Extracellular Vesicles as Therapeutic Agents in Systemic Lupus Erythematosus
Abstract
:1. Introduction
2. Types of Extracellular Vesicles
3. Extracellular Vesicles in Systemic Lupus Erythematosus
3.1. Extracellular Vesicles in Immune Modulation
3.2. Extracellular Vesicles as Biomarkers of Systemic Lupus Erythematosus
4. Extracellular Vesicles as Cell-Free Therapy in Autoimmunity
4.1. Mesenchymal Stem Cell Derived Extracellular Vesicles
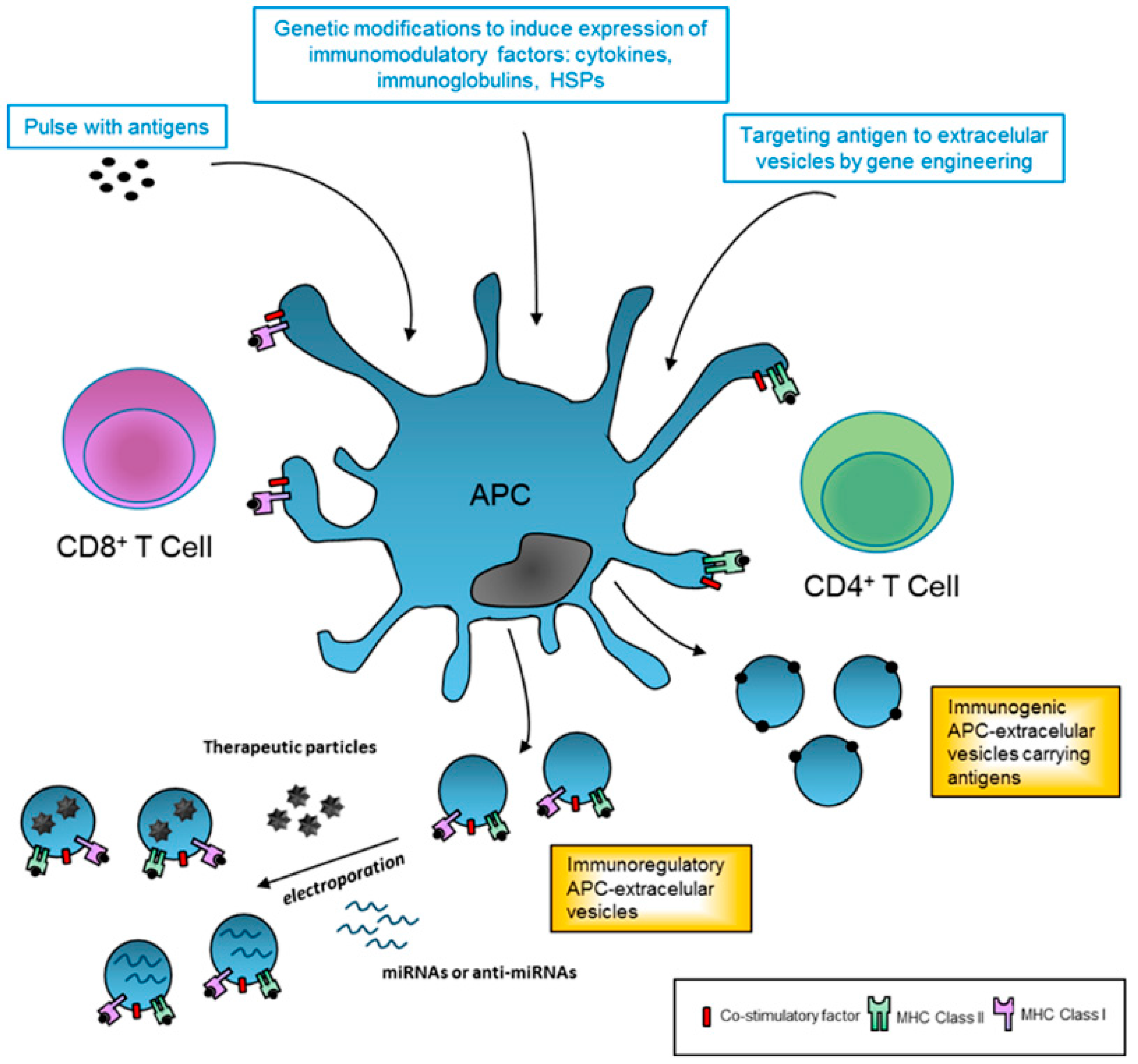
4.2. Extracellular Vesicles Derived from Professional Antigen-Presenting Cells
5. Future Research
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Al-Shobaili, H.A.; Al Robaee, A.A.; Alzolibani, A.A.; Rasheed, Z. Antibodies against 4-hydroxy-2-nonenal modified epitopes recognized chromatin and its oxidized forms: Role of chromatin, oxidized forms of chromatin and 4-hydroxy-2-nonenal modified epitopes in the etiopathogenesis of SLE. Dis. Markers 2012, 33, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Colasanti, T.; Maselli, A.; Conti, F.; Sanchez, M.; Alessandri, C.; Barbati, C.; Vacirca, D.; Tinari, A.; Chiarotti, F.; Giovannetti, A.; et al. Autoantibodies to estrogen receptor alpha interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Carls, G.S.; Panopalis, P.; Wang, S.; Gibson, T.B.; Goetzel, R.Z. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: A five-year analysis of a large medicaid population. Arthritis Rheum. 2009, 61, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.Y.; Choi, J.Y.; Park, S.; Choi, J.; Hong, S.J.; Lee, H.S.; Choi, C.B.; Bae, S.C. Brief report: Influence of HLA-DRB1 susceptibility alleles on the clinical subphenotypes of systemic lupus erythematosus in Koreans. Arthritis Rheumatol. 2016, 68, 1190–1196. [Google Scholar] [PubMed]
- Yih Chen, J.; Ling Wu, Y.; Yin Mok, M.; Jan Wu, Y.J.; Lintner, K.E.; Wang, C.M.; Chung, E.K.; Yang, Y.; Zhou, B.; Wang, H.; et al. Effects of complement C4 gene copy number variations, size dichotomy, and C4A deficiency on genetic risk and clinical presentation of systemic lupus erythematosus in East Asian populations. Arthritis Rheumatol. 2016, 68, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Moser, K.L.; Kelly, J.A.; Lessard, C.J.; Harley, J.B. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun. 2009, 10, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wu, A.; Richardson, B.C. Demethylation of the same promoter sequence increases CD70 expression in lupus T cells and T cells treated with lupus-inducing drugs. J. Immunol. 2005, 174, 6212–6219. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wu, A.; Tesmer, L.; Ray, D.; Yousif, N.; Richardson, B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J. Immunol. 2007, 179, 6352–6358. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Qiu, X.; Luo, Y.; Yuan, J.; Li, Y.; Lei, W.; Zhang, G.; Zhou, Y.; Su, Y.; Lu, Q. Abnormal histone modification patterns in lupus CD4+ T cells. J. Rheumatol. 2008, 35, 804–810. [Google Scholar] [PubMed]
- Duroux-Richard, I.; Cuenca, J.; Ponsolles, C.; Pineiro, A.B.; Gonzalez, F.; Roubert, C.; Areny, R.; Chea, R.; Pefaur, J.; Pers, Y.M.; et al. MicroRNA profiling of B cell subsets from systemic lupus erythematosus patients reveals promising novel biomarkers. Int. J. Mol. Sci. 2015, 16, 16953–16965. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, X.; Cui, H.; Ni, X.; Yuan, M.; Guo, Y.; Huang, X.; Zhou, H.; de Vries, N.; Tak, P.P.; et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009, 60, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, M.; Costenbader, K.H. Environmental exposures and the development of systemic lupus erythematosus. Curr. Opin. Rheumatol. 2016, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.; Tay, S.H. Environmental factors, toxicants and systemic lupus erythematosus. Int. J. Mol. Sci. 2014, 15, 16043–16056. [Google Scholar] [CrossRef] [PubMed]
- Jonsen, A.; Bengtsson, A.A.; Nived, O.; Truedsson, L.; Sturfelt, G. Gene-environment interactions in the aetiology of systemic lupus erythematosus. Autoimmunity 2007, 40, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Paley, M.A.; Strand, V.; Kim, A.H. From mechanism to therapies in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2017, 29, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Rostamzadeh, D.; Razavi, S.R.; Esmaeili, S.; Dolati, S.; Ahmahi, M.; Sadreddini, S.; Jadidi-Niaragh, F.; Yousefi, M. Application of nanoparticle technology in the treatment of systemic lupus erythematous. Biomed. Pharmacother. 2016, 83, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Kalunian, K.C.; Merrill, J.T.; Maciuca, R.; McBride, J.M.; Townsend, M.J.; Wei, X.; Davis, J.C., Jr.; Kennedy, W.P. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 2016, 75, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; van Vollenhoven, R.F.; Aranow, C.; Wagner, C.; Gordon, R.; Zhuang, Y.; Belkowski, S.; Hsu, B. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheumatol. 2016, 68, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.H. Belimumab for systemic lupus erythematosus. N. Engl. J. Med. 2013, 368, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Lasser, C.; Alikhani, V.S.; Ekstrom, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjostrand, M.; Gabrielsson, S.; Lotvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 5876. [Google Scholar] [CrossRef] [PubMed]
- Lazaro-Ibanez, E.; Sanz-Garcia, A.; Visakorpi, T.; Escobedo-Lucea, C.; Siljander, P.; Ayuso-Sacido, A.; Yliperttula, M. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: Apoptotic bodies, microvesicles, and exosomes. Prostate 2014, 74, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Loyer, X.; Vion, A.C.; Tedgui, A.; Boulanger, C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Lim, J.W.; Tauro, B.J.; Ji, H.; Moritz, R.L.; Simpson, R.J. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol. Cell. Proteom. 2010, 9, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, S.A.; Coleman, B.M.; Hill, A.F. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012, 40, 10937–10949. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 2014, 1841, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, N.; Tan, S.; Boudreau, L.H.; Cramb, C.; Subbaiah, R.; Lahey, L.; Albert, A.; Shnayder, R.; Gobezie, R.; Nigrovic, P.A.; et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol. Med. 2013, 5, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Ullal, A.J.; Pisetsky, D.S. The role of microparticles in the generation of immune complexes in murine lupus. Clin. Immunol. 2013, 146, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M.; Heyder, P.; Ziegler, S.; Niessen, A.; Classen, L.; Lauffer, A.; Lorenz, H.M. During apoptosis HMGB1 is translocated into apoptotic cell-derived membranous vesicles. Autoimmunity 2013, 46, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Munoz, L.E.; Janko, C.; Grossmayer, G.E.; Frey, B.; Voll, R.E.; Kern, P.; Kalden, J.R.; Schett, G.; Fietkau, R.; Herrmann, M.; et al. Remnants of secondarily necrotic cells fuel inflammation in systemic lupus erythematosus. Arthritis Rheum. 2009, 60, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Dieker, J.; Hilbrands, L.; Thielen, A.; Dijkman, H.; Berden, J.H.; van der Vlag, J. Enhanced activation of dendritic cells by autologous apoptotic microvesicles in MRL/lpr mice. Arthritis Res. Ther. 2015, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.T.; Ostergaard, O.; Stener, L.; Iversen, L.V.; Truedsson, L.; Gullstrand, B.; Jacobsen, S.; Heegaard, N.H. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 2012, 64, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Ullal, A.J.; Reich, C.F.; Clowse, M.; Criscione-Schreiber, L.G.; Tochacek, M.; Monestier, M.; Pisetsky, D.S. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J. Autoimmun. 2011, 36, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, J.K.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Circulating exosomes from patients with systemic lupus erythematosus induce an proinflammatory immune response. Arthritis Res. Ther. 2016, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’Donnell, E.; Farndale, R.W.; Ware, J.; et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Evankovich, J.; Yan, W.; Nace, G.; Zhang, L.; Ross, M.; Liao, X.; Billiar, T.; Xu, J.; Esmon, C.T.; et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology 2011, 54, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Goh, F.G.; Midwood, K.S. Intrinsic danger: Activation of Toll-like receptors in rheumatoid arthritis. Rheumatology 2012, 51, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Prados-Rosales, R.; Baena, A.; Martinez, L.R.; Luque-Garcia, J.; Kalscheuer, R.; Veeraraghavan, U.; Camara, C.; Nosanchuk, J.D.; Besra, G.S.; Chen, B.; et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Investig. 2011, 121, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Sabapatha, A.; Gercel-Taylor, C.; Taylor, D.D. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am. J. Reprod. Immunol. 2006, 56, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Marazuela, E.G.; Segura, E.; Fernandez-Garcia, H.; Villalba, M.; Thery, C.; Rodriguez, R.; Batanero, E. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J. Immunol. 2008, 181, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Ostman, S.; Taube, M.; Telemo, E. Tolerosome-induced oral tolerance is MHC dependent. Immunology 2005, 116, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; Cortes, R. Extracellular vesicles as biomarkers of systemic lupus erythematosus. Dis. Markers 2015, 2015, 613536. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.T.; Ostergaard, O.; Johnsen, C.; Jacobsen, S.; Heegaard, N.H. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3067–3077. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, O.; Nielsen, C.T.; Iversen, L.V.; Tanassi, J.T.; Knudsen, S.; Jacobsen, S.; Heegaard, N.H. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis Rheum. 2013, 65, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Dye, J.R.; Ullal, A.J.; Pisetsky, D.S. The role of microparticles in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Scand. J. Immunol. 2013, 78, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.; Al-Husain, A.; Pemberton, P.; Yates, A.P.; Ho, P.; Gorodkin, R.; Teh, L.S.; Alexander, M.Y.; Bruce, I.N. Suppression of inflammation reduces endothelial microparticles in active systemic lupus erythematosus. Ann. Rheum. Dis. 2014, 73, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Vinuela-Berni, V.; Doniz-Padilla, L.; Figueroa-Vega, N.; Portillo-Salazar, H.; Abud-Mendoza, C.; Baranda, L.; Gonzalez-Amaro, R. Proportions of several types of plasma and urine microparticles are increased in patients with rheumatoid arthritis with active disease. Clin. Exp. Immunol. 2015, 180, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Dieker, J.; Tel, J.; Pieterse, E.; Thielen, A.; Rother, N.; Bakker, M.; Fransen, J.; Dijkman, H.B.; Berden, J.H.; de Vries, J.M.; et al. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol. 2016, 68, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Ortega, L.M.; Schultz, D.R.; Lenz, O.; Pardo, V.; Contreras, G.N. Review: Lupus nephritis: Pathologic features, epidemiology and a guide to therapeutic decisions. Lupus 2010, 19, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Fortin, P.R.; Cloutier, N.; Bissonnette, V.; Aghdassi, E.; Eder, L.; Simonyan, D.; Laflamme, N.; Boilard, E. Distinct subtypes of microparticle-containing immune complexes are associated with disease activity, damage, and carotid intima-media thickness in systemic lupus erythematosus. J. Rheumatol. 2016, 43, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.T.; Ostergaard, O.; Rekvig, O.P.; Sturfelt, G.; Jacobsen, S.; Heegaard, N.H. Galectin-3 binding protein links circulating microparticles with electron dense glomerular deposits in lupus nephritis. Lupus 2015, 24, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.T.; Rasmussen, N.S.; Heegaard, N.H.; Jacobsen, S. “Kill” the messenger: Targeting of cell-derived microparticles in lupus nephritis. Autoimmun. Rev. 2016, 15, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; Forner, M.J.; Pinto, C.; Chaves, F.J.; Cortes, R.; Redon, J. Increased urinary exosomal MicroRNAs in patients with systemic lupus erythematosus. PLoS ONE 2015, 10, e0138618. [Google Scholar] [CrossRef] [PubMed]
- Miranda, K.C.; Bond, D.T.; McKee, M.; Skog, J.; Paunescu, T.G.; Da Silva, N.; Brown, D.; Russo, L.M. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010, 78, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Tricarico, M.; Corbelli, A.; Annaratone, L.; Pinach, S.; Grimaldi, S.; Bruno, G.; Cimino, D.; Taverna, D.; Deregibus, M.C.; et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS ONE 2013, 8, e73798. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sun, X.; Scicluna, B.J.; Coleman, B.M.; Hill, A.F. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int. 2014, 86, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Ichii, O.; Otsuka-Kanazawa, S.; Horino, T.; Kimura, J.; Nakamura, T.; Matsumoto, M.; Toi, M.; Kon, Y. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS ONE 2014, 9, e110383. [Google Scholar] [CrossRef] [PubMed]
- Sole, C.; Cortes-Hernandez, J.; Felip, M.L.; Vidal, M.; Ordi-Ros, J. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol. Dial. Transplant. 2015, 30, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell. Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Farini, A.; Sitzia, C.; Erratico, S.; Meregalli, M.; Torrente, Y. Clinical applications of mesenchymal stem cells in chronic diseases. Stem Cell Int. 2014, 2014, 306573. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.H.; Levy, O.; Inamdar, M.S.; Karp, J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Stem Cell 2012, 10, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Perez Lanzon, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Xu, W.; Wang, B.; Wu, H.; Tao, Y.; Zhang, B.; Wang, M.; Mao, F.; Yan, Y.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther. 2013, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Jarmalaviciute, A.; Tunaitis, V.; Pivoraite, U.; Venalis, A.; Pivoriunas, A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy 2015, 17, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yin, Y.; Lai, R.C.; Tan, S.S.; Choo, A.B.; Lim, S.K. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014, 23, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.E.; Cuenca Moreno, J.; la Cava, A. Novel approaches to lupus drug discovery using stem cell therapy. Role of mesenchymal-stem-cell-secreted factors. Expert Opin. Drug Discov. 2014, 9, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.A.; Mardani, K. Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunol. Lett. 2012, 147, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, D.; Chen, C.; Hamamura, K.; Moshaverinia, A.; Yang, R.; Liu, Y.; Jin, Y.; Shi, S. MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab. 2015, 22, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Buschow, S.I.; van Balkom, B.W.; Aalberts, M.; Heck, A.J.; Wauben, M.; Stoorvogel, W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 2010, 88, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lechman, E.R.; Bianco, N.; Menon, R.; Keravala, A.; Nash, J.; Mi, Z.; Watkins, S.C.; Gambotto, A.; Robbins, P.D. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J. Immunol. 2005, 174, 6440–6448. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Bianco, N.R.; Shufesky, W.J.; Morelli, A.E.; Robbins, P.D. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J. Immunol. 2007, 179, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Bianco, N.; Menon, R.; Lechman, E.R.; Shufesky, W.J.; Morelli, A.E.; Robbins, P.D. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol. Ther. 2006, 13, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Bianco, N.R.; Kim, S.H.; Ruffner, M.A.; Robbins, P.D. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009, 60, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Hung, M.E.; Breakefield, X.O.; Leonard, J.N. Therapeutic applications of extracellular vesicles: Clinical promise and open questions. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 439–464. [Google Scholar] [CrossRef] [PubMed]
- Nolte-‘t Hoen, E.N.; Buschow, S.I.; Anderton, S.M.; Stoorvogel, W.; Wauben, M.H. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood 2009, 113, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Vallhov, H.; Gutzeit, C.; Johansson, S.M.; Nagy, N.; Paul, M.; Li, Q.; Friend, S.; George, T.C.; Klein, E.; Scheynius, A.; et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J. Immunol. 2011, 186, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
| Type and Size | Biogenesis | Markers | Contents |
|---|---|---|---|
| Exosomes (40–130 nm) | Endolysosomal pathway. Release by exocytosis of multivesicular bodies | Tetraspanins (CD63, CD9, CD81), Alix, TSG101, Hsp60, Hsp70, Hsp90 | miRNA and mRNA; lipids (cholesterol, ceramide, sphingomyelin), cytokines receptors, MHC molecules |
| Microvesicles (100–1000 nm) | Cell surface. Outward budding of plasma membrane | Integrins, selectins, metalloproteinases, Phosphatidyl-serine | mRNA, non-coding RNAs, membrane receptors, cytoplasmic proteins (cytokines) |
| Apoptotic bodies (50–5000 nm) | Cell surface. Release from cellular blebs during apoptosis | Phosphatidyl-serine | Nuclear fractions, cell organelles, DNA, rRNA, mRNA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Hernandez, J.; Redon, J.; Cortes, R. Extracellular Vesicles as Therapeutic Agents in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2017, 18, 717. https://doi.org/10.3390/ijms18040717
Perez-Hernandez J, Redon J, Cortes R. Extracellular Vesicles as Therapeutic Agents in Systemic Lupus Erythematosus. International Journal of Molecular Sciences. 2017; 18(4):717. https://doi.org/10.3390/ijms18040717
Chicago/Turabian StylePerez-Hernandez, Javier, Josep Redon, and Raquel Cortes. 2017. "Extracellular Vesicles as Therapeutic Agents in Systemic Lupus Erythematosus" International Journal of Molecular Sciences 18, no. 4: 717. https://doi.org/10.3390/ijms18040717






