Increased Regenerative Capacity of the Olfactory Epithelium in Niemann–Pick Disease Type C1
Abstract
:1. Introduction
2. Results
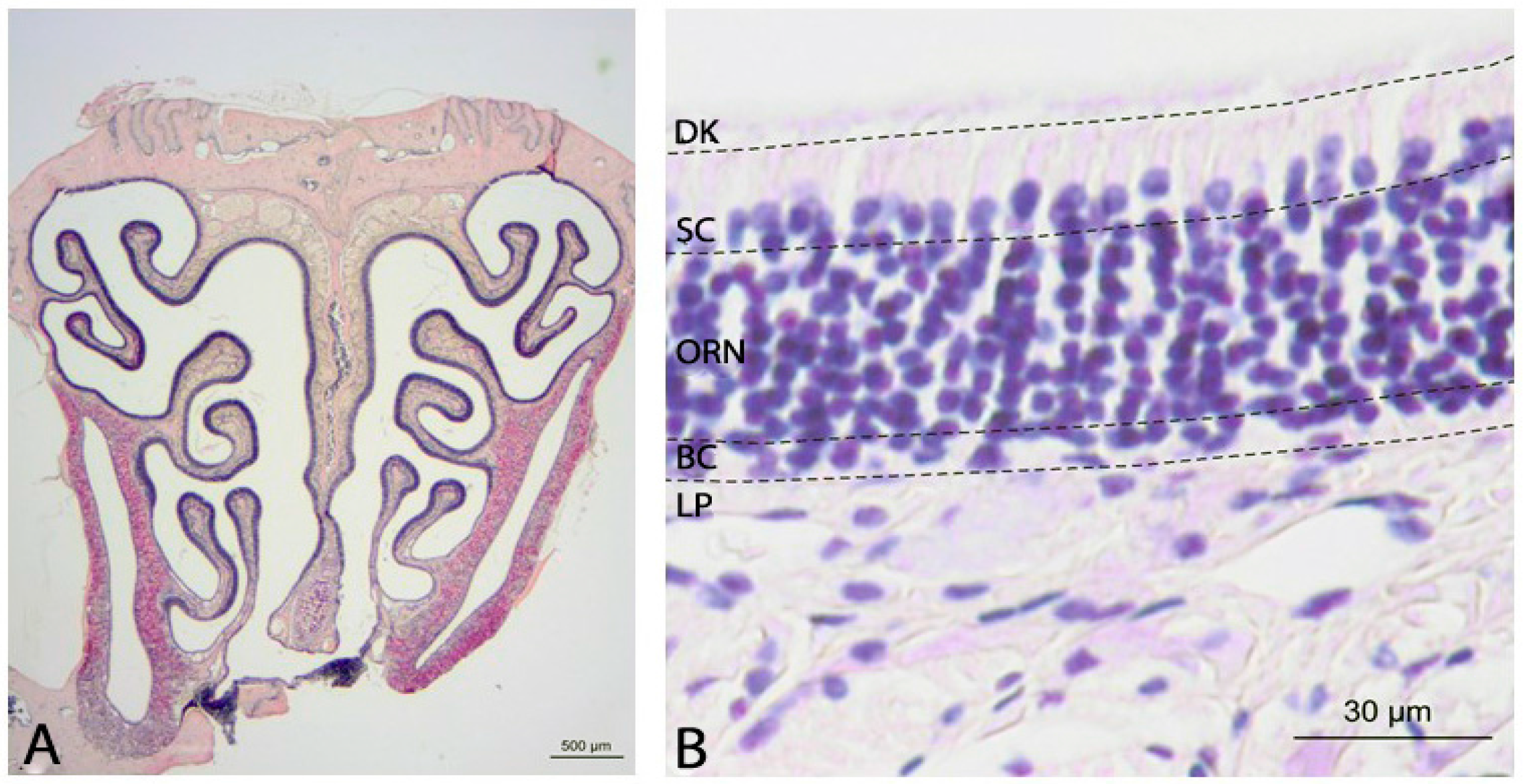
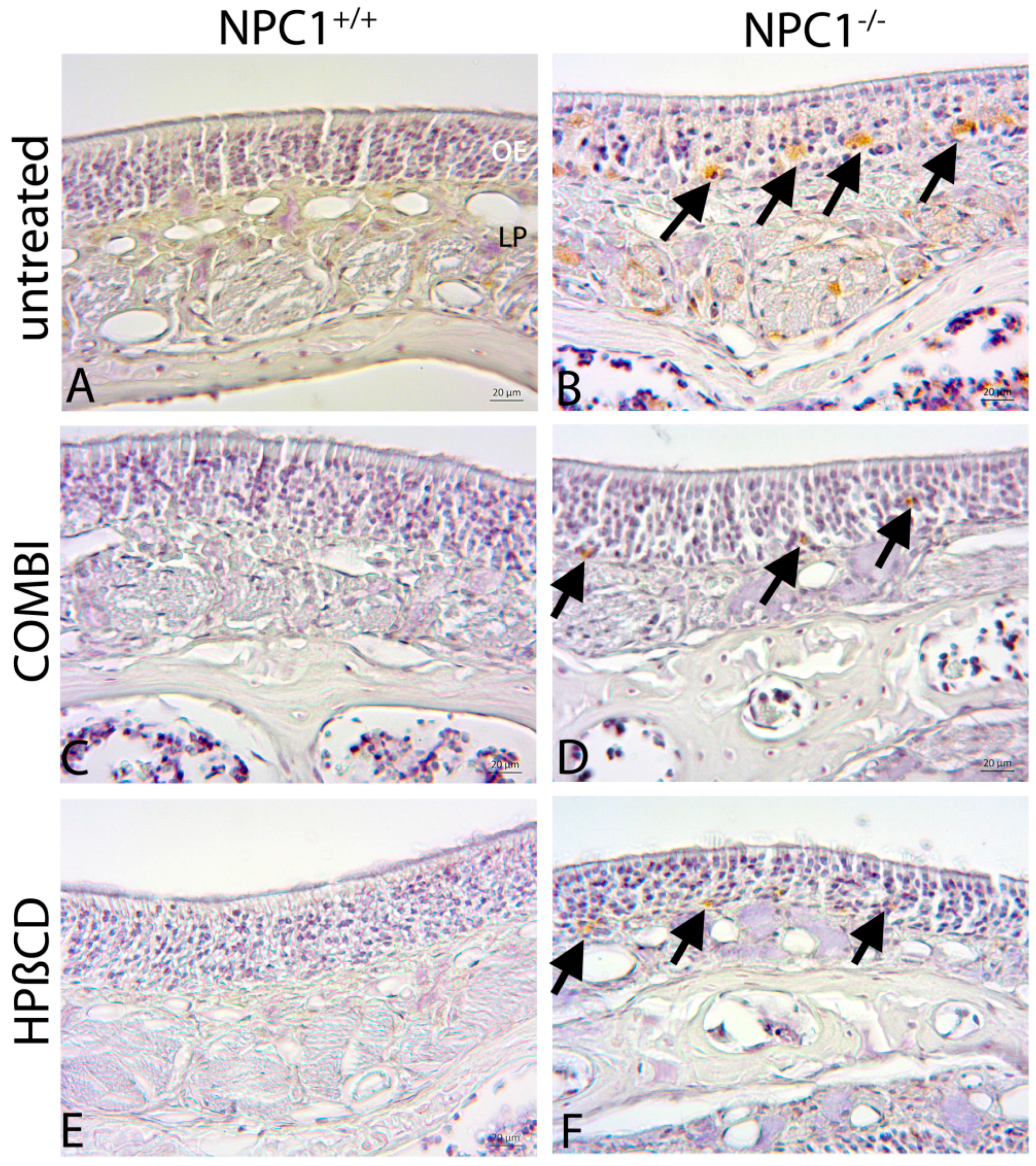
2.1. Histology of the Olfactory Mucosa
2.2. Quantification of BrdU(+) Proliferating Cells
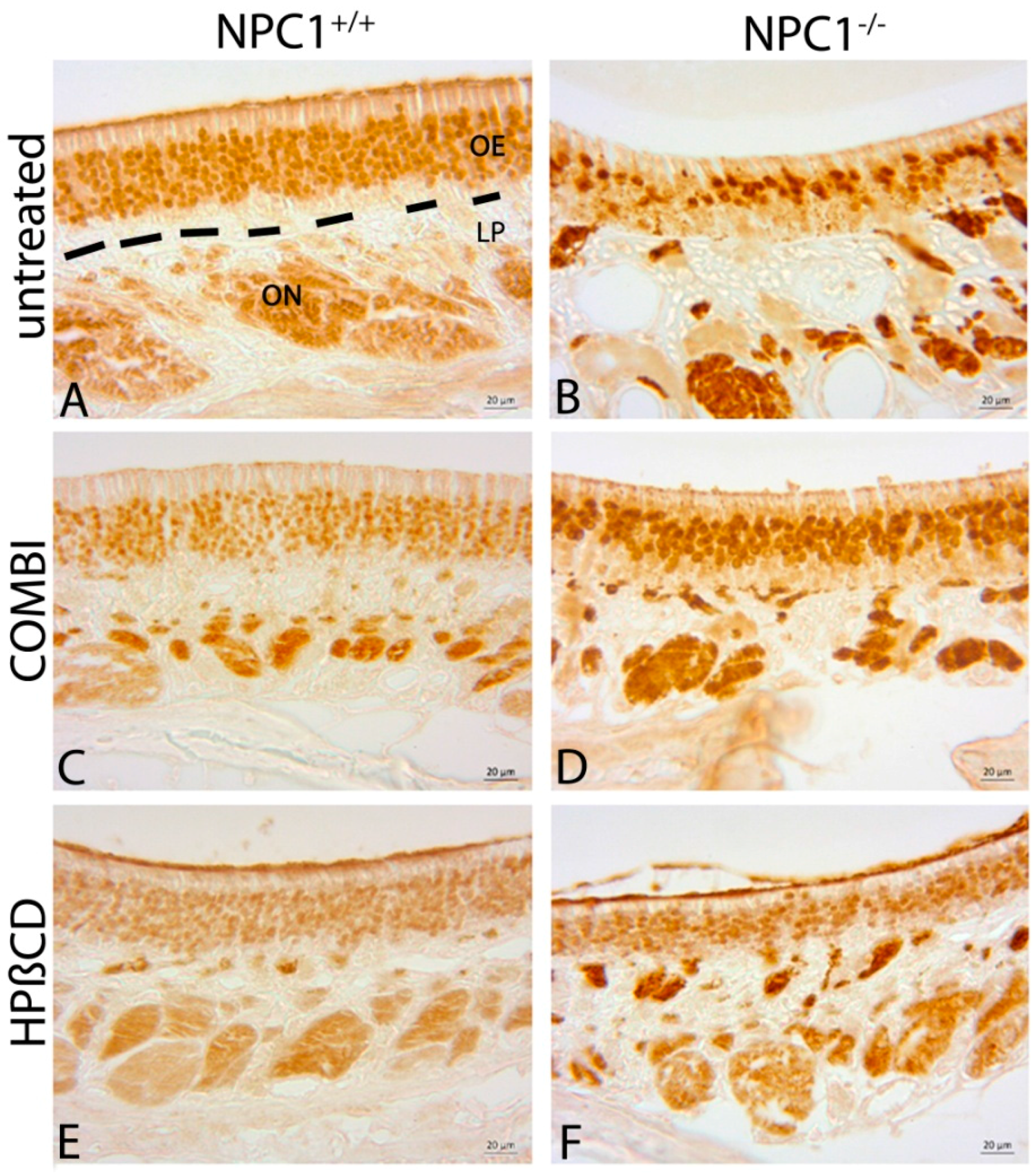
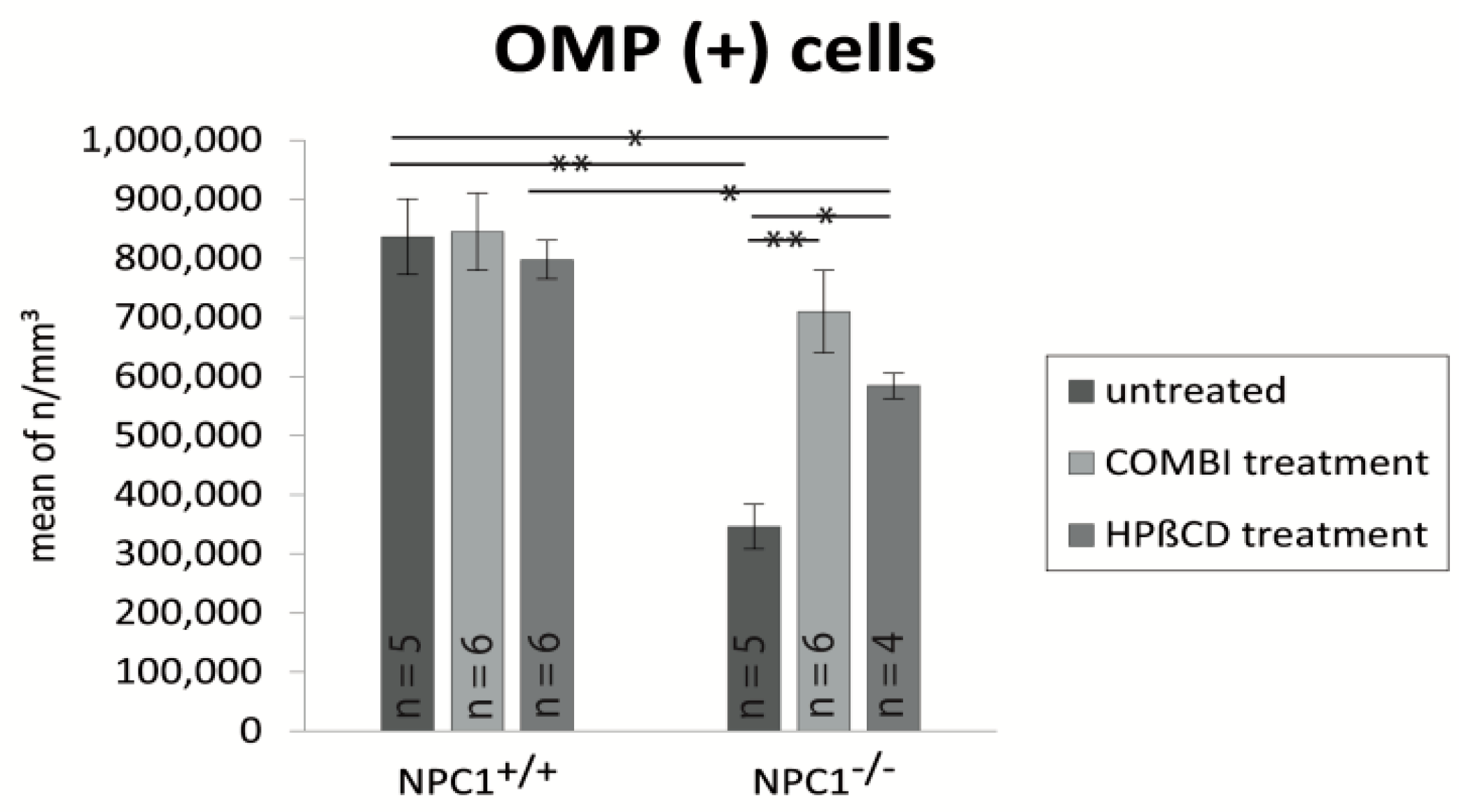
2.3. Quantification of OMP(+) Mature ORNs
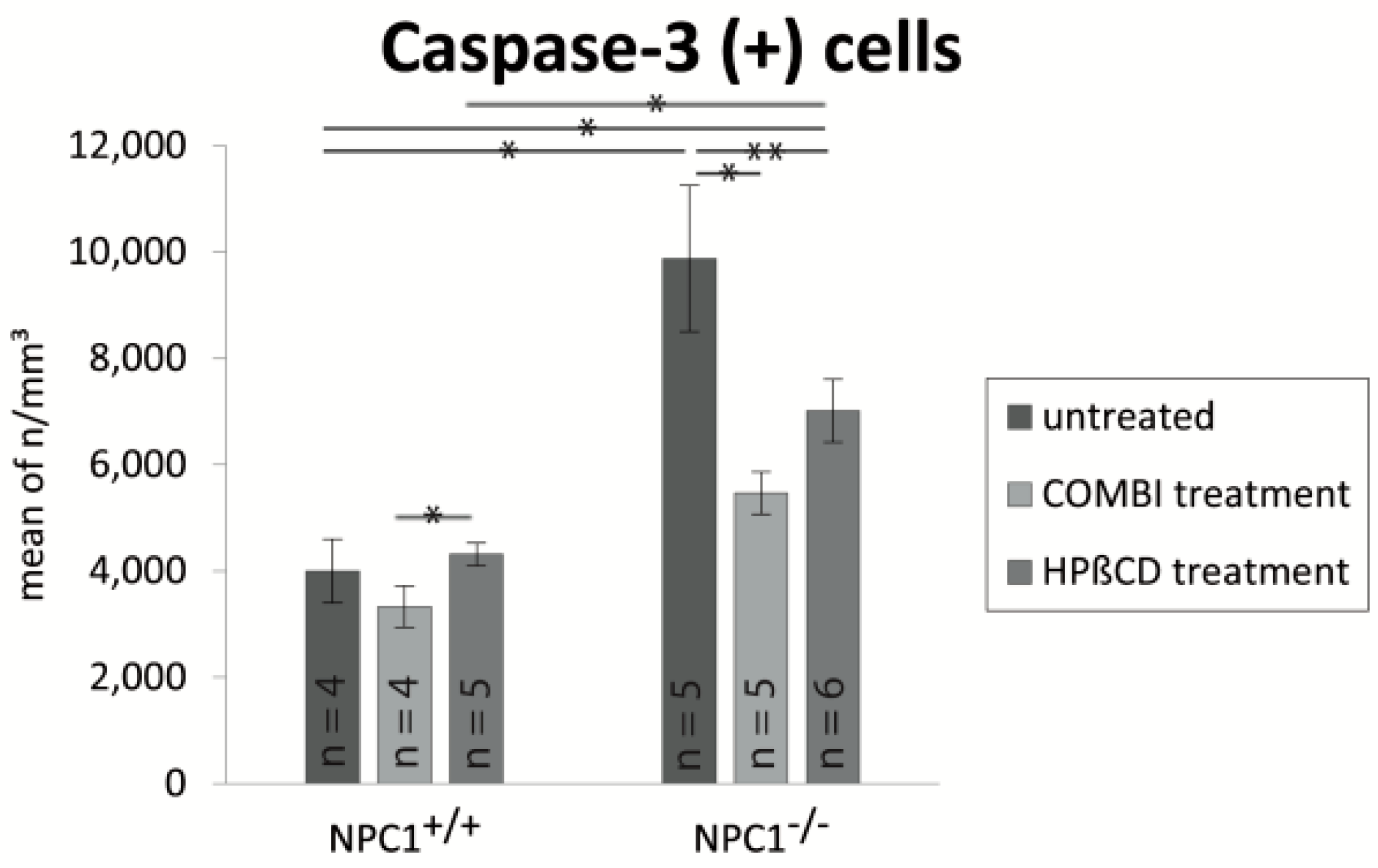
2.4. Quantification of Cas-3(+) Apoptotic Cells
2.5. Cathepsin D
3. Discussion
3.1. Olfactory Epithelium in NPC1+/+ Control Animals
3.2. Morphological Alterations in NPC1−/− Animals
3.3. Combination Treatment of NPC1−/− Mice Shows Beneficial Effects
3.4. Monotherapy with HPβCD
3.5. Limitations of Symptomatic Treatments
3.6. Therapy-Induced Changes in NPC1+/+ Mice
3.7. Differences between the Therapeutic Effects
4. Methods
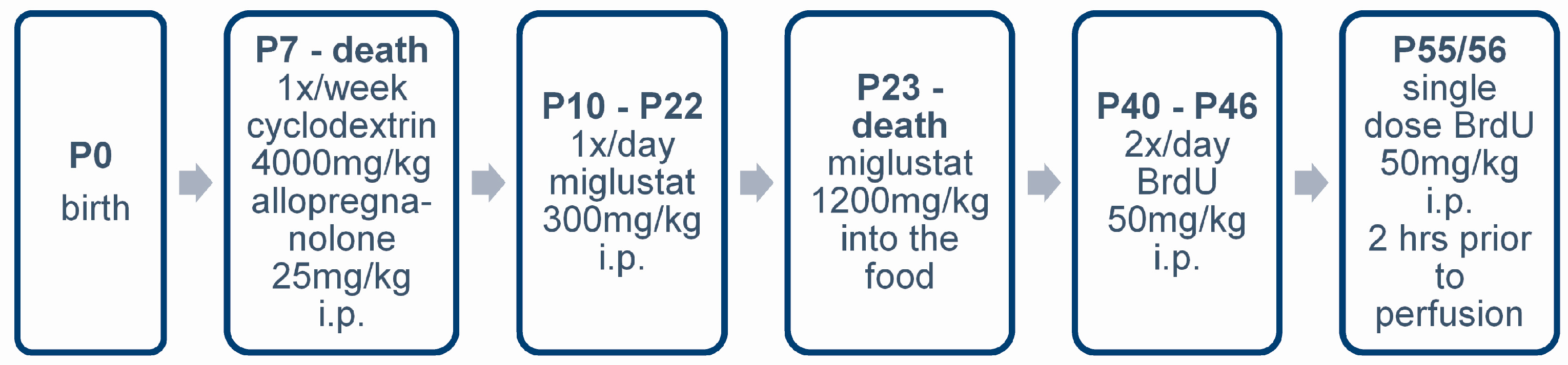
4.1. Animals
4.2. Genotyping
4.3. Pharmacologic Treatment
4.4. BrdU Injections
4.5. Sample Preparation
4.6. Immunohistochemistry
4.7. Stereology and Statistic Evaluation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ross, G.W.; Petrovitch, H.; Abbott, R.D.; Tanner, C.M.; Popper, J.; Masaki, K.; Launer, L.; White, L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 2008, 63, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H.; Shephard, B.C.; Daniel, S.E. Is Parkinson’s disease a primary olfactory disorder? QJM 1999, 92, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Deems, D.A.; Stellar, S. Olfactory dysfunction in Parkinsonism: A general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 1988, 38, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Mesholam, R.I.; Moberg, P.J.; Mahr, R.N.; Doty, R.L. Olfaction in neurodegenerative disease: A meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch. Neurol. 1998, 55, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Jones-Gotman, M.; de Sousa, K.; Chertkow, H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2008, 29, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Moberg, P.J.; Pearlson, G.D.; Speedie, L.J.; Lipsey, J.R.; Strauss, M.E.; Folstein, S.E. Olfactory recognition: Differential impairments in early and late Huntington’s and Alzheimer’s diseases. J. Clin. Exp. Neuropsychol. 1987, 9, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory disorders and quality of life—An updated review. Chem. Sens. 2014, 39, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Karpa, M.J.; Gopinath, B.; Rochtchina, E.; Jie Jin, W.; Cumming, R.G.; Sue, C.M.; Mitchell, P. Prevalence and neurodegenerative or other associations with olfactory impairment in an older community. J. Aging Health 2010, 22, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Loo, A.T.; Youngentob, S.L.; Kent, P.F.; Schwob, J.E. The aging olfactory epithelium: Neurogenesis, response to damage, and odorant-induced activity. Int. J. Dev. Neurosci. 1996, 14, 881–900. [Google Scholar] [CrossRef]
- Graziadei, P.P.; Monti Graziadei, G.A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 1979, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Carr, V.M.; Farbman, A.I. The dynamics of cell death in the olfactory epithelium. Exp. Neurol. 1993, 124, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Sim, A.; St John, J.; Schwob, J.E. Neurogenesis in the adult olfactory epithelium. In Handbook of Olfaction and Gustation, 3rd ed.; Doty, R.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 133–156. [Google Scholar]
- Schwob, J.E.; Jang, W.; Holbrook, E.H.; Lin, B.; Herrick, D.B.; Peterson, J.N.; Hewitt Coleman, J. Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license. J. Comp. Neurol. 2017, 525, 1034–1054. [Google Scholar] [CrossRef] [PubMed]
- Hovakimyan, M.; Meyer, A.; Lukas, J.; Luo, J.; Gudziol, V.; Hummel, T.; Rolfs, A.; Wree, A.; Witt, M. Olfactory deficits in Niemann-Pick Pick type C1 (NPC1) disease. PLoS ONE 2013, 8, e82216. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, H.-S.; Shin, Y.; Kang, I.; Choi, S.W.; Yu, K.-R.; Seo, K.-W.; Kang, K.-S. Excessive microglial activation aggravates olfactory dysfunction by impeding the survival of newborn neurons in the olfactory bulb of Niemann-Pick disease type C1 mice. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.W.; Gordon, R.E.; Ioannou, Y.A. NPC1 late endosomes contain elevated levels of non-esterified (’free’) fatty acids and an abnormally glycosylated form of the NPC2 protein. Biochem. J. 2005, 390, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, E.B.; Wastney, M.; Patel, S.; Suresh, S.; Cooney, A.M.; Dwyer, N.K.; Roff, C.F.; Ohno, K.; Morris, J.A.; Carstea, E.D.; et al. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem. 1999, 274, 9627–9635. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Yao, Y.; Liu, J.; Yu, Z.; Cheung, S.; Xie, A.; Liang, X.; Bi, X. Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in NPC1−/− mouse brain. Am. J. Pathol. 2007, 171, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Sokol, J.; Blanchette-Mackie, J.; Kruth, H.S.; Dwyer, N.K.; Amende, L.M.; Butler, J.D.; Robinson, E.; Patel, S.; Brady, R.O.; Comly, M.E.; et al. Type C Niemann-Pick disease. Lysosomal accumulation and defective intracellular mobilization of low density lipoprotein cholesterol. J. Biol. Chem. 1988, 263, 3411–3417. [Google Scholar] [PubMed]
- Te Vruchte, D.; Lloyd-Evans, E.; Veldman, R.J.; Neville, D.C.; Dwek, R.A.; Platt, F.M.; van Blitterswijk, W.J.; Sillence, D.J. Accumulation of glycosphingolipids in Niemann-Pick C disease disrupts endosomal transport. J. Biol. Chem. 2004, 279, 26167–26175. [Google Scholar] [CrossRef] [PubMed]
- Maass, F.; Petersen, J.; Hovakimyan, M.; Schmitt, O.; Witt, M.; Hawlitschka, A.; Lukas, J.; Rolfs, A.; Wree, A. Reduced cerebellar neurodegeneration after combined therapy with cyclodextrin/allopregnanolone and miglustat in NPC1: A mouse model of Niemann-Pick type C1 disease. J. Neurosci. Res. 2015, 93, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Elleder, M.; Jirasek, A.; Smid, F.; Ledvinova, J.; Besley, G.T. Niemann-Pick disease type C. Study on the nature of the cerebral storage process. Acta Neuropathol. 1985, 66, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Nakamura, H.; Miyawaki, S. Cerebellar involvement in murine sphingomyelinosis: A new model of Niemann-Pick disease. J. Neuropathol. Exp. Neurol. 1988, 47, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Murayama, S.; Pentchev, P.G.; Suzuki, K. Cerebellar degeneration in the Niemann-Pick type C mouse. Acta Neuropathol. 1993, 85, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Sarna, J.R.; Larouche, M.; Marzban, H.; Sillitoe, R.V.; Rancourt, D.E.; Hawkes, R. Patterned Purkinje cell degeneration in mouse models of Niemann-Pick type C disease. J. Comp. Neurol. 2003, 456, 279–291. [Google Scholar] [CrossRef] [PubMed]
- German, D.C.; Quintero, E.M.; Liang, C.L.; Ng, B.; Punia, S.; Xie, C.; Dietschy, J.M. Selective neurodegeneration, without neurofibrillary tangles, in a mouse model of Niemann-Pick C disease. J. Comp. Neurol. 2001, 433, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Saji, M.; Ukita, Y.; Shinoda, Y.; Taniguchi, M.; Higaki, K.; Ninomiya, H.; Ohno, K. Progressive neuronal loss in the ventral posterior lateral and medial nuclei of thalamus in Niemann-Pick disease type C mouse brain. Brain Dev. 2001, 23, 288–297. [Google Scholar] [CrossRef]
- Love, S.; Bridges, L.R.; Case, C.P. Neurofibrillary tangles in Niemann-Pick disease type C. Brain 1995, 118, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Lukas, J.; Witt, M.; Wree, A.; Hübner, R.; Frech, M.; Köhling, R.; Rolfs, A.; Luo, J. Decreased expression of myelin gene regulatory factor in Niemann-Pick type C 1 mouse. Metab. Brain Dis. 2011, 26, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Goodrum, J.F.; Pentchev, P.G. Cholesterol reutilization during myelination of regenerating pns axons is impaired in Niemann-Pick disease type C mice. J. Neurosci. Res. 1997, 49, 389–392. [Google Scholar] [CrossRef]
- Yu, T.; Lieberman, A.P. NPC1 acting in neurons and glia is essential for the formation and maintenance of cns myelin. PLoS Genet. 2013, 9, e1003462. [Google Scholar] [CrossRef] [PubMed]
- German, D.C.; Liang, C.L.; Song, T.; Yazdani, U.; Xie, C.; Dietschy, J.M. Neurodegeneration in the Niemann-Pick C mouse: Glial involvement. Neuroscience 2002, 109, 437–450. [Google Scholar] [CrossRef]
- Platt, F.M.; Jeyakumar, M. Substrate reduction therapy. Acta Paediatr. 2008, 97, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef] [PubMed]
- Liu, B. Therapeutic potential of cyclodextrins in the treatment of Niemann–Pick type C disease. Clin. Lipidol. 2012, 7, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Turley, S.D.; Burns, D.K.; Miller, A.M.; Repa, J.J.; Dietschy, J.M. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc. Natl. Acad. Sci. USA 2009, 106, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.I.; Zhang, G.; Warren, J.D.; Maxfield, F.R. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5477–5482. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Liu, B.; Mari, Y.; Liu, B.; Repa, J.J. Cyclodextrin mediates rapid changes in lipid balance in NPC1−/− mice without carrying cholesterol through the bloodstream. J. Lipid Res. 2012, 53, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Vtesse. Inc. Study of 2-Hydroxypropyl-Beta-Cyclodextrin (vts-270) to Treat Niemann-Pick Type C1 (NPC1) Disease. Clinicaltrials. Government National Institutes of Health. Available online: https://clinicaltrials.Gov/ct2/show/study/nct02534844 (accessed on 6 March 2017).
- Aqul, A.; Liu, B.; Ramirez, C.M.; Pieper, A.A.; Estill, S.J.; Burns, D.K.; Liu, B.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 2011, 31, 9404–9413. [Google Scholar] [CrossRef] [PubMed]
- Baldisseri, D.M.; Margolis, J.W.; Weber, D.J.; Koo, J.H.; Margolis, F.L. Olfactory marker protein (OMP) exhibits a beta-clam fold in solution: Implications for target peptide interaction and olfactory signal transduction. J. Mol. Biol. 2002, 319, 823–837. [Google Scholar] [CrossRef]
- Margolis, F.L. Olfactory marker protein (OMP). Scand. J. Immunol. 1982, 9, 181–199. [Google Scholar] [CrossRef]
- Arnold, S.E.; Lee, E.B.; Moberg, P.J.; Stutzbach, L.; Kazi, H.; Han, L.Y.; Lee, V.M.; Trojanowski, J.Q. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann. Neurol. 2010, 67, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Jellinger, K.A. Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin. Neuropathol. 2006, 25, 265–271. [Google Scholar] [PubMed]
- Devanand, D.P.; Lee, S.; Manly, J.; Andrews, H.; Schupf, N.; Doty, R.L.; Stern, Y.; Zahodne, L.B.; Louis, E.D.; Mayeux, R. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 2015, 84, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Berendse, H.W.; Booij, J.; Francot, C.M.; Bergmans, P.L.; Hijman, R.; Stoof, J.C.; Wolters, E.C. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann. Neurol. 2001, 50, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Witt, M.; Reichmann, H.; Welge-Luessen, A.; Haehner, A. Immunohistochemical, volumetric, and functional neuroimaging studies in patients with idiopathic Parkinson’s disease. J. Neurol. Sci. 2010, 289, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.M.; Youngentob, S.L.; Goldstein, B.J.; Luskin, M.B.; Schwob, J.E. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J. Comp. Neurol. 1998, 400, 469–486. [Google Scholar] [CrossRef]
- Kawagishi, K.; Ando, M.; Yokouchi, K.; Sumitomo, N.; Karasawa, M.; Fukushima, N.; Moriizumi, T. Stereological estimation of olfactory receptor neurons in rats. Chem. Sens. 2015, 40, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cluzeau, C.V.; Watkins-Chow, D.E.; Fu, R.; Borate, B.; Yanjanin, N.; Dail, M.K.; Davidson, C.D.; Walkley, S.U.; Ory, D.S.; Wassif, C.A.; et al. Microarray expression analysis and identification of serum biomarkers for Niemann-Pick disease, type C1. Hum. Mol. Genet. 2012, 21, 3632–3646. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, A.M.; Paskevich, P.A.; Kominami, E.; Nixon, R.A. Lysosomal hydrolases of different classes are abnormally distributed in brains of patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 1991, 88, 10998–11002. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.M.; Vaules, W.A.; Coleman, P.D. Quantitative decrease in synaptophysin message expression and increase in cathepsin D message expression in Alzheimer disease neurons containing neurofibrillary tangles. J. Neuropathol. Exp. Neurol. 1999, 58, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Hemby, S.E.; Lee, V.M.; Eberwine, J.H.; Trojanowski, J.Q. Expression profile of transcripts in Alzheimer’s disease tangle-bearing CA1 neurons. Ann. Neurol. 2000, 48, 77–87. [Google Scholar] [CrossRef]
- Vitner, E.B.; Dekel, H.; Zigdon, H.; Shachar, T.; Farfel-Becker, T.; Eilam, R.; Karlsson, S.; Futerman, A.H. Altered expression and distribution of cathepsins in neuronopathic forms of Gaucher disease and in other sphingolipidoses. Hum. Mol. Genet. 2010, 19, 3583–3590. [Google Scholar] [CrossRef] [PubMed]
- Amritraj, A.; Wang, Y.; Revett, T.J.; Vergote, D.; Westaway, D.; Kar, S. Role of Cathepsin D in U18666A-induced neuronal cell death: Potential implication in Niemann-Pick type C disease pathogenesis. J. Biol. Chem. 2012, 288, 3136–3152. [Google Scholar] [CrossRef] [PubMed]
- Hovakimyan, M.; Maass, F.; Petersen, J.; Holzmann, C.; Witt, M.; Lukas, J.; Frech, M.J.; Hübner, R.; Rolfs, A.; Wree, A. Combined therapy with cyclodextrin/allopregnanolone and miglustat improves motor but not cognitive functions in Niemann-Pick type C1 mice. Neuroscience 2013, 252, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Hovakimyan, M.; Petersen, J.; Maass, F.; Reichard, M.; Witt, M.; Lukas, J.; Stachs, O.; Guthoff, R.; Rolfs, A.; Wree, A. Corneal alterations during combined therapy with cyclodextrin/allopregnanolone and miglustat in a knock-out mouse model of npc1 disease. PLoS ONE 2011, 6, e28418. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamada, Y.; Ishitsuka, Y.; Matsuo, M.; Shiraishi, K.; Wada, K.; Uchio, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; et al. Efficacy of 2-hydroxypropyl-beta-cyclodextrin in Niemann-Pick disease type C model mice and its pharmacokinetic analysis in a patient with the disease. Biol. Pharm. Bull. 2015, 38, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Maarup, T.J.; Chen, A.H.; Porter, F.D.; Farhat, N.Y.; Ory, D.S.; Sidhu, R.; Jiang, X.; Dickson, P.I. Intrathecal 2-hydroxypropyl-beta-cyclodextrin in a single patient with Niemann-Pick C1. Mol. Genet. Metab. 2015, 116, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Togawa, M.; Hirabaru, K.; Mochinaga, S.; Narita, A.; Adachi, M.; Egashira, M.; Irie, T.; Ohno, K. Effects of cyclodextrin in two patients with Niemann-Pick type C disease. Mol. Genet. Metab. 2013, 108, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Crumling, M.A.; Liu, L.; Thomas, P.V.; Benson, J.; Kanicki, A.; Kabara, L.; Halsey, K.; Dolan, D.; Duncan, R.K. Hearing loss and hair cell death in mice given the cholesterol-chelating agent hydroxypropyl-beta-cyclodextrin. PLoS ONE 2012, 7, e53280. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.; O’Donnell, P.; Fernandez, S.; Vite, C.H. 2-hydroxypropyl-beta-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr. Res. 2010, 68, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Vite, C.H.; Bagel, J.H.; Swain, G.P.; Prociuk, M.; Sikora, T.U.; Stein, V.M.; O’Donnell, P.; Ruane, T.; Ward, S.; Crooks, A.; et al. Intracisternal cyclodextrin prevents cerebellar dysfunction and purkinje cell death in feline Niemann-Pick type C1 disease. Sci. Transl. Med. 2015, 7, 276ra226. [Google Scholar] [CrossRef] [PubMed]
- Megias-Vericat, J.E.; Garcia-Robles, A.; Company-Albir, M.J.; Fernandez-Megia, M.J.; Perez-Miralles, F.C.; Lopez-Briz, E.; Casanova, B.; Poveda, J.L. Early experience with compassionate use of 2 hydroxypropyl-beta-cyclodextrin for Niemann-Pick type C disease: Review of initial published cases. Neurol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Farbman, A.I. Olfactory neurogenesis: Genetic or environmental controls? Trends Neurosci. 1990, 13, 362–365. [Google Scholar] [CrossRef]
- Hinds, J.W.; Hinds, P.L.; McNelly, N.A. An autoradiographic study of the mouse olfactory epithelium: Evidence for long-lived receptors. Anat. Rec. 1984, 210, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Lipinski, M.; Degterev, A. Diversity in the mechanisms of neuronal cell death. Neuron 2003, 40, 401–413. [Google Scholar] [CrossRef]
- Schlegel, V.; Thieme, M.; Holzmann, C.; Witt, M.; Grittner, U.; Rolfs, A.; Wree, A. Pharmacologic treatment assigned for Niemann Pick type C1 disease partly changes behavioral traits in wild-type mice. Int. J. Mol. Sci. 2016, 17, 1866. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.D.; Fishman, Y.I.; Puskas, I.; Szeman, J.; Sohajda, T.; McCauliff, L.A.; Sikora, J.; Storch, J.; Vanier, M.T.; Szente, L.; et al. Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann. Clin. Transl. Neurol. 2016, 3, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Hipler, U.C.; Schonfelder, U.; Hipler, C.; Elsner, P. Influence of cyclodextrins on the proliferation of HaCaT keratinocytes in vitro. J. Biomed. Mater. Res. A 2007, 83, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, M.; Kubota, Y.; Motoyama, K.; Higashi, T.; Taniyoshi, M.; Tokumaru, H.; Nishiyama, R.; Tabe, Y.; Mochinaga, S.; Sato, A.; et al. 2-hydroxypropyl-beta-cyclodextrin acts as a novel anticancer agent. PLoS ONE 2015, 10, e0141946. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Johnston, P.B.; Ball, B.G.; Brinton, R.D. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J. Neurosci. 2005, 25, 4706–4718. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, S.; Mellon, S.H.; Butters, T.D.; Nevyjel, M.; Covey, D.F.; Bembi, B.; Dardis, A. Oxidative stress in Npc1 deficient cells: Protective effect of allopregnanolone. J. Cell. Mol. Med. 2009, 13, 3786–3796. [Google Scholar] [CrossRef] [PubMed]
- Dover, R.; Patel, K. Improved methodology for detecting bromodeoxyuridine in cultured cells and tissue sections by immunocytochemistry. Histochemistry 1994, 102, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Onda, K.; Davis, R.L.; Shibuya, M.; Wilson, C.B.; Hoshino, T. Correlation between the bromodeoxyuridine labeling index and the Mib-1 and Ki-67 proliferating cell indices in cerebral gliomas. Cancer 1994, 74, 1921–1926. [Google Scholar] [CrossRef]




| Treatment Group | BrdU (Cells/mm3 ± SEM) | OMP (Cells/mm3 ± SEM) | Cas-3 (Cells/mm3 ± SEM) |
|---|---|---|---|
| untreated NPC1+/+ | 17,417 ± 1317 | 836,392 ± 63,784 | 3999 ± 596 |
| untreated NPC1−/− | 25,180 ± 1605 | 346,129 ± 37,812 | 9879 ± 1373 |
| COMBI NPC1+/+ | 67,972 ± 7694 | 844,819 ± 65,313 | 3327 ± 387 |
| COMBI NPC1−/− | 44,693 ± 4191 | 709,729 ± 69,558 | 5471 ± 397 |
| HPΒCD NPC1+/+ | 75,871 ± 1865 | 797,470 ± 32,568 | 4316 ± 215 |
| HPΒCD NPC1−/− | 70,558 ± 8159 | 584,268 ± 21,134 | 7016 ± 591 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, A.; Wree, A.; Günther, R.; Holzmann, C.; Schmitt, O.; Rolfs, A.; Witt, M. Increased Regenerative Capacity of the Olfactory Epithelium in Niemann–Pick Disease Type C1. Int. J. Mol. Sci. 2017, 18, 777. https://doi.org/10.3390/ijms18040777
Meyer A, Wree A, Günther R, Holzmann C, Schmitt O, Rolfs A, Witt M. Increased Regenerative Capacity of the Olfactory Epithelium in Niemann–Pick Disease Type C1. International Journal of Molecular Sciences. 2017; 18(4):777. https://doi.org/10.3390/ijms18040777
Chicago/Turabian StyleMeyer, Anja, Andreas Wree, René Günther, Carsten Holzmann, Oliver Schmitt, Arndt Rolfs, and Martin Witt. 2017. "Increased Regenerative Capacity of the Olfactory Epithelium in Niemann–Pick Disease Type C1" International Journal of Molecular Sciences 18, no. 4: 777. https://doi.org/10.3390/ijms18040777







