Possible Roles of CC- and CXC-Chemokines in Regulating Bovine Endometrial Function during Early Pregnancy
Abstract
:1. Introduction
2. Results
2.1. Expression of Chemokines and Their Receptors in the Bovine Endometrium during Early Pregnancy
2.2. Effects of IFNT and FMP on Chemokine Expression in Cultured Endometrial Tissues
2.3. Effects of Chemokines, IFNT, FMP, TNF, and LIF on ISG15, MX1, COX2, OTR, and ESR1 Expression in Cultured Endometrial Tissues
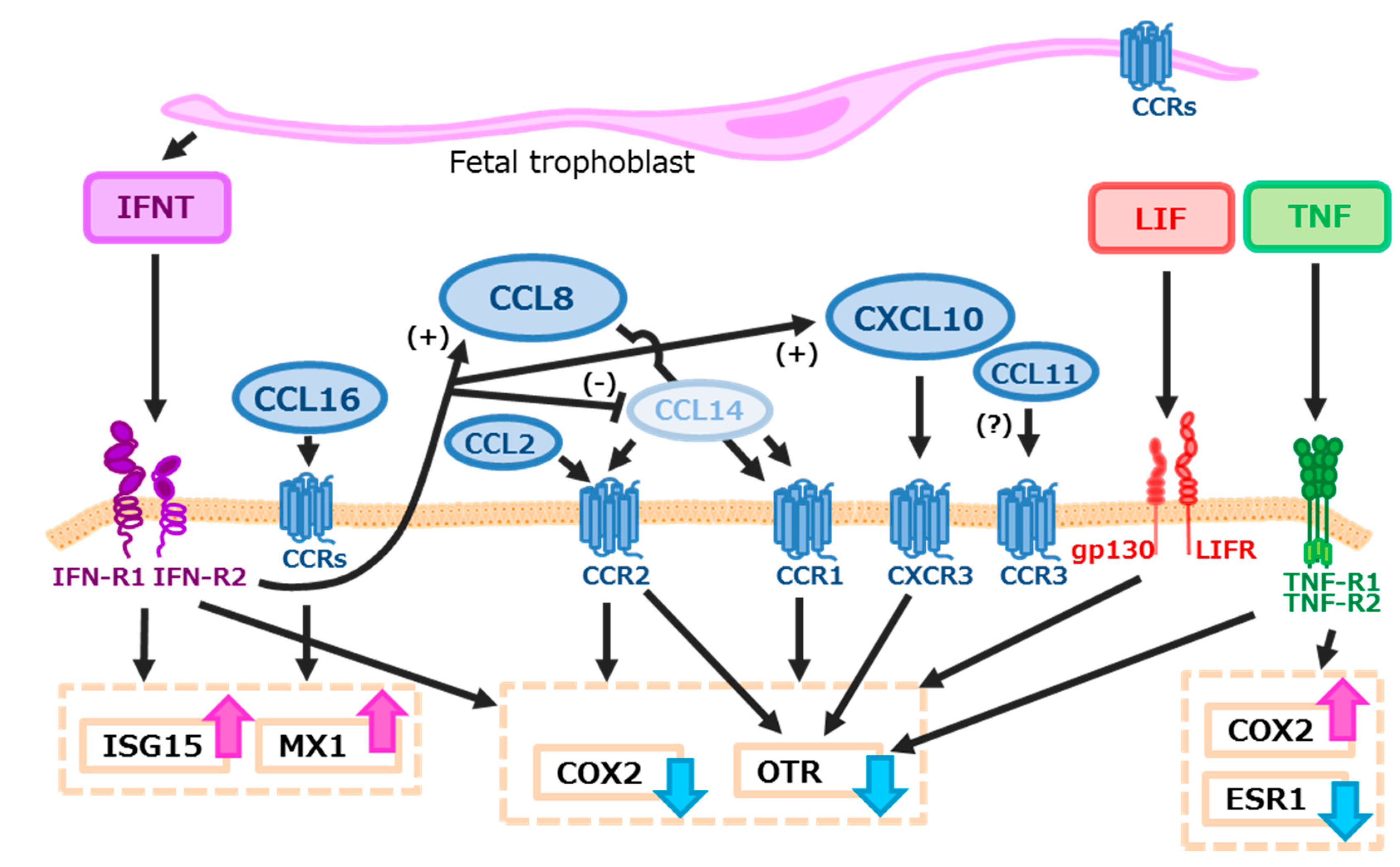
3. Discussion
4. Materials and Methods
4.1. Collection of the Bovine Uterus and Fetal Trophoblast
4.2. Microarray Analysis
4.3. Real-Time PCR
4.4. Immunohistochemistry
4.5. Endometrial Tissue Culture
4.6. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IFNT | interferon τ |
| COX2 | cyclooxygenase 2 |
| OTR | oxytocin receptor |
| ESR1 | estrogen receptor α |
| CL | corpus luteum |
| PG | prostaglandin |
| TNF | tumor necrosis factor α |
| LIF | leukemia inhibitory factor |
| FMP | supernatant derived from homogenized fetal trophoblast |
| MCP | monocyte chemoattractant protein |
| MIP | macrophage inflammatory protein |
| E2 | estradiol-17β |
References
- Thatcher, W.W.; Bartol, F.F.; Knickerbocker, J.J.; Curl, J.S.; Wolfenson, D.; Bazer, F.W.; Roberts, R.M. Maternal recognition of pregnancy in cattle. J. Dairy Sci. 1984, 67, 2797–2811. [Google Scholar] [CrossRef]
- Bazer, F.W. Mediators of maternal recognition of pregnancy in mammals. Proc. Soc. Exp. Biol. Med. 1992, 199, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Demmers, K.J.; Derecka, K.; Flint, A. Trophoblast interferon and pregnancy. Reproduction 2001, 121, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Spencer, T.E.; Ott, T.L. Interferon tau: A novel pregnancy recognition signal. Am. J. Reprod. Immunol. 1997, 37, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, S.; Ulbrich, S.E.; Gross, K.; Schmidt, S.E.; Meyer, H.H.; Wenigerkind, H.; Vermehren, M.; Sinowatz, F.; Blum, H.; Wolf, E. Embryo-induced transcriptome changes in bovine endometrium reveal species-specific and common molecular markers of uterine receptivity. Reproduction 2006, 132, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.; Henkes, L.E.; Ashley, R.L.; Purcell, S.H.; Smirnova, N.P.; Veeramachaneni, D.N.; Anthony, R.V.; Hansen, T.R. Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-τ release from the uterine vein. Endocrinology 2008, 149, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; Carter, F.; Spencer, T.E.; Bazer, F.W.; Sandra, O.; Mansouri-Attia, N.; Okumu, L.A.; McGettigan, P.A.; Mehta, J.P.; McBride, R.; et al. Conceptus-induced changes in the endometrial transcriptome: How soon does the cow know she is pregnant? Biol. Reprod. 2011, 85, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, S.; Ulbrich, S.E.; Reichenbach, H.D.; Reichenbach, M.; Büttner, M.; Meyer, H.H.; Spencer, T.E.; Minten, M.; Sax, G.; Winter, G.; et al. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol. Reprod. 2012, 86, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cerri, R.L.; Thompson, I.M.; Kim, I.H.; Ealy, A.D.; Hansen, P.J.; Staples, C.R.; Li, J.L.; Santos, J.E.; Thatcher, W.W. Effects of lactation and pregnancy on gene expression of endometrium of Holstein cows at day 17 of the estrous cycle or pregnancy. J. Dairy Sci. 2012, 95, 5657–5675. [Google Scholar] [CrossRef] [PubMed]
- Forde, N.; Lonergan, P. Transcriptomic analysis of the bovine endometrium: What is required to establish uterine receptivity to implantation in cattle? J. Reprod. Dev. 2012, 58, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.J.; Mansourri-Attia, N.; Fahey, A.G.; Browne, J.; Forde, N.; Roche, J.F.; Lonergan, P.; Fair, T. Characterization of the Th profile of the bovine endometrium during the oestrous cycle and early pregnancy. PLoS ONE 2013, 8, e75571. [Google Scholar] [CrossRef] [PubMed]
- Ealy, A.D.; Yang, Q.E. Control of interferon-tau expression during early pregnancy in ruminants. Am. J. Reprod. Immunol. 2009, 61, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Cobb, S.P.; Watson, E.D. Immunohistochemical study of immune cells in the bovine endometrium at different stages of the oestrous cycle. Res. Vet. Sci. 1995, 59, 238–241. [Google Scholar] [CrossRef]
- Asselin, E.; Johnson, G.A.; Spencer, T.E.; Bazer, F.W. Monocyte chemotactic protein-1 and -2 messenger ribonucleic acids in the ovine uterus: Regulation by pregnancy, progesterone, and interferon-τ. Biol. Reprod. 2001, 64, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, M.L.; Balestrieri, A.; Mancini, F.P.; Napoli, C. Understanding the immunoangiostatic CXC chemokine network. Cardiovasc. Res. 2008, 78, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Asselin, E.; Drolet, P.; Fortier, M.A. Cellular mechanisms involved during oxytocin-induced prostaglandin F2α production in endometrial epithelial cells in vitro: Role of cyclooxygenase-2. Endocrinology 1997, 138, 4798–4805. [Google Scholar] [PubMed]
- Nagaoka, K.; Sakai, A.; Nojima, H.; Suda, Y.; Yokomizo, Y.; Imakawa, K.; Sakai, S.; Christenson, R.K. A chemokine, interferon (IFN)-γ-inducible protein 10 kDa, is stimulated by IFN-τ and recruits immune cells in ovine endometrium. Biol. Reprod. 2003, 68, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Nagaoka, K.; Nojima, H.; Hara, Y.; Christenson, R.K. Changes in immune cell distribution and IL-10 production are regulated through endometrial IP-10 expression in the goat uterus. Am. J. Reprod. Immunol. 2005, 53, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Imai, M.; Sakai, A.; Suzuki, M.; Nagaoka, K.; Sakai, S.; Lee, S.R.; Chang, K.T.; Echternkamp, S.E.; Christenson, R.K. Regulation of conceptus adhesion by endometrial CXC chemokines during the implantation period in sheep. Mol. Reprod. Dev. 2006, 73, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, K.; Nojima, H.; Watanabe, F.; Chang, K.T.; Christenson, R.K.; Sakai, S.; Imakawa, K. Regulation of blastocyst migration, apposition, and initial adhesion by a chemokine, interferon γ-inducible protein 10 kDa (IP-10), during early gestation. J. Biol. Chem. 2003, 278, 29048–29056. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Martínez, S.; Quiñonero, A.; Loro, F.; Horcajadas, J.A.; Pellicer, A.; Simón, C. CXCL10 and IL-6 induce chemotaxis in human trophoblast cell lines. Mol. Hum. Reprod. 2008, 14, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Caballero-Campo, P.; Garcia-Velasco, J.A.; Pellicer, A. Potential implications of chemokines in reproductive function: An attractive idea. J. Reprod. Immunol. 1998, 38, 169–193. [Google Scholar] [CrossRef]
- Hannan, N.J.; Salamonsen, L.A. Role of chemokines in the endometrium and in embryo implantation. Curr. Opin. Obstet. Gynecol. 2007, 19, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Jones, R.L.; Critchley, H.O.; Kovacs, G.J.; Rogers, P.A.; Affandi, B.; Salamonsen, L.A. Coexpression of fractalkine and its receptor in normal human endometrium and in endometrium from users of progestin-only contraception supports a role for fractalkine in leukocyte recruitment and endometrial remodeling. J. Clin. Endocrinol. Metab. 2004, 89, 6119–6129. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Jones, R.L.; White, C.A.; Salamonsen, L.A. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol. Reprod. 2006, 74, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Lathbury, L.J.; Salamonsen, L.A. Expression of the chemokine eotaxin and its receptor, CCR3, in human endometrium. Biol. Reprod. 2000, 62, 404–411. [Google Scholar] [CrossRef]
- Salcedo, R.; Young, H.A.; Lourdes Ponce, M.; Ward, J.M.; Kleinman, H.K.; Murphy, W.J.; Oppenheim, J.J. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J. Immunol. 2001, 166, 7571–7578. [Google Scholar] [CrossRef] [PubMed]
- Gouon-Evans, V.; Pollard, J.W. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology 2001, 142, 4515–4521. [Google Scholar] [CrossRef] [PubMed]
- Chau, S.E.; Murthi, P.; Wong, M.H.; Whitley, G.S.; Brennecke, S.P.; Keogh, R.J. Control of extravillous trophoblast function by the eotaxins CCL11, CCL24 and CCL26. Hum. Reprod. 2013, 28, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Chand, A.L.; Murray, A.S.; Jones, R.L.; Hannan, N.J.; Salamonsen, L.A.; Rombauts, L. Laser capture microdissection and cDNA array analysis of endometrium identify CCL16 and CCL21 as epithelial-derived inflammatory mediators associated with endometriosis. Reprod. Biol. Endocrinol. 2007, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Cappello, P.; Caorsi, C.; Bosticardo, M.; de Angelis, S.; Novelli, F.; Forni, G.; Giovarelli, M. CCL16/LEC powerfully triggers effector and antigen-presenting functions of macrophages and enhances T cell cytotoxicity. J. Leukoc. Biol. 2004, 75, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Mäkikallio, K.; Kaukola, T.; Tuimala, J.; Kingsmore, S.F.; Hallman, M.; Ojaniemi, M. Umbilical artery chemokine CCL16 is associated with preterm preeclampsia and fetal growth restriction. Cytokine 2012, 60, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bussolino, F.; Mantovani, A.; Persico, G. Molecular mechanisms of blood vessel formation. Trends Biochem. Sci. 1997, 22, 251–256. [Google Scholar] [CrossRef]
- Strasly, M.; Doronzo, G.; Cappello, P.; Valdembri, D.; Arese, M.; Mitola, S.; Moore, P.; Alessandri, G.; Giovarelli, M.; Bussolino, F. CCL16 activates an angiogenic program in vascular endothelial cells. Blood 2004, 103, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, M.; Goff, A.K. Effect of pregnancy in oxytocin-induced release of prostaglandin F2α in heifers. Biol. Reprod. 1985, 33, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Skarzynski, D.J.; Okuda, K. Is tumor necrosis factor α a trigger for the initiation of endometrial prostaglandin F2α release at luteolysis in cattle? Biol. Reprod. 2000, 62, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, D.J.; Miyamoto, Y.; Okuda, K. Production of prostaglandin F2α by cultured bovine endometrial cells in response to tumor necrosis factor-α: Cell type specificity and intracellular mechanisms. Biol. Reprod. 2000, 62, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Kasahara, Y.; Murakami, S.; Takahashi, H.; Woclawek-Potocka, I.; Skarzynski, D.J. Interferon-τ blocks the stimulatory effect of tumor necrosis factor-α on prostaglandin F2α synthesis by bovine endometrial stromal cells. Biol. Reprod. 2004, 70, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Sakumoto, R. Multiple roles of TNF super family members in corpus luteum function. Reprod. Biol. Endocrinol. 2003, 1, 95. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Shuya, L.L.; Menkhorst, E.M.; Yap, J.; Li, P.; Lane, N.; Dimitriadis, E. Leukemia inhibitory factor enhances endometrial stromal cell decidualization in humans and mice. PLoS ONE 2011, 6, e25288. [Google Scholar] [CrossRef] [PubMed]
- Sakumoto, R.; Hayashi, K.G.; Takahashi, T. Different expression of PGE synthase, PGF receptor, TNF, Fas and oxytocin in the bovine corpus luteum of the estrous cycle and pregnancy. Reprod. Biol. 2014, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, K.; Shichijo-Kizaki, A.; Furusawa, T.; Takahashi, T.; Hosoe, M.; Hashizume, K. Differential neutrophil gene expression in early bovine pregnancy. Reprod. Biol. Endocrinol. 2013, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Sakumoto, R.; Hayashi, K.G.; Hosoe, M.; Iga, K.; Kizaki, K.; Okuda, K. Gene expression profiles in the bovine corpus luteum (CL) during the estrous cycle and pregnancy: Possible roles of chemokines in regulating CL function during pregnancy. J. Reprod. Dev. 2015, 61, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sakumoto, R.; Komatsu, T.; Kasuya, E.; Saito, T.; Okuda, K. Expression of mRNAs for interleukin-4, interleukin-6 and their receptors in porcine corpus luteum during the estrous cycle. Domest. Anim. Endocrinol. 2006, 31, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.G.; Meier, S.; Mitchell, M.D.; Roche, J.R.; Littlejohn, M. Evaluation of real-time PCR endogenous control genes for analysis of gene expression in bovine endometrium. BMC Mol. Biol. 2009, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Ushizawa, K.; Takahashi, T.; Hosoe, M.; Kizaki, K.; Hashizume, K. Characterization and expression analysis of SOLD1, a novel member of the retrotransposon-derived Ly-6 superfamily, in bovine placental villi. PLoS ONE 2009, 4, e5814. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Sakumoto, R.; Hayashi, K.G.; Hosoe, M.; Shirai, J.; Hashizume, K. Generation of recombinant bovine interferon tau in the human embryonic kidney cell line and its biological activity. Anim. Sci. J. 2017, in press. [Google Scholar]
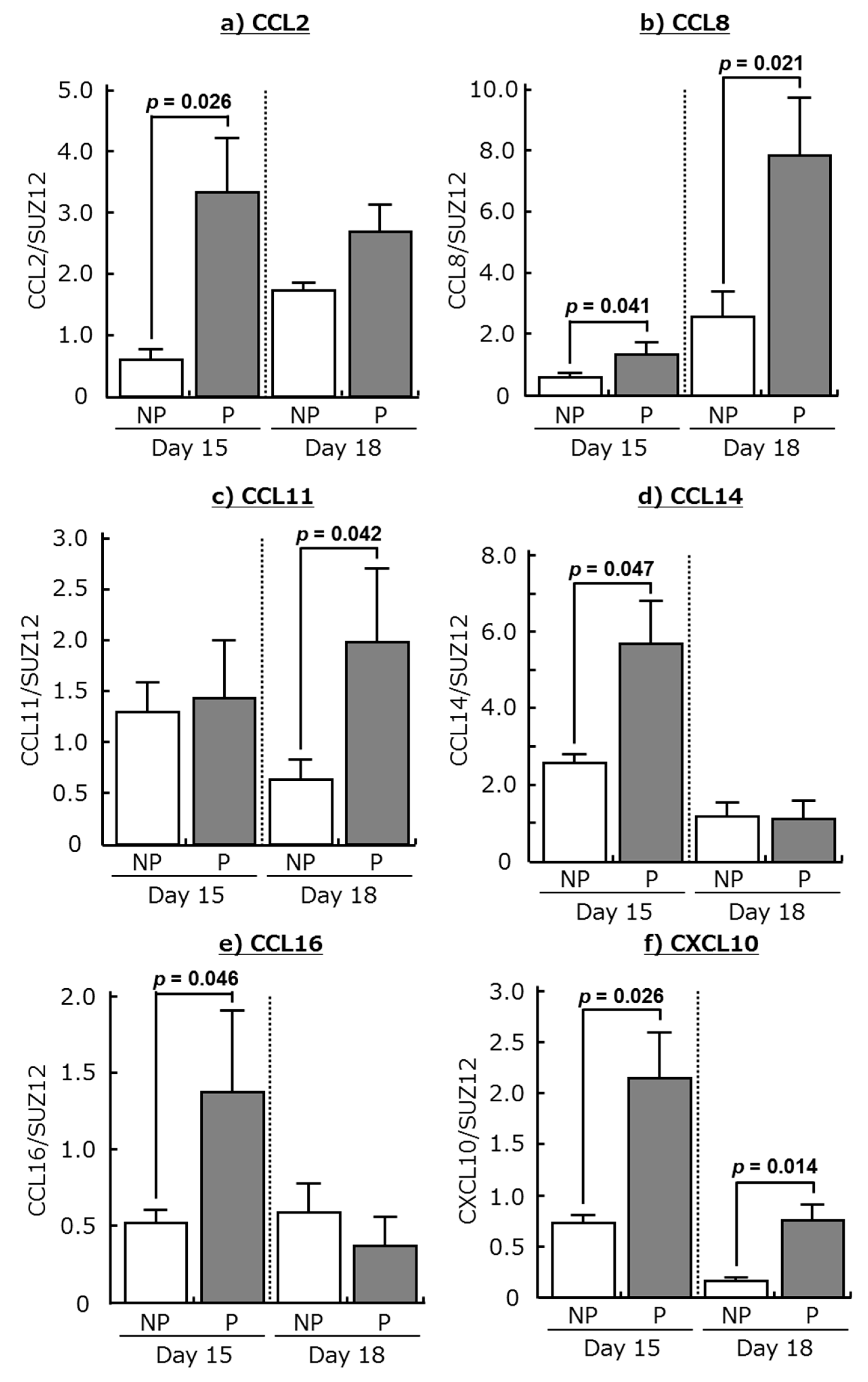
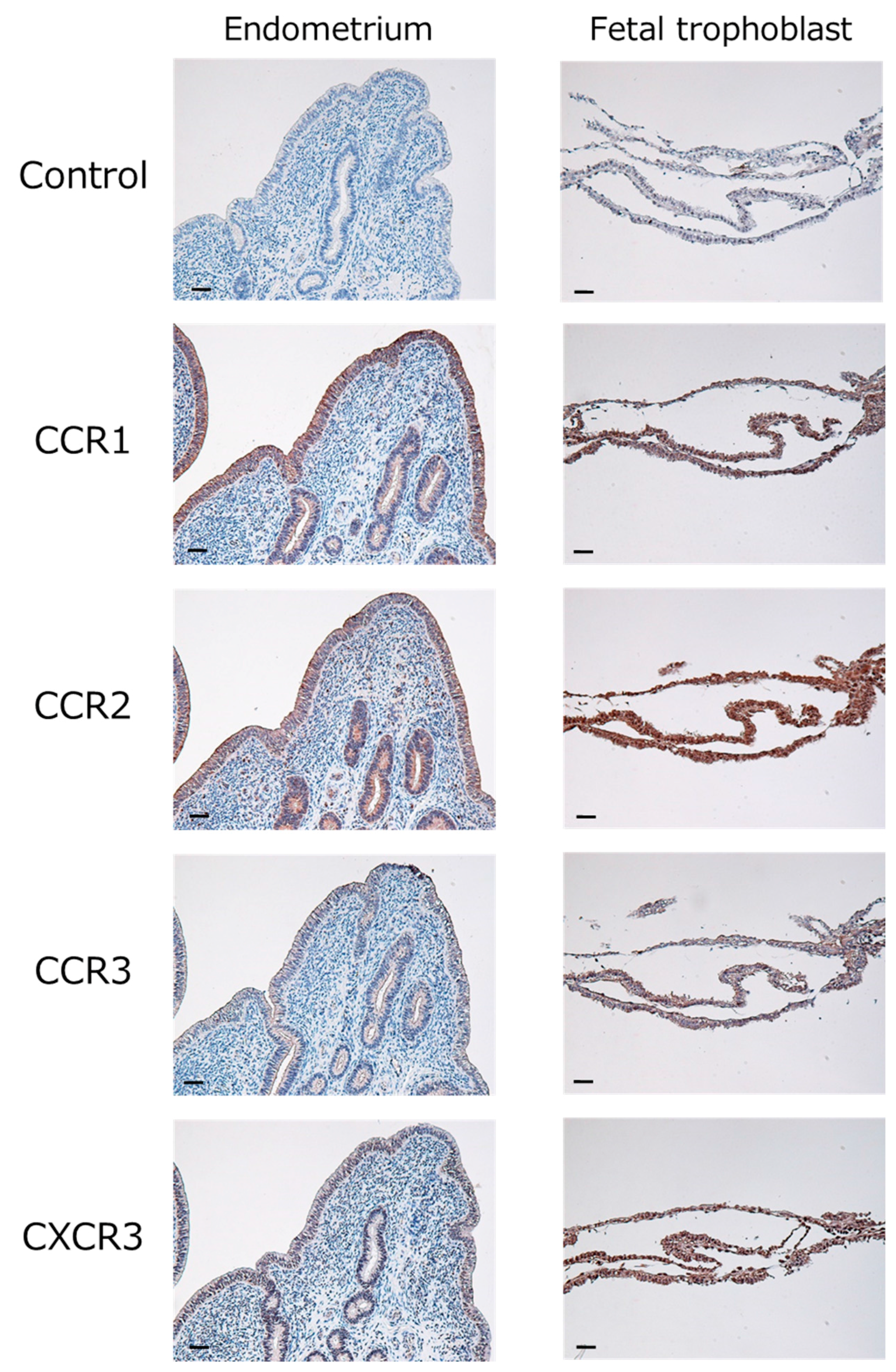
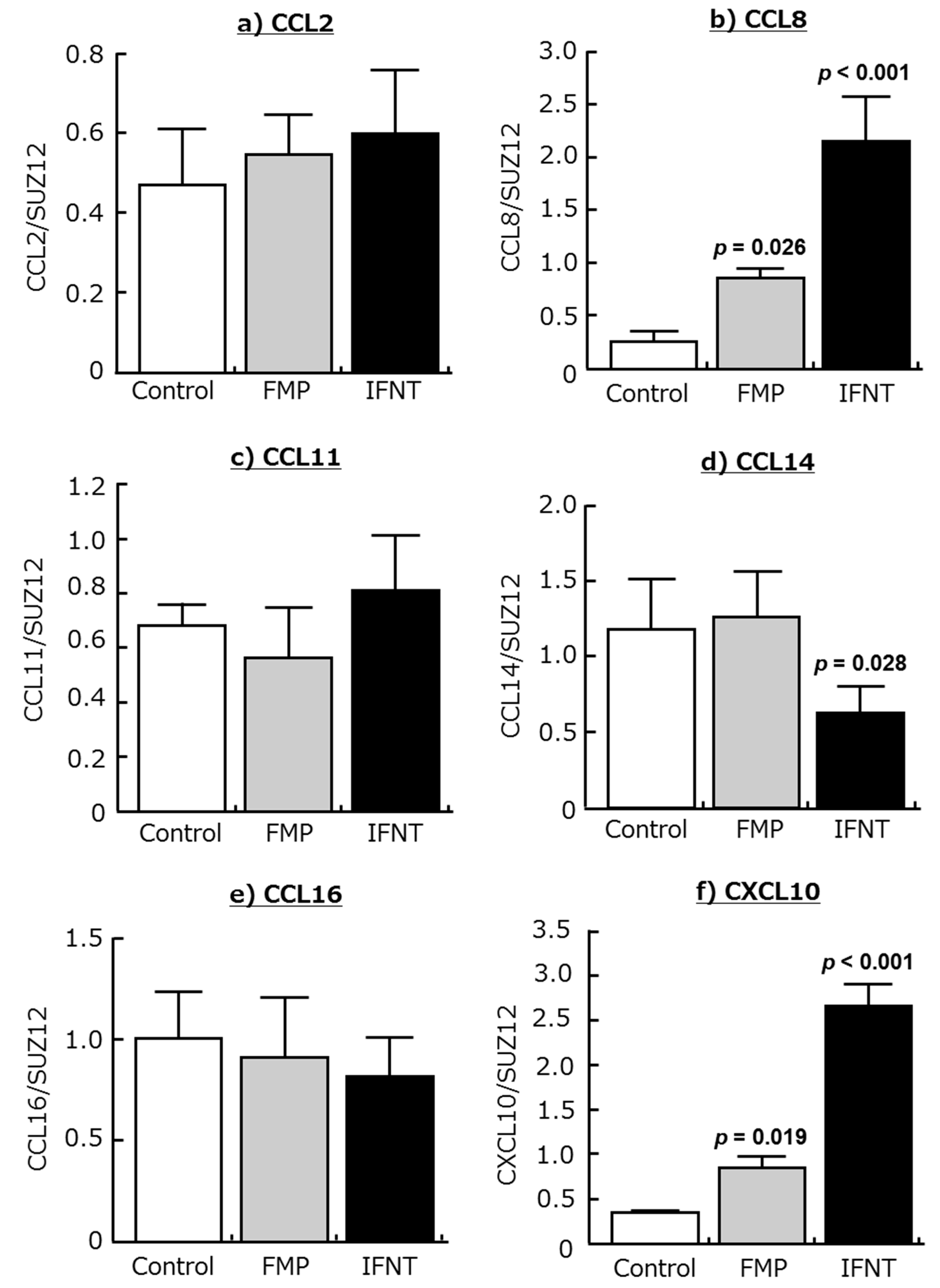

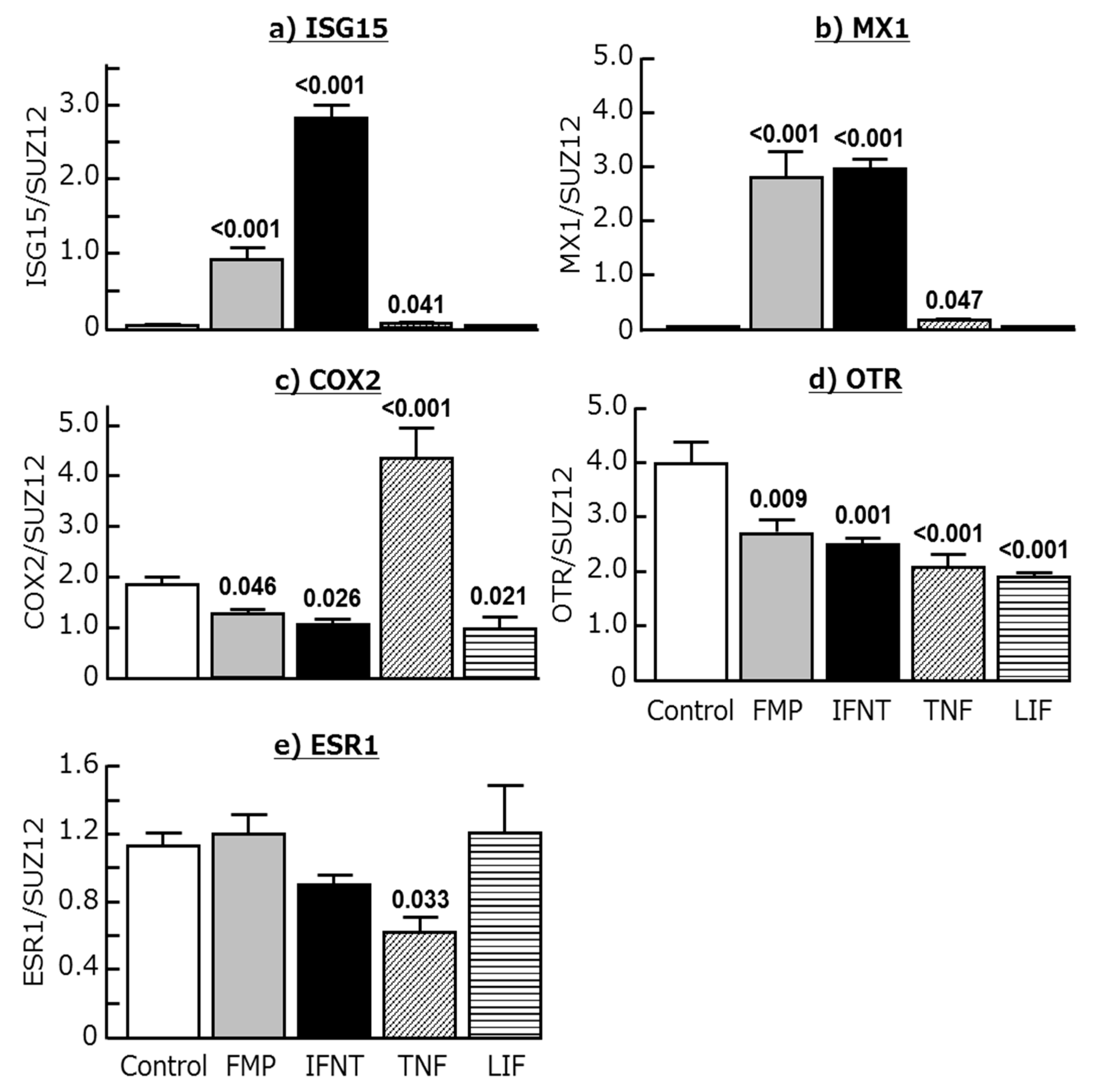
| Genes | Day 15 (344 Genes) | Day 18 (1336 Genes) |
|---|---|---|
| CCL2 (MCP-1) | 3.58 | - |
| CCL8 (MCP-2) | 4.91 | 12.9 |
| CCL11 (Eotaxin) | - | 61.1 |
| CCL14 (HCC-1) | 2.67 | - |
| CCL16 (HCC-4) | 2.55 | - |
| CXCL10 (IP-10) | 2.10 | 21.1 |
| Genes | Sequence (5′-3′) | GenBank Accession Number | Size (bp) |
|---|---|---|---|
| CCL2 | Forward: TGCAGACCCCAAGCAGAAAT | NM_174006 | 144 |
| Reverse: AGAGGGCAGTTAGGGAAAGC | |||
| CCL8 | Forward: AACATGAAGGTCTCCGCTGG | NM_174007 | 108 |
| Reverse: GCAGCAGGTGATTGGGGTAG | |||
| CCL11 | Forward: TCACGAGCAGCAAATGTCCT | NM_205773 | 101 |
| Reverse: CATGGCATTCTGGACCCACT | |||
| CCL14 | Forward: ACTAAATTTCCCCGCTCGCT | NM_001046585 | 121 |
| Reverse: TGGCCAAACTTCTGCAGAGT | |||
| CCL16 | Forward: GCCCACTGAGAGGATGAAGG | XM_002695627 | 129 |
| Reverse: TACTTCAGGCAGCAGTTGGG | |||
| CXCL10 | Forward: CTCGAACACGGAAAGAGGCA | NM_001046551 | 117 |
| Reverse: TCCACGGACAATTAGGGCTT | |||
| ISG15 | Forward: GCAGACCAGTTCTGGCTGTCT | NM_174366 | 58 |
| Reverse: CCAGCGGGTGCTCATCAT | |||
| MX1 | Forward: GAGGTGGACCCCCAAGGA | NM_173940 | 58 |
| Reverse: CCACCAGATCGGGCTTTGT | |||
| COX2 | Forward: TGTGAAAGGGAGGAAAGAGC | AF004944 | 115 |
| Reverse: GGCAAAGAATGCAAACATCA | |||
| OTR | Forward: TGTGCTGGACGCCATTCTT | NM_174134 | 93 |
| Reverse: GGAGCATGGCGATGATGAAAG | |||
| ESR1 | Forward: CAGGCACATGAGCAACAAAG | NM_001001443 | 84 |
| Reverse: TCCAGCAGCAGGTCGTAGAG | |||
| SUZ12 | Forward: GAACACCTATCACACACATTCTTGT | NM_001205587 | 130 |
| Reverse: TAGAGGCGGTTGTGTCCACT |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakumoto, R.; Hayashi, K.-G.; Fujii, S.; Kanahara, H.; Hosoe, M.; Furusawa, T.; Kizaki, K. Possible Roles of CC- and CXC-Chemokines in Regulating Bovine Endometrial Function during Early Pregnancy. Int. J. Mol. Sci. 2017, 18, 742. https://doi.org/10.3390/ijms18040742
Sakumoto R, Hayashi K-G, Fujii S, Kanahara H, Hosoe M, Furusawa T, Kizaki K. Possible Roles of CC- and CXC-Chemokines in Regulating Bovine Endometrial Function during Early Pregnancy. International Journal of Molecular Sciences. 2017; 18(4):742. https://doi.org/10.3390/ijms18040742
Chicago/Turabian StyleSakumoto, Ryosuke, Ken-Go Hayashi, Shiori Fujii, Hiroko Kanahara, Misa Hosoe, Tadashi Furusawa, and Keiichiro Kizaki. 2017. "Possible Roles of CC- and CXC-Chemokines in Regulating Bovine Endometrial Function during Early Pregnancy" International Journal of Molecular Sciences 18, no. 4: 742. https://doi.org/10.3390/ijms18040742





