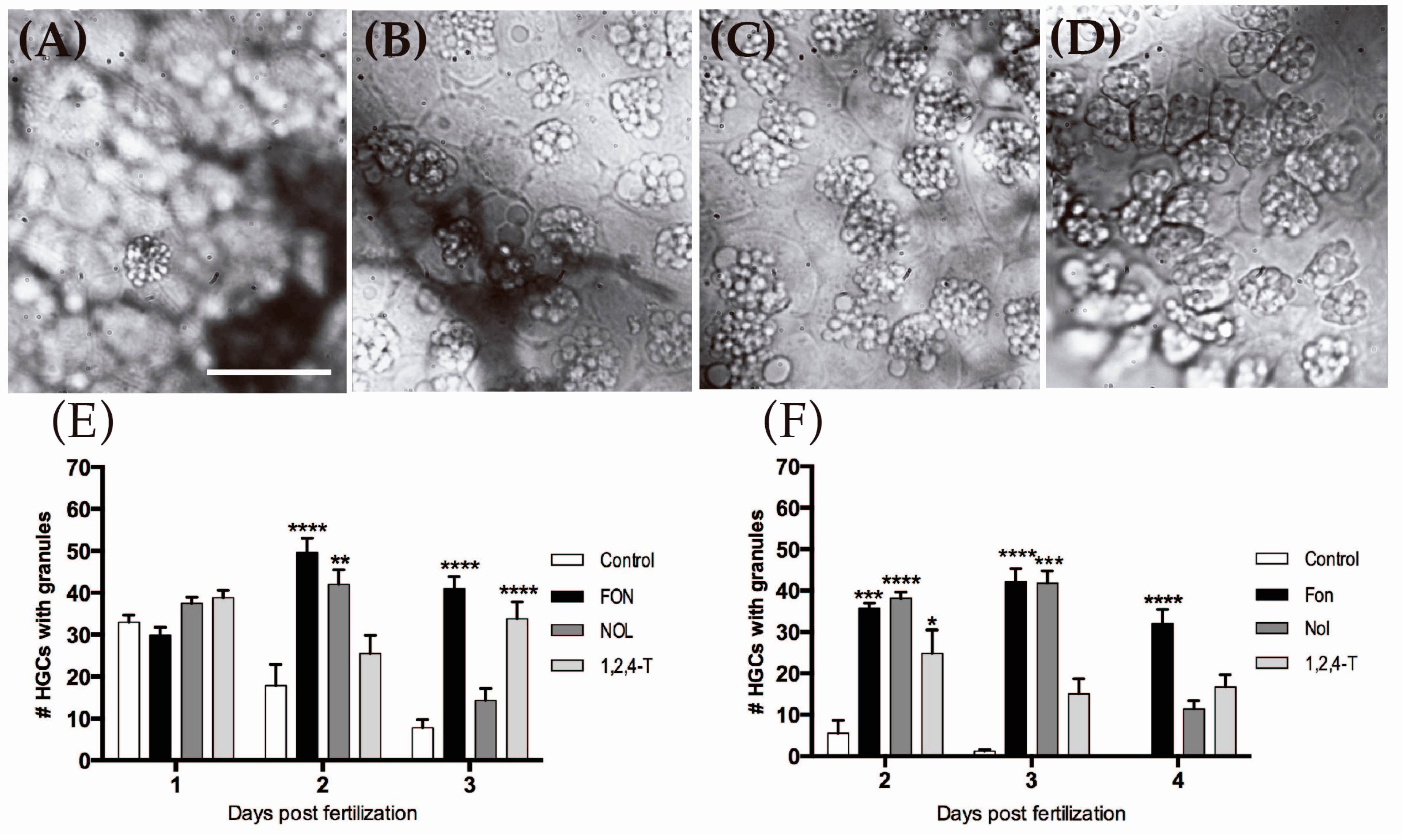
2.5. Triazole-Treated Embryos Retain Granular Content in the Cytoplasm of HGCs
2.6. Triadimefon Affects Locomotor Activity in Zebrafish Larvae
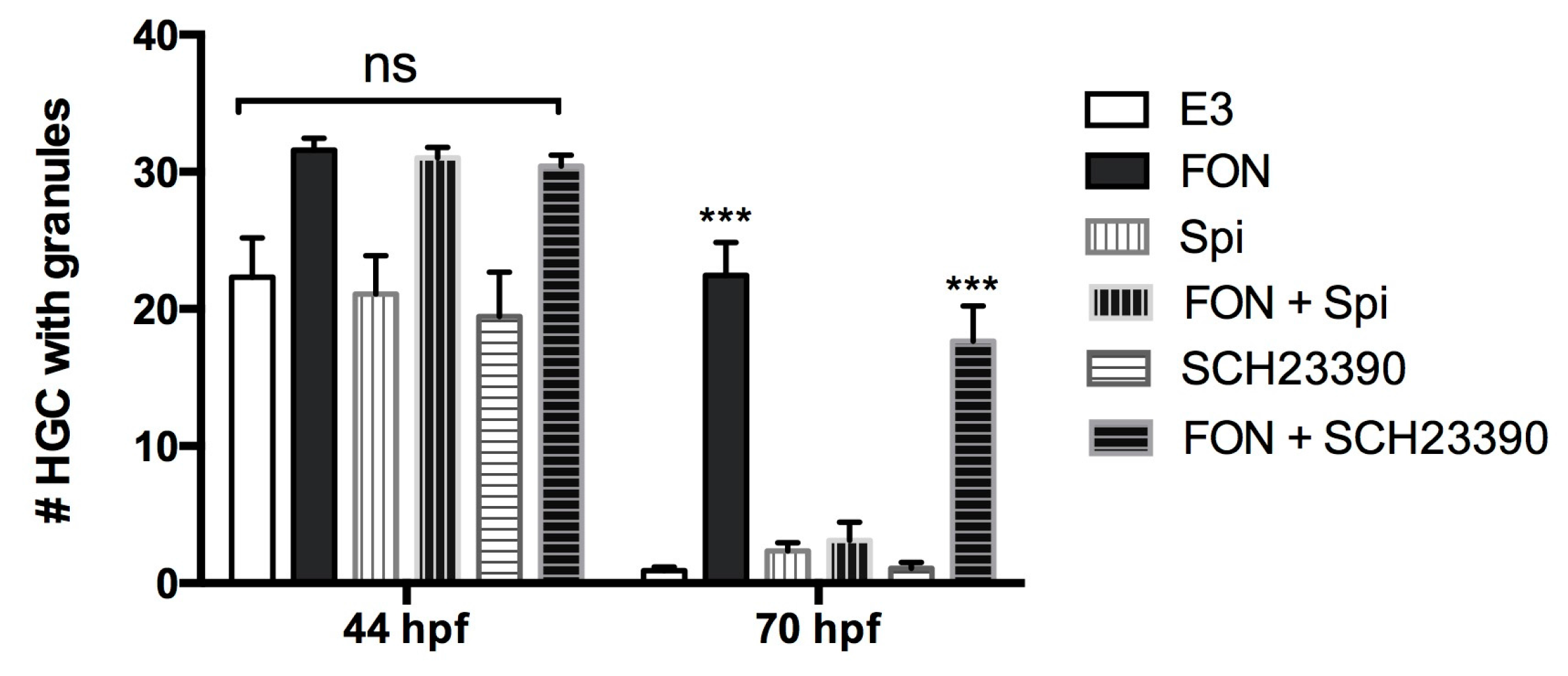
2.7. A Dopamine Receptor Antagonist Rescues FON-Induced HGC Granule Release Inhibition
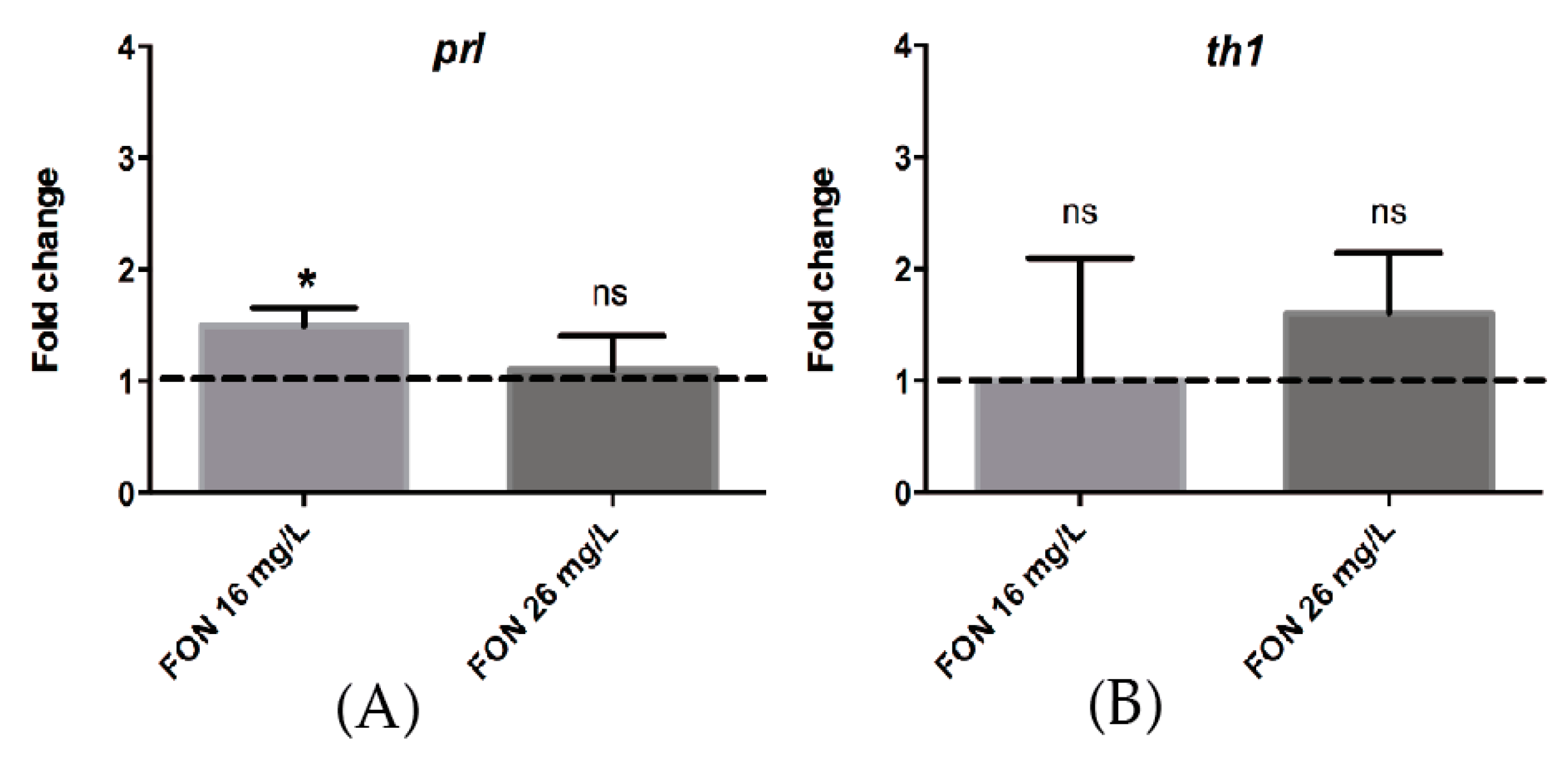
2.8. Acute FON Exposure Induces a Significant Change in the Expression of Prolactin mRNA.
3. Discussion
3.1. Triazoles Alter Gene Expression, Metabolism, and Development
3.2. HGCs as a Target of Environmental Pollutants
3.3. FON Reduces HE1 Secretion from HGCs by a Dopaminergic Pathway
3.4. FON Modulates prl Expression
4. Materials and Methods
4.1. Animals
4.2. Chemicals and Determination of Exposure Concentrations
4.3. Hatching Success Assessment
4.4. Cell Death Detection
4.5. Counting and Measurement of HGCs
4.6. Proteolytic Enzyme Secretion Assay
4.7. Quantification of HGCs with Granular Content
4.8. Quantitative PCR
4.9. Locomotor Activity Quantification
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 1,2,4-T | 1,2,4-Triazole |
| DA | Dopamine |
| FON | Triadimefon |
| GFP | Green fluorescent protein |
| HE1 | Hatching enzyme 1 |
| HGC | Hatching gland cells |
| hpf | Hours post-fertilization |
| HPT | Hypothalamic-pituitary-thyroid |
| IC50 | Inhibition concentration 50 |
| LC50 | Lethal concentration 50 |
| LMA | Locomotor Activity |
| NOL | Triadimenol |
| PRL | Prolactin |
| PVS | Perivitelline space |
| SPI | Spiperone |
| SCH | SCH23390 |
References
- Gilbert, S.F. Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000; Available online: https://www.ncbi.nlm.nih.gov/books/NBK10044/ (accessed on 1 March 2017).
- Schoots, A.F.; Stikkelbroeck, J.J.; Bekhuis, J.F.; Denucé, J.M. Hatching in teleostean fishes: Fine structural changes in the egg envelope during enzymatic breakdown in vivo and in vitro. J. Ultrastruct. Res. 1982, 80, 185–196. [Google Scholar] [CrossRef]
- Mizell, M.; Romig, E.S. The aquatic vertebrate embryo as a sentinel for toxins: Zebrafish embryo dechorionation and perivitelline space microinjection. Int. J. Dev. Biol. 1997, 41, 411–423. [Google Scholar] [PubMed]
- He, J.; Yang, D.; Wang, C.; Liu, W.; Liao, J.; Xu, T.; Bai, C.; Chen, J.; Lin, K.; Huang, C.; et al. Chronic zebrafish low dose decabrominated diphenyl ether (BDE-209) exposure affected parental gonad development and locomotion in F1 offspring. Ecotoxicology 2011, 20, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Nagler, J.J.; Cyr, D.G. Exposure of male American plaice (Hippoglossoides platessoides) to contaminated marine sediments decreases the hatching success of their progeny. Environ. Toxicol. Chem. 1997, 16, 1733–1738. [Google Scholar] [CrossRef]
- Segner, H. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: Report from the European IDEA project. Ecotox. Environ. Saf. 2003, 54, 302–314. [Google Scholar] [CrossRef]
- Blüthgen, N.; Zucchi, S.; Fent, K. Effects of the UV filter benzophenone-3 (oxybenzone) at low concentrations in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2012, 263, 184–194. [Google Scholar] [CrossRef] [PubMed]
- De la Paz, J.F. Bioassays with Zebrafish Embryos (Danio rerio) as Tools to Assess Toxicological State of Water Water Bodys and Mechanisms of Hatching Inhbition. Master’s Thesis, Sciences, Science Faculty, University of Chile, Santiago, Chile, 2012. (In Spanish). [Google Scholar]
- DiMichele, L.; Taylor, M. The environmental control of hatching in Fundulus heteroclitus. J. Exp. Zool. 1980, 214, 181–187. [Google Scholar] [CrossRef]
- DiMichele, L.; Taylor, M. The mechanism of hatching in Fundulus heteroclitus: Development and physiology. Dev. Biol. 1981, 217, 73–79. [Google Scholar]
- Schoots, A.F.; Meijer, R.C.; Denucé, J.M. Dopaminergic regulation of hatching in fish embryos. Dev. Biol. 1983, 100, 59–63. [Google Scholar] [CrossRef]
- Schoots, A.; de Bont, R.; van Eys, G.J.; Denucé, M. Evidence for a stimulating effect of prolactin on teleostean hatching enzyme secretion. J. Exp. Zool. 1982, 219, 129–132. [Google Scholar] [CrossRef]
- Christian, F.; Tate, T. Effects of fluometuron on embryonic development and hatching of Fasciola hepatica’s miracidia. Bull. Environ. Contam. Toxicol. 1982, 28, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.M.C.; Bevilaqua, C.M.L.; Morais, S.M.; Costa, C.T.C.; Silva, A.R.A.; Braz-Filho, R. Anthelmintic acetogenin from Annona squamosa L. Seeds. Anais da Academia Brasileira de Ciências 2008, 80, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, R.; Goicoechea, O.; Garrido, O.; Molinari, E. Salmo salar: Morfología ultraestructural de la pared del corion en ovas normales y con problemas de eclosión. Arch. Med. Vet. 2009, 41, 67–71. (In Spanish) [Google Scholar] [CrossRef]
- Oppen-Berntsen, D.; Bogsnes, A.; Walther, B. The effects of hypoxia, alkalinity and neurochemicals on hatching of Atlantic salmon (Salmo salar) eggs. Aquaculture 1990, 86, 417–430. [Google Scholar] [CrossRef]
- Czerkiesa, P.; Brzuzanb, P.; Kordalskib, K.; Luczynskib, M. Critical partial pressures of oxygen causing precocious hatching in Coregonus lavaretus and C. albula embryos. Aquaculture 2001, 196, 151–158. [Google Scholar] [CrossRef]
- Bresch, H. Investigation of the long-term action of xenobiotics on fish with special regard to reproduction. Ecotox. Environ. Saf. 1982, 6, 102–112. [Google Scholar] [CrossRef]
- Landner, L.; Neilson, A.H.; Sörensen, L.; Tärnholm, A.; Viktor, T. Short-term test for predicting the potential of xenobiotics to impair reproductive success in fish. Ecotox. Environ. Saf. 1985, 9, 282–293. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals; Test N° 210: Fish, Early-Life Stage Toxicity Test; OECD: París, France, 1992. [Google Scholar]
- OECD. Guideline for Testing of Chemicals; Test N° 212: Fish, Short-Term Toxicity Test on Embryo and Sac-Fry Stages; OECD: París, France, 1998. [Google Scholar]
- OECD. Guideline for Testing of Chemicals; Test N° 236: Fish Embryo Acute Toxicity (FET) Test; OECD: París, France, 2006. [Google Scholar]
- Bourrachot, S.; Simon, O.; Gilbin, R. The effects of waterborne uranium on the hatching success, development, and survival of early life stages of zebrafish (Danio rerio). Aquat. Toxicol. 2008, 90, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dong, Q.; Li, S.; Guo, J.; Wang, X.; Zhu, G. Developmental toxicity of cartap on zebrafish embryos. Aquat. Toxicol. 2009, 95, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.D.; von Westernhagen, H.; Rosenthal, H. Chlorinated hydrocarbons and hatching success in Baltic herring spring spawners. Mar. Environ. Res. 1985, 15, 59–76. [Google Scholar] [CrossRef]
- Brix, K.V.; Gerdes, R.M.; Adams, W.J.; Grosell, M. Effects of copper, cadmium, and zinc on the hatching success of brine shrimp (Artemia franciscana). Arch. Environ. Contam. Toxicol. 2006, 51, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Inohaya, K.; Kawaguchi, M.; Yoshizaki, N.; Iuchi, I.; Yasumasu, S. Purification and characterization of zebrafish hatching enzyme—An evolutionary aspect of the mechanism of egg envelope digestion. FEBS J. 2008, 275, 5934–5946. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dynam. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Inohaya, K.; Yasumasu, S.; Ishimaru, M.; Ohyama, A.; Iuchi, I.; Yamagami, K. Temporal and spatial patterns of gene expression for the hatching enzyme in the teleost embryo, Oryzias latipes. Dev. Biol. 1995, 171, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Willemse, M.T.M.; Denuce, J.M. Hatching glands in the teleosts Brachydanio rerio, Danio malabaricus, Moenkhausia oligolepis and Barbus schuberti. Dev. Growth Differ. 1973, 15, 169–177. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (U.S. EPA). Triadimefon. Preliminary Human Health Risk Assessment Revised; U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- Liu, S.Y.; Jin, Q.; Huang, X.H.; Zhu, G.N. Disruption of zebrafish (Danio rerio) sexual development after full life-cycle exposure to environmental levels of triadimefon. Environ. Toxicol. Pharmacol. 2014, 37, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Watschke, T.L.; Mumma, R.O.; Linde, D.T.; Borger, J.A.; Harrison, S.A. Surface runoff of selected pesticides applied to turfgrasses. Fate Manag. Turfgrass Chem. 2000, 743, 94–105. [Google Scholar]
- Lepesheva, G.; Waterman, M. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim. Biophys. Acta 2007, 1770, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Zarn, J.A.; Brüschweiler, B.J.; Schlatter, J.R. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 α-demethylase and aromatase. Environ. Health Perspect. 2003, 111, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Vinggaard, A.M.; Breinholt, V.; Larsen, J.C. Screening of selected pesticides for oestrogen receptor activation in vitro. Food Addit. Contam. 1999, 16, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Vinggaard, A.M.; Hnida, C.; Breinholt, V.; Larsen, J.C. Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol. In Vitro 2000, 14, 227–234. [Google Scholar] [CrossRef]
- Goetz, A.K.; Rockett, J.C.; Ren, H.; Thillainadarajah, I.; Dix, D.J. Inhibition of rat and human steroidogenesis by triazole antifungals. Syst. Biol. Reprod. Med. 2009, 55, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.T.; Brennan, R.J.; Hu, W.Y.; Ayanoglu, E.; Lau, C.; Ren, H.Z.; Wood, C.R.; Corton, J.C.; Kavlock, R.J.; Dix, D.J. Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol. Sci. 2007, 97, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.S.; Chiou, B.H.; Hsu, T.W.; Wang, C.C.; Chen, S.C. The regulation of transcriptome responses in zebrafish embryo exposure to triadimefon. Environ. Toxicol. 2016, 32, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, Q.; Jiao, F.; Zhu, G. Impact of co-exposure with butachlor and triadimefon on thyroid endocrine system in larval zebrafish. Exp. Toxicol. Pathol. 2016, 68, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chang, J.; Zhao, Y.; Zhu, G. Changes of thyroid hormone levels and related gene expression in zebrafish on early life stage exposure to triadimefon. Environ. Toxicol. Pharmacol. 2011, 32, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Pennati, R.; Groppelli, S.; Zega, G.; Biggiogero, M.; de Bernardi, F.; Sotgia, C. Toxic effects of two pesticides, imazalil and triadimefon, on the early development of the ascidian Phallusia mammillata (Chordata, Ascidiacea). Aquat. Toxicol. 2006, 79, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Groppelli, S.; Pennati, R.; de Bernardi, F.; Menegola, E.; Giavini, E.; Sotgia, C. Teratogenic effects of two antifungal triazoles, triadimefon and triadimenol, on Xenopus laevis development: Craniofacial defects. Aquat. Toxicol. 2005, 73, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Menegola, E.; Broccia, M.L.; di Renzo, F.; Massa, V.; Giavini, E. Craniofacial and axial skeletal defects induced by the fungicide triadimefon in the mouse. Birth Defects Res. 2005, 74, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Menegola, E.; Broccia, M.L.; di Renzo, F.; Giavini, E. Postulated pathogenic pathway in triazole fungicide induced dysmorphogenic effects. Reprod. Toxicol. 2006, 22, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, S.A.; van den Brandhof, E.J.; van der Ven, L.T.; Piersma, A.H. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo. Toxicol. In Vitro 2011, 37, 45–53. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. TOXNET Toxicology Data Network. Available online: https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+6857 (accessed on 1 March 2017).
- Crofton, K.M.; Boncek, V.M.; Reiter, L.W. Hyperactivity induced by triadimefon, a triazole fungicide. Fundam. Appl. Toxicol. 1988, 10, 459–465. [Google Scholar] [CrossRef]
- Ikaiddi, M.U.; Akunne, H.C.; Soliman, K.F.A. Behavioral and neurochemical effects of acute and repeated administration of triadimefon in the male rat. Neurotoxicology 1997, 18, 771–780. [Google Scholar] [PubMed]
- Walker, Q.D.; Lewis, M.H.; Crofton, K.M.; Mailman, R.B. Triadimefon, a triazole fungicide, induces stereotyped behavior and alters monoamine metabolism in rats. Toxicol. Appl. Pharmacol. 1990, 102, 474–485. [Google Scholar] [CrossRef]
- Ensor, D.M. Comparative Endocrinology of Prolactin; Chapman and Hall: London, UK, 1978; pp. 309–310. [Google Scholar]
- Ball, J.N. Hypothalamic control of the pars distalis in fishes, amphibians and reptiles. Gen. Comp. Endocrinol. 1981, 44, 135–170. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2009; Available online: https://zfin.org/zf_info/zfbook/cont.html#cont2 (accessed on 1 March 2017).
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Haas, P.; Gilmour, D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev. Cell 2006, 10, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kolosov, D.; Bui, P.; Chasiotis, H.; Kelly, S.P. Claudins in teleost fishes. Tissue Barriers 2013, 1, e25391. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.T.; Hwang, J.; Ock, J.; Lee, M.G.; Lee, W.H.; Suk, K. The antipsychotic spiperone attenuates inflammatory response in cultured microglia via the reduction of proinflammatory cytokine expression and nitric oxide production. J. Neurochem. 2008, 107, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Terai, M.; Usuda, S.; Kuroiwa, I.; Noshiro, O.; Maeno, H. Selective binding of YM-09151-2, a new potent neuroleptic, to D2-dopaminergic receptors. Jpn. J. Pharmacol. 1983, 33, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.A. SCH 23390: The first selective dopamine D1-like receptor antagonist. CNS Drug Rev. 2001, 7, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Irons, T.D.; Kelly, P.E.; Hunter, D.L.; Macphail, R.C.; Padilla, S. Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol. Biochem. Behav. 2013, 103, 792–813. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.; Dinan, T.G. Prolactin and dopamine: What is the connection? A review article. J Psychopharmacol. 2008, 2, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chou, P.H.; Chen, P.J. Two azole fungicides (carcinogenic triadimefon and non-carcinogenic myclobutanil) exhibit different hepatic cytochrome P450 activities in Medaka fish. J. Hazard. Mater. 2014, 277, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Yashwanth, B.; Pamanji, R.; Rao, J.V. Toxicomorphomics and toxicokinetics of quinalphos on embryonic development of zebrafish (Danio rerio) and its binding affinity towards hatching enzyme, ZHE1. Aquat. Toxicol. 2016, 180, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.; Thiruchelvam, M.; Cory-Slechta, D.A. Development of behavioral sensitization to the cocaine-like fungicide triadimefon is prevented by AMPA, NMDa, DA D1 but not DA D2 receptor antagonists. Toxicol. Sci. 2004, 79, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Zúñiga, S. Center for Genome Regulation, Facultad de Ciencias, Universidad de Chile, Santiago Chile. Master’s Thesis, Biological Sciences, Science Faculty, University of Chile, Santiago, Chile, 2017. (In Spanish). [Google Scholar]
- Fontaine, R.; Affaticati, P.; Bureau, C.; Colin, I.; Demarque, M.; Dufour, S.; Vernier, P.; Yamamoto, K.; Pasqualini, C. Dopaminergic neurons controlling anterior pituitary functions: Anatomy and ontogenesis in zebrafish. Endocrinology 2015, 156, 2934–2948. [Google Scholar] [CrossRef] [PubMed]
- De la Paz, J.F. Assessment of Teratogenic Effects of Fungicides Using Bioassays with Zebrafish (Danio rerio). Master’s Thesis, Environmental Biologist, Science Faculty, University of Chile, Santiago, Chile, 2012. (In Spanish). [Google Scholar]
- Chen, Y.C.; Priyadarshini, M.; Panula, P. Complementary developmental expression of the two tyrosine hydroxylase transcripts in zebrafish. Histochem. Cell. Biol. 2009, 132, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Hoshijima, K.; Hirose, S. Expression of endocrine genes in zebrafish larvae in response to environmental salinity. J. Endocrinol. 2007, 193, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, S.H.; Golombek, D.A. An automated tracking system for C. elegans locomotor behavior and circadian studies application. J. Neurosci. Meth. 2007, 161, 273–280. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Paz, J.F.; Beiza, N.; Paredes-Zúñiga, S.; Hoare, M.S.; Allende, M.L. Triazole Fungicides Inhibit Zebrafish Hatching by Blocking the Secretory Function of Hatching Gland Cells. Int. J. Mol. Sci. 2017, 18, 710. https://doi.org/10.3390/ijms18040710
De la Paz JF, Beiza N, Paredes-Zúñiga S, Hoare MS, Allende ML. Triazole Fungicides Inhibit Zebrafish Hatching by Blocking the Secretory Function of Hatching Gland Cells. International Journal of Molecular Sciences. 2017; 18(4):710. https://doi.org/10.3390/ijms18040710
Chicago/Turabian StyleDe la Paz, Javiera F., Natalia Beiza, Susana Paredes-Zúñiga, Misque S. Hoare, and Miguel L. Allende. 2017. "Triazole Fungicides Inhibit Zebrafish Hatching by Blocking the Secretory Function of Hatching Gland Cells" International Journal of Molecular Sciences 18, no. 4: 710. https://doi.org/10.3390/ijms18040710





