1. Background
Osteoarthritis (OA) is a degenerative joint disorder that affects all tissues in synovial joints [
1,
2,
3]. Symptomatically, OA is characterized by joint pain and stiffness with eventual deformity and loss of mobility [
4]. During the onset of OA, it has been demonstrated that articular chondrocytes initiate apoptotic cascades which eventually leads to alterations in articular cartilage composition, structure, and function [
5]. Pathophysiologically, both cartilage degeneration and accelerated bone turnover have been identified as causal elements in the progression of OA [
2]. Risk factors for OA include age, obesity, and previous joint trauma; however, the interactions among these factors is unknown [
6,
7,
8,
9]. Another tissue commonly affected in the pathogenesis of OA is the synovium. Synovium is the soft tissue lining the spaces of diarthrodial joints and contains highly metabolically active cells called synoviocytes that are further separated as macrophage-like (Type A) and fibroblast-like (Type B) synoviocytes. These cell types and the synovium as a whole play a key role in normal joint physiology as they facilitate nourishment for chondrocytes and the removal of waste products through the synovial fluid [
10]. Inflammation of the synovium, known as synovitis, is commonly found in the early stages of OA, and while a number of animal and clinical studies have found a correlation between synovitis and disease severity and/or progression others have not [
11,
12]. However, because of the synovium’s influential role in maintaining the health of the joint, it is thought that this chronic inflammation of the synovium (and/or alternation of normal synovial function) is detrimental to the overall health of the joint itself.
Although historically OA was considered a degenerative disorder, more recently it has been proposed that OA is significantly more complex than previously thought. Ayral et al. performed a systematic review in which they stated that the processes involved in OA are seen as dynamic biological and biochemical processes [
9]. Of significant importance in OA pathology is the interaction of articular cartilage and synovium [
9,
13,
14]. Osteoarthritis-related synovitis results in a positive feedback loop: (a) cartilage breakdown releases tissue and molecular fragments into the synovial fluid; (b) phagocytes/macrophages in the synovium remove these fragments, inducing further signaling that promotes further synovial inflammation. The newly activated synoviocytes release catabolic and pro-inflammatory cytokines (including interleukin (IL)-1 and tumor necrosis factor-α (TNF-α)), which can lead to excess production of proteolytic enzymes (matrix metalloproteinases (MMPs); a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) and others) and to remodeling of the matrix, as well as triggering apoptosis in chondrocytes and thereby further effect cartilage breakdown. Additionally, osteoarthritic synovium produces fewer antagonists of the IL-1 receptor, intensifying the catabolic effect of the above inflammatory cytokines in OA [
15]. As cartilage breaks down further, the cycle continues [
5,
15]. Synovitis is thought to be responsible for many of the clinical symptoms of OA and has been shown to reflect the structural progression of the disease [
5]. Clinically, synovitis is rarely observed without cartilage degradation [
5], further solidifying the link between cartilage pathology and synovitis in OA.
As mentioned, the synovium is comprised of Type B synovial fibroblasts which produce lubricating molecules such as Hyaluronic acid (HA) and lubricin, but this population of cells also contains a sub-population of mesenchymal stem/progenitor cells (MSC/MPC), which are believed to be an important part of regeneration and healing in multiple tissues types. In OA, synovial MSCs/MPCs increase in number but demonstrate decreased chondrogenic potential [
16]. Synovial macrophages are a likely regulator in this phenomenon, as it was recently found that MSC chondrogenesis can be inhibited by pro-inflammatory macrophages [
17,
18]. This suggests a relationship between degeneration (apoptosis) and repair in the joint with inflammation potentially acting as the intermediary. However, to date, there is no direct evidence in humans that cartilage can repair itself once injured/damaged, furthermore, a recent study has suggested that cartilage has little to no turnover after skeletal maturity regardless of the disease state of the individual (OA vs. normal) [
19]. Therefore, it is necessary to determine the role of these MSCs/MPCs and macrophages in normal and arthritic synovium, and a logical first step is to enumerate these cells throughout the stages of OA. It is also important to note that we chose to use the terminology mesenchymal progenitor cell (MPC) instead of mesenchymal stem cell (MSC). This was done intentionally, since the name MSC is defined by the functional properties of the cell and not solely by marker expression. A histological examination of the spatial and temporal distribution and quantity of marker cannot define the functional capacity of a cell.
Previously, Hermida-Gomez et al. [
20] quantified cells expressing MSC/MPC markers in healthy and OA synovium from hip joints and found that cells positive for MSC/MPC markers were increased in OA compared to normal synovium. This result is consistent with other findings [
16,
21], that suggest an increase in stem/progenitor cells with severity of cartilage damage/OA. However, no study to date has evaluated the differences in MPC levels in pre-OA versus normal knee synovium, and furthermore, to our knowledge no study has examined the localization of MPCs in relation to synovial macrophages throughout disease progression. In the current study, we therefore sought to examine if synovial MPCs and macrophage populations are also increased in pre-OA since it has been previously hypothesized that, as the severity of OA increases, so does the number of MPCs and immune cells present within the synovium. We have attempted to address this hypothesis by quantifying the number of cells expressing markers of MPC and macrophages present in normal, pre-OA, and OA synovium. Additionally, the localization of the cells (intima vs. subintimal) and overall inflammatory state of the tissue was also examined.
3. Discussion
A number of previous studies have demonstrated that synovial MSC/MPC populations increase in OA. In the majority of these studies, a normal/control group was compared to a clinically diagnosed (typically end stage) OA patient cohort. While the results of the current study agree with previous finding between normal and OA joints, no increase in MPCs was observed in a pre-OA patient population that presented with cartilage damage and synovial inflammation, yet were asymptomatic and demonstrated no radiographic changes associated with OA. Furthermore, the same trend was observed with synovial macrophages between normal and OA knee synovium, however, fewer macrophages were observed in pre-OA patients compared to normal controls. The results and limitations of this study will be discussed in relation the published literature below.
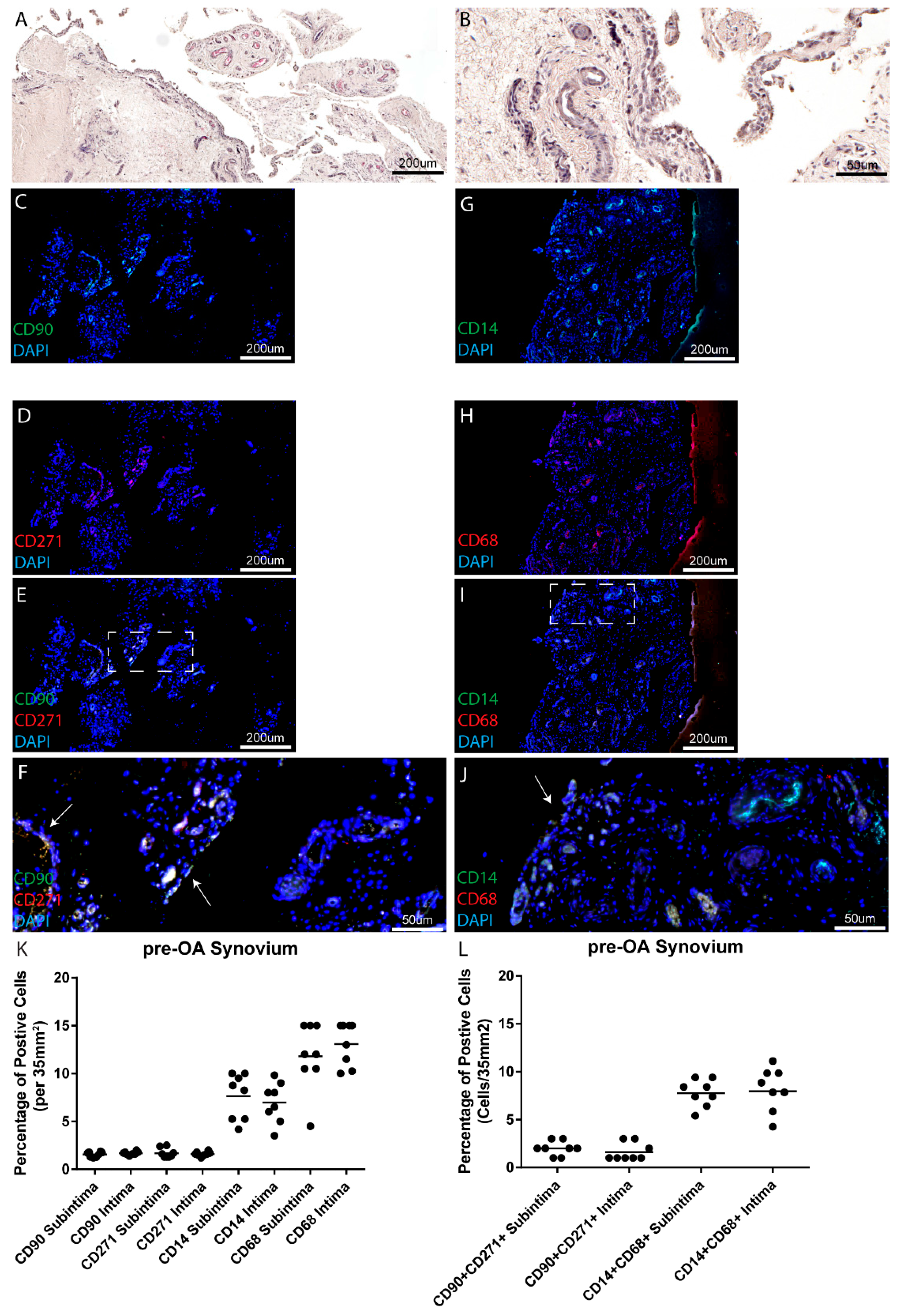
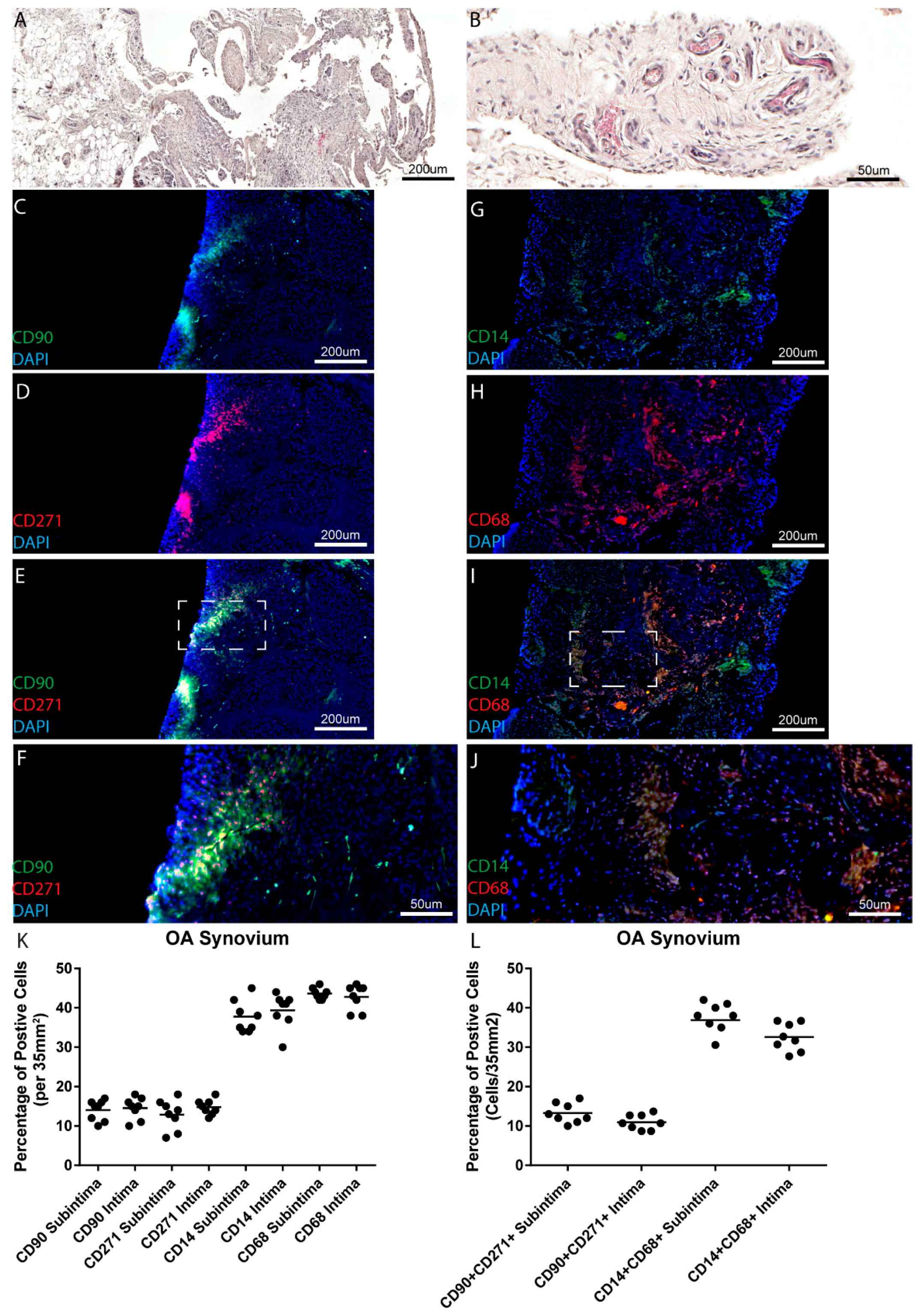
In this study, we chose to examine the MSC/MPC markers CD90 and CD271 for a number of reasons. Primarily, both our group and others have demonstrated that synovial cells purified based on the basis of CD90
+ demonstrated increased chondrogenic potential compared to the CD90
− population [
22,
23,
24]. Additionally, it has been previously demonstrated in hip synovium that the CD90
+ CD271
+ double positive population is not only present throughout the synovium (intima and subintima) [
25], but when CD271
+ or CD271
− bone marrow-derived cells were used to treat a chondral defect, the CD271
+ positive population demonstrated significantly increased repair potential [
25]. While CD90 and CD271 are promising markers to identify MSC/MPC populations from synovium and other tissues, there are many additional marker normally used to characterize these cell population including but not limited to CD44, CD73, CD105, CD146. In this study, the main limitation was that we were not able to perform co-localization with more markers; however, that being said, it is widely agreed upon that the marker expression of a cell does not correlate to function, and to determine if a cell is truly an MSC, functional analysis must be undertaken. Therefore, in this study we have defined these cell populations as progenitor cells based on the previous literature demonstrating that synovial cells selected for by CD90 and/or CD271 meet the functional definition of a progenitor. However, it is important to be clear that although we have enumerated these cell populations, there is no guarantee that all CD90
+, CD271
+ or CD90
+ CD271
+ positive cells represent the same cell type and/or demonstrate a conserved function. Additionally, it is more than likely that the cells (MPCs and/or macrophages) examined in this study represent only a sub-population of a larger population that might not be accurately represented by the markers selected for this study.
Increased numbers of MSCs/MPCs have been found in OA joints as compared to normal joints, in both synovial fluid and the synovium itself, findings which have been confirmed in this study. Several studies have examined the quantity of stem cells found in synovial fluid. Jones et al. compared OA synovial fluid with rheumatoid arthritis (RA) synovial fluid and found increased numbers of MSCs in the OA synovial fluid [
26]. Quantification revealed that MSCs were increased ten-fold in OA as compared to RA synovial fluid. It is unknown whether these MSCs are from the synovium itself or whether they could be recruited from bone, periosteum or bone marrow. Jones et al. [
27] explored the above idea when they examined MSC in normal and OA human joints. The study was unique in that it examined synovial fluid in early OA, rather than late stage OA. They attempted to understand whether normal synovial fluid has a resident stem cell population, or whether an increase in stem cells in the fluid was directly linked to the disease state of the joints. After identifying the cell signature of the endogenous synovial fluid cells, they determined that the MSCs likely came directly from the synovial fluid [
27].
Similarly, Sekiya et al. found that synovial fluid MSC increased as the severity of OA increased [
21], and Hermida-Gomez et al. [
20] found that there were significantly more CD44 and CD90 positive cells in OA synovium than in normal synovium. This effect was reversed, however, regarding cells positive for CD73; healthy synovium has greater numbers of CD73 positive cells than OA synovium [
20]. This is interesting to note, considering that the presence of CD44, CD90 and CD73 all indicate MSCs. However, it has been shown using clonal analysis in vitro that multiple distinct MSCs populations reside within a given tissue. Therefore, one plausible hypothesis is that specific populations of MSCs were influenced by the disease state of the synovium and/or joint as a whole. Singh et al. characterized normal knee synovium using immuno-histochemistry (IHC). After removing synovial biopsies from 12 healthy subjects, samples were stained and analyzed for lymphocytes (T cells and B cells) and macrophages [
28]. Findings indicated the presence of T cells and macrophages, but no B cells. Osteoarthritic tissue containing increased numbers of macrophages has been put forth as a mechanism by which endogenous MSCs in OA joints become dysfunctional [
17]. Benito et al. [
29] compared early stage OA synovium to late stage OA synovium. Findings showed that there were increased numbers of macrophages in the early OA synovium as compared to the late stage OA synovium. Macrophages in synovium contribute to the overall inflammatory cascade seen in OA. Products of cartilage breakdown are phagocytosed by the synoviocytes (macrophage-like), which inflames the synovium. Production of proinflammatory cytokines then amplifies this effect. Activated synovial T cells, B cells and macrophages increase the inflammatory response and contribute to a positive feedback loop [
10]. In addition, macrophages themselves produce growth factors such as vascular endothelial growth factor and inflammatory cytokines such as IL-1B and TNF-a [
30]. While in the current study, increased numbers of CD68
+ CD14
+ cells were observed in normal vs. OA synovium, however, OA synovium presented with a higher inflammatory score. Since CD68 and CD14 do not describe if a macrophage is M1 vs. M2 polarized, it is therefore possible that the CD68
+ CD14
+ cells detected in normal synovium may be M2 anti-inflammatory macrophages that are lost during the onset and progression of OA; however, further study with specific polarization markers will be needed to address this hypothesis. Additionally, we cannot discount the possibility that other immune cells and/or synovial fibroblasts are contributing to the increased synovial inflammation observed in the pre-OA cohort. Furthermore, it is also quite possible that pre-OA patients might have been self-medicating with various pharmacological agents (none of the pre-OA cohort were on prescription anti-inflammatories) which led to a decrease in macrophage/monocytic cells in this cohort.
The results of the current study using immunofluorescence staining showed the presence of both MPCs and macrophages. There was no correlation between synovial inflammation in pre-OA and increased numbers of the cell types examined. Additionally, there was no significant difference between the apparent cellularity of pre-OA synovium and normal synovium. These findings conflict with much of the current literature. Sekiya et al. [
21] and Jones et al. [
26] found increased numbers of stem cell type cells present in the synovial fluid of OA patients as compared to normal controls. The Hermida-Gomez study described above illustrated increased numbers of stem cells in OA synovium as compared to normal control synovium [
20]. This being said, most of the existing studies were performed on late stage OA synovium or exclusively on synovial fluid. To date, there has been no study which has compared MPCs in pre-OA synovium to normal controls. However, in this study, when an OA cohort was examined (radiographic OA with confirmed cartilage changes during arthroscopy) increased numbers of both MPCs and macrophages were observed consistent with the published literature. Therefore, there could be a number of potential reasons explaining the findings in the current study. As we aimed to quantify cells found in synovium at the earliest stages of OA, most of the pre-OA patients used in this study demonstrated very low levels of synovitis in the joint (although increased compared to normal joints, but decreased compared to OA joints). The inflammatory threshold for cellular infiltration has not been established and therefore there may not have been sufficient inflammation to lead to increased cellularity. In this study, ‘pre-OA’ was asymptomatic. Considering that synovitis is typically associated with pain, this could indicate that subclinical OA synovium has few differences from ‘normal synovium’. As there have been no studies that have examined pre-OA synovium and normal controls, it is difficult to know whether any cellular differences would be significant. Another potential confounding variable is patient heterogeneity and our relatively low sample size (
n = 8 per group). Although our sample size is on par with a number of the studies cited within this manuscript, OA is known to be a heterogeneous disease potentially encompassing many sub-types. It is unlikely that our sample size has captured the breadth of heterogeneity observed within this disease and that must be considered as a limitation. Furthermore, since we sampled many sites within each patient (48 data points per patient) we did observe heterogeneity in marker expression (MPC and macrophage) both within and between patients. Specifically, we did observe some areas of cells positive for only one marker and others that were predominantly double positive (
Figures S1 and S2); however, when this was quantified, the majority of cells (MPCs or macrophages) in all cohort were double positive for both markers (either CD90
+ CD271
+ or CD14
+ CD68
+). However, this does suggest that there are other populations in the synovium that express only one marker and it would of interest to determine if these different populations exhibit different functions.
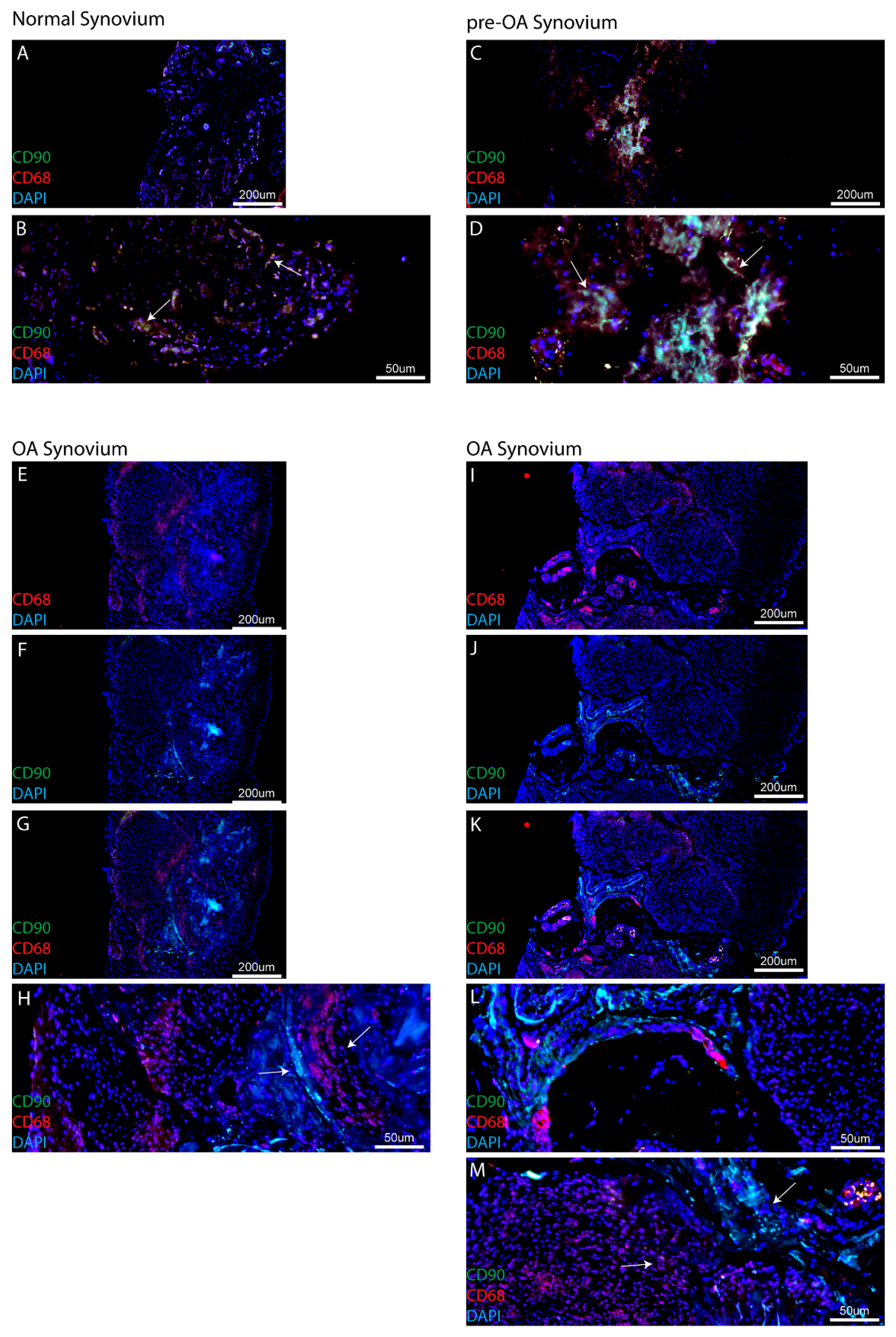
One finding of this study that has not been discussed previously to our knowledge is the co-localization (or lack thereof) of MPCs and macrophages within the synovium. While MPCs and macrophages have both been observed throughout the synovium, in this study using CD90 and CD68 staining, it was observed that these two cell types are in close proximity to each other in normal and pre-OA synovium. While this does not directly indicate a relationship between the two cell types, it has been previously demonstrated the synovial macrophages can regulate the chondrogenic and inflammatory state of synovial MSCs [
17,
18]. However, in OA synovial specimens, it was observed that MPC and macrophage populations were no longer in close proximity to each other and each population appeared to be located within distinct clusters of similar cells types with defined boarders within the synovium. It is also important to note, that this result was consistent across all the eight OA patients examined, with no clear example of MPC-macrophage intermixing observed within this cohort. While this result in itself does not suggest that macrophages and MPCs are no longer interacting with each other, this observation does merit further examination to determine if in OA, the relationship between MPCs and macrophages has been altered, if the same pathways are active in both cell types (e.g., growth factors/cytokines), and whether the added distance between the cells affected the ability of each cell type to effectively communicate with the other.
Recently, studies have begun to dissect the relationship between MSCs and macrophages in the joint environment [
17,
18]. While there is still not direct evidence that endogenous MSCs can repair cartilage defects in humans, it is clear that these synovial MSCs/MPCs present with increased chondrogenic capacity compared to their bone marrow-derived relatives. Additionally, since it is known that macrophages can secrete factors that trigger chondrocytes to undergo apoptosis (such as Inducible nitric oxide synthase (iNOS)), this highlights the macrophage as a potential signaling ‘lynchpin’ in the joint environment that is not only capable for inducing degeneration (through chondrocyte cell death), but potentially regulating the repair capacity of local MSCs. While this study did not functionally test this hypothesis, to our knowledge we are the first to demonstrate that, in addition to the numbers of cells (MPCs, macrophages) present in the synovium at any given stage of the disease, the localization and interaction of the cells with each other may play a role in the loss of homeostasis in the arthritic joint as it progresses from pre- to post-radiographic OA.
4. Methods
4.1. Ethics Statement
Informed consent to participate was obtained by written agreement. The study protocol was approved by the University of Calgary Research Ethics Board (University of Calgary ethics #21987).
4.2. Subjects
A total of 24 human subjects were used for the current study. Synovial biopsies were harvested from normal (
n = 8), pre-OA (
n = 8) and osteoarthritic (
n = 8) knees. Normal subjects ranged in age from 32 years to 80 years of age; pre-OA subjects had an age range of 37 years to 66 years, while patients OA had an age range of 51–82 (
Table 1).
Normal control tissue samples were obtained from the Southern Alberta Tissue Donation Program. Criteria for control cadaveric donations were: no history of arthritis, joint injury or surgery (including visual inspection of the cartilage surfaces during recovery), no prescription anti-inflammatory medications, no co-morbidities (such as diabetes/cancer), and availability within 4 h of death. A minimum of four synovial biopsies (total) were taken by the recovery team from the medial and lateral compartments of the joint adjacent to the capsule [
16], and care was taken to avoid the fat pad.
Synovial biopsies from pre-OA subjects were taken during scheduled diagnostic arthroscopic surgery. Surgery was performed for numerous reasons, including low grade pain, clicking or crepitus. The inclusion criterion was the absence of radiographic OA defined as a Kellgren Lawrence (K/L) grade of 0 or 1, but with an Outerbridge score of 1 or 2 based on arthroscopic examination performed by an orthopedic surgeon at the University of Calgary. Synovial biopsies from OA subjects were taken during arthroscopic surgery. The inclusion criteria for OA was meeting the American College of Rheumatology (ACR) criteria with a K/L grade of 3 or 4. Based on ethics approval from the University of Calgary, a maximum of four synovial biopsies were obtained from OA patients and these were collected from the medial and lateral compartments of the joint adjacent to the capsule.
4.3. Tissue Fixation and Processing
Synovium from each individual patient was fixed in 4% paraformaldahyde (PFA) at 4 °C overnight, rinsed in phosphate-buffered saline, infiltrated using an automatic tissue processor (Leica Biosystems, Wetzlar, Germany), and embedded in paraffin wax. Paraffin blocks were sectioned at a thickness of 7 μm sections using a rotary microtome (Leica RM2255). A minimum of 12 slides (48 serial sections) were cut from each block.
4.4. Staining
Slides were stained with hematoxylin and eosin (H&E) and by immunofluorescence (IF). For IF, sections (7 μm) were deparaffinized in CitriSolv (Fisher Scientific; Fairlawn, NJ, USA) and rehydrated through a series of graded ethanol to distilled water steps. Antigen retrieval (10 mM sodium citrate, pH 6.0, Sigma-Aldrich; St. Louis, Missouri, USA) and blocking (1:500 dilution; 100 μL goat serum: 50 mL TRIS-buffered saline, 0.1% Tween 20 (TBST) for 1 h), steps were performed prior to going through sequential wash (TBST) and the application of primary antibody. Primary antibodies conjugated to fluorophores included: CD14-FITC (Macrophage), CD68-PE (Macrophage), CD90-Alexa488 (MPC) or CD271-Alexa568 (MPC) (all BD Biosciences; Franklin Lakes, NJ, USA), and all slides were counterstained with the nucleic acid stain DAPI (4′,6-diamidino-2-phenylindole) (Sigma-Aldrich; St. Louis, MS, USA) and mounted using FluorSave reagent (Calbiochem; Darmstadt, Germany). Isotype controls for fluorescein isothiocyanate (FITC) and phycoerythrin (PE) demonstrated little to no reactivity (
Figure S5).
Imaging: Slides were imaged using a Plan-Apochromat objective (20×/0.8 M27) on an Axio Scan.Z1 Slide Scanner microscope (Carl Zeiss; Oberkochen, Germany); DAPI (excitation 353 nm, emission 465 nm), Alexa488 (excitation 493 nm, emission 517 nm), and Alexa568 (excitation 565 nm, emission 576 nm).
4.5. Evaluation/Cell Counting
Counts of cell profiles staining positive for each antibody marker were made for each section. Each section was examined at low magnification (approximately 2×) for the purpose of choosing four random areas, two from the outer layer of the synovium (subintima) and two from the inner layer (adjacent to the synovial fluid) of the synovium (intima). This allowed for examination of two areas on the periphery and two in the central area of the tissue. Counting was performed at 40× magnification which resulted in a field of area of 35 mm
2. Twelve sections per synovial biopsy were examined using this methodology with 4 fields of view examined per section (2 intima, 2 subintima), therefore, 48 data points were generated per patient. Positive cell counts are presented as the percentage of marker positive cells over the total number of cells (DAPI) in a 35 mm
2 field of view. Subjective scoring of synovitis was performed on H&E stained synovium using the scale of Krenn et al. [
31]. Briefly, three features of synovitis: enlargement of lining cell layer, cellular density of synovial stroma and leukocytic infiltrate were evaluated from 0—absent, to 3—strong and each feature was graded separately. The sum provided the synovitis score, which was interpreted as: 0–1, no synovitis; 2–4, low-grade synovitis; 5–9, high-grade synovitis. Grading was performed by three individuals in total, 2 were blinded.
4.6. Analysis
To determine if cells were single and double positive for a given marker, image analysis was performed in the Zen software suite (Carl Zeiss; Oberkochen, Germany). DAPI-positive cells that expressed at least one marker were identified and marked. The signal intensity of the FITC and PE channels was quantified after autofluorescence correction and if a cell demonstrated a signal intensity in FITC or PE of greater than 95% of the background level (based on an area intensity histogram) it was called positive for that marker. Clear areas of intense autofluorescence (e.g., blood cells) and/or process/staining artefacts were excluded from the analysis.
All statistics were performed in GraphPad Prism 6.05 (La Jolla, CA, USA). A two-way analysis of variance was used to determine if significant differences existed between the mean numbers of cell profiles for each cell type. Comparisons were made both within a joint, as well as between osteoarthritic and normal joints. A Bonferroni analysis was used for all post-hoc analyses. Alpha was set at 0.05.