Prp19 Arrests Cell Cycle via Cdc5L in Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Results
2.1. The Positive Correlation of Prp19 and Cdc5L in HCC
2.2. Prp19 Binds with Cdc5L and Modulates Cdc5L Expression in HCC Cells
2.3. Prp19 Modulates Cdc5L Expression Via Inhibiting mRNA Translation and Facilitating Lysosome-Mediated Degradation in HCC Cells
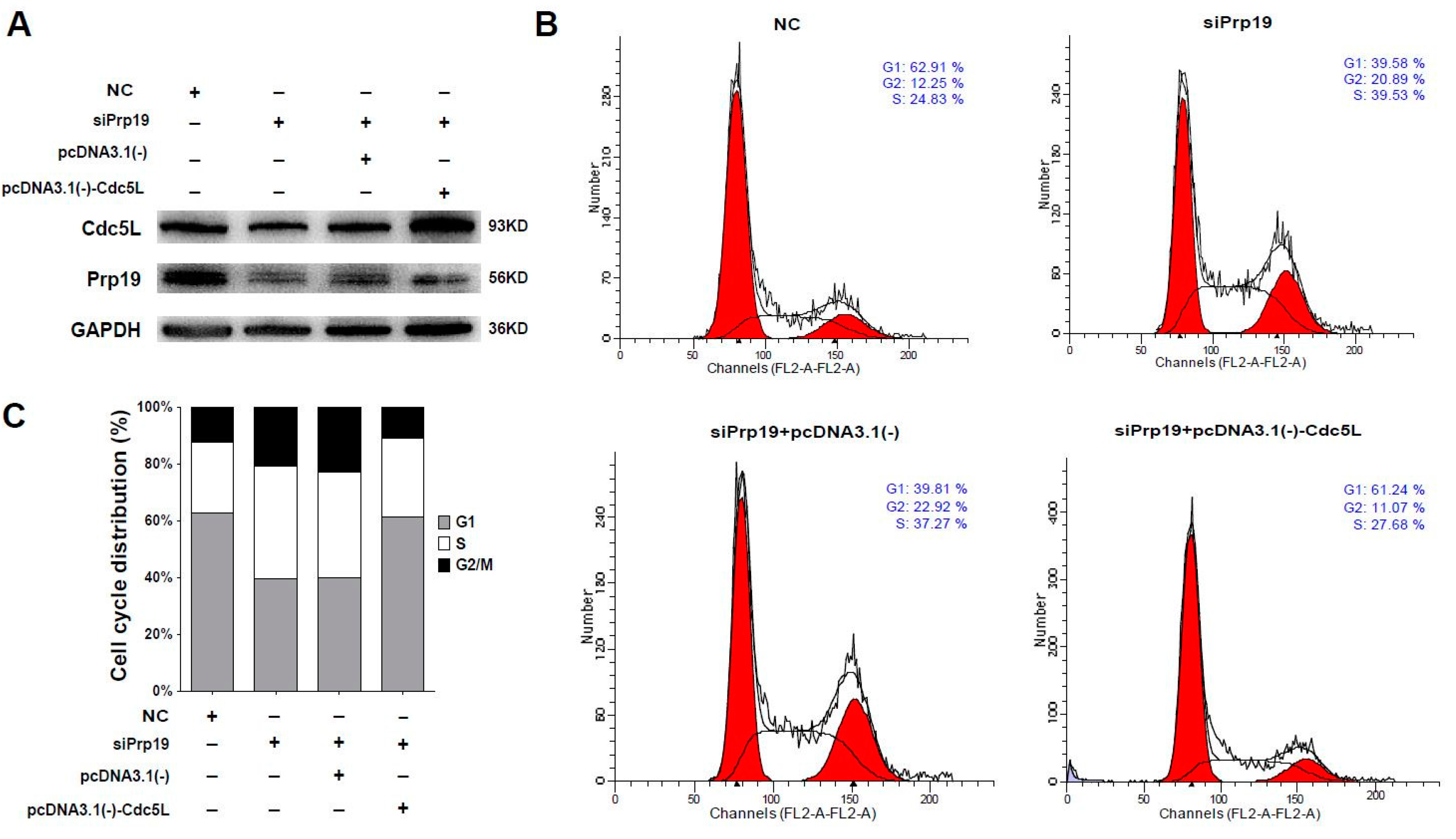
2.4. Prp19 Regulates Mitotic Progression via Cdc5L in HCC Cells
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Immunochemistry and Immunofluorescence
4.3. Cell Lines, Plasmids, siRNAs, Reagents and Chemical Agents
4.4. Real-Time PCR (qPCR)
4.5. Western Blot Assay
4.6. Immunoprecipitation Analysis
4.7. Dual Luciferase Analysis
4.8. Flow Cytometric Analysis
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Merle, P.; Trepo, C. Molecular mechanisms underlying hepatocellular carcinoma. Viruses 2009, 1, 852–872. [Google Scholar] [CrossRef] [PubMed]
- Manchado, E.; Guillamot, M.; Malumbres, M. Killing cells by targeting mitosis. Cell Death Differ. 2012, 19, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Chanarat, S.; Strasser, K. Splicing and beyond: The many faces of the Prp19 complex. Biochim. Biophys. Acta 2013, 1833, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, K. hPso4/hPrp19: A critical component of DNA repair and DNA damage checkpoint complexes. Oncogene 2015, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Huang, J. The PSO4 protein complex associates with replication protein A (RPA) and modulates the activation of ataxia telangiectasia-mutated and Rad3-related (ATR). J. Biol. Chem. 2014, 289, 6619–6626. [Google Scholar] [CrossRef] [PubMed]
- Jurica, M.S.; Moore, M.J. Pre-mRNA splicing: Awash in a sea of proteins. Mol. Cell 2003, 12, 5–14. [Google Scholar] [CrossRef]
- Ohi, M.D.; Kooi, C.W.V.; Rosenberg, J.A.; Ren, L.P.; Hirsch, J.P.; Chazin, W.J.; Walz, T.; Gould, K.L. Structural and functional analysis of essential pre-mRNA splicing factor Prp19p. Mol. Cell. Biol. 2005, 25, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ohi, M.D.; Vander Kooi, C.W.; Rosenberg, J.A.; Chazin, W.J.; Gould, K.L. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat. Struct. Biol. 2003, 10, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Vander Kooi, C.W.; Ohi, M.D.; Rosenberg, J.A.; Oldham, M.L.; Newcomer, M.E.; Gould, K.L.; Chazin, W.J. The Prp19 U-box crystal structure suggests a common dimeric architecture for a class of oligomeric E3 ubiquitin ligases. Biochemistry 2006, 45, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.C.; Will, C.L.; Luehrmann, R. The Spliceosome: Design Principles of a Dynamic RNP Machine. Cell 2009, 136, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Kaur, R.; Lu, X.; Shen, X.; Li, L.; Legerski, R.J. The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J. Biol. Chem. 2005, 280, 40559–40567. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, Y.A.; Liu, T.T.; Zhu, J.M.; Shen, X.Z. DNA damage induces down-regulation of Prp19 via impairing Prp19 stability in hepatocellular carcinoma cells. PLoS ONE 2014, 9, e89976. [Google Scholar] [CrossRef] [PubMed]
- Ajuh, P.; Lamond, A.I. Identification of peptide inhibitors of pre-mRNA splicing derived from the essential interaction domains of CDC5L and PLRG1. Nucleic Acids Res. 2003, 31, 6104–6116. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhang, X.; Ni, W.; Shi, W.; Fan, H.; Xu, J.; Chen, Y.; Ni, R.; Tao, T. Expression and Clinical Role of Cdc5L as a Novel Cell Cycle Protein in Hepatocellular Carcinoma. Dig. Dis. Sci. 2016, 61, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.S.; Coughlin, S.R. A mammalian homolog of fission yeast Cdc5 regulates G2 progression and mitotic entry. J. Biol. Chem. 1998, 273, 4666–4671. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, L.; Zhu, J.M.; Yu, Q.; Xue, R.Y.; Fang, Y.; Zhang, Y.A.; Chen, Y.J.; Liu, T.T.; Dong, L.; et al. Prp19 facilitates invasion of hepatocellular carcinoma via p38 mitogen-activated protein kinase/twist1 pathway. Oncotarget 2016, 7, 21939–21951. [Google Scholar] [PubMed]
- De Benedetti, A.; Graff, J.R. eIF-4E expression and its role in malignancies and metastases. Oncogene 2004, 23, 3189–3199. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Wang, Y.B.; Wu, M.; Yang, Y.; Song, W.; Li, T.; Zhang, W.N.; Tan, B.; Li, A.L.; Wang, N.; et al. Depletion of pre-mRNA splicing factor Cdc5L inhibits mitotic progression and triggers mitotic catastrophe. Cell Death Dis. 2014, 5, e1151. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhu, J.M.; Shen, X.Z. New insights into pre-mRNA processing factor 19: A multi-faceted protein in humans. Biol. Cell 2012, 104, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Gotzmann, J.; Gerner, C.; Meissner, M.; Holzmann, K.; Grimm, R.; Mikulits, W.; Sauermann, G. hNMP 200: A novel human common nuclear matrix protein combining structural and regulatory functions. Exp. Cell Res. 2000, 261, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, J.; Zhu, Y. The JNK Signaling pathway is a novel molecular target for S-propargyl-l-cysteine, a naturally-occurring garlic derivatives: Link to its anticancer activity in pancreatic cancer in vitro and in vivo. Curr. Cancer Drug Targets 2015, 15, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Hong, J.Y.; Bae, S.Y.; Kang, S.S.; Park, H.J.; Lee, S.K. Antitumor activity of americanin a isolated from the seeds of phytolacca americana by regulating the ATM/ATR signaling pathway and the Skp2-p27 axis in human colon cancer cells. J. Nat. Prod. 2015, 78, 2983–2993. [Google Scholar] [CrossRef] [PubMed]
- Chelsky, Z.L.; Yue, P.; Kondratyuk, T.P.; Paladino, D.; Pezzuto, J.M.; Cushman, M.; Turkson, J. A resveratrol analogue promotes ERKMAPK-dependent STAT3 serine and tyrosine phosphorylation alterations and antitumor effects in vitro against human tumor cells. Mol. Pharmacol. 2015, 88, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Sun, L.; Hao, Y.; Wang, L.; Xu, J.; Zhang, W.; Xie, J.; Guo, L.; Zhou, L.; Yun, X.; et al. Ribosomal RACK1 promotes chemoresistance and growth in human hepatocellular carcinoma. J. Clin. Investig. 2012, 122, 2554–2566. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Xue, R.; Qu, D.; Yin, J.; Shen, X.-Z. Prp19 Arrests Cell Cycle via Cdc5L in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2017, 18, 778. https://doi.org/10.3390/ijms18040778
Huang R, Xue R, Qu D, Yin J, Shen X-Z. Prp19 Arrests Cell Cycle via Cdc5L in Hepatocellular Carcinoma Cells. International Journal of Molecular Sciences. 2017; 18(4):778. https://doi.org/10.3390/ijms18040778
Chicago/Turabian StyleHuang, Renzheng, Ruyi Xue, Di Qu, Jie Yin, and Xi-Zhong Shen. 2017. "Prp19 Arrests Cell Cycle via Cdc5L in Hepatocellular Carcinoma Cells" International Journal of Molecular Sciences 18, no. 4: 778. https://doi.org/10.3390/ijms18040778




