pH-Dependent Antimicrobial Properties of Copper Oxide Nanoparticles in Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. CuO Nanoparticles Inhibit Growth
2.2. CuO Nanoparticles Alter the Redox Status in S. aureus
2.3. Analysis of CuO Nanoparticle Fine Structure in S. aureus Cells
3. Discussion
4. Materials and Methods
4.1. CuO-NP Characterization
4.2. Assessment of CuO-NP Antibacterial Effects
4.3. RedoxSensor Measurement
4.4. XANES and EXAFS Analyses
4.5. Measurement of Soluble Copper
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schlievert, P.M.; Shands, K.N.; Dan, B.B.; Schmid, G.P.; Nishimura, R.D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J. Infect. Dis. 1981, 143, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Concia, E.; Cristini, F.; de Rosa, F.G.; Esposito, S.; Menichetti, F.; Petrosillo, N.; Tumbarello, M.; Venditti, M.; Viale, P.; et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin. Microbiol. Infect. 2016, 22, 27–36. [Google Scholar] [CrossRef]
- Rello, J.; Nieto, M.; Solé-Violán, J.; Wan, Y.; Gao, X.; Solem, C.T.; de Salas-Cansado, M.; Mesa, F.; Charbonneau, C.; Chastre, J. Nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus treated with linezolid or vancomycin: A secondary economic analysis of resource use from a Spanish perspective. Medicina Intensiva/Sociedad Espanola de Medicina Intensiva y Unidades Coronarias 2016, 40, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Foschi, F. Does gender affect the outcome of community-acquired Staphylococcus aureus bacteraemia? Clin. Microbiol. Infect. 2017, 23, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Ward, P.B.; Charles, P.G.; Korman, T.M.; Fuller, A.; du Cros, P.; Grabsch, E.A.; Roberts, S.A.; Robson, J.; Read, K.; et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 2004, 38, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Razzak, M.S.A.; Al-Charrakh, A.H.; AL-Greitty, B.H. Relationship between Lactobacilli and opportunistic bacterial pathogens associated with vaginitis. N. Am. J. Med. Sci. 2011, 3, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Schlievert, P.M.; Osterholm, M.T.; Kelly, J.A.; Nishimura, R.D. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann. Intern. Med. 1982, 96, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Hansmann, M.A.; Delaney, M.L.; Modern, P.A.; Dubois, A.M.; Wieland-Alter, W.; Wissemann, K.W.; Wild, J.E.; Jones, M.B.; Seymour, J.L.; et al. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J. Clin. Microbiol. 2005, 43, 4628–4634. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Sarkar, R.K.; Chattopadhyay, A.P.; Aich, P.; Chakraborty, R.; Basu, T. A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency against E. coli. Nanotechnology 2012, 23, 085103. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Park, J.-E.; Osaka, T.; Park, S.-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Usman, M. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar]
- Borkow, G.; Gabbay, J. Copper, an ancient remedy returning to fight microbial, fungal and viral infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.; Karuppiah, P.; Al-Dhabi, N.A. Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J. Nanomater. 2014, 2014, 17. [Google Scholar] [CrossRef]
- Ren, G.; Hu, D.; Cheng, E.W.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B Biointerfaces 2013, 101, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Von Dem Bussche, A.; Kabadi, P.K.; Kane, A.B.; Hurt, R.H. Biological and environmental transformations of copper-based nanomaterials. ACS Nano 2013, 7, 8715–8727. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Albalghiti, E.M.; Fishman, Z.S.; Perreault, F.; Corredor, C.; Posner, J.D.; Elimelech, M.; Pfefferle, L.D.; Zimmerman, J.B. Shape-dependent surface reactivity and antimicrobial activity of nano-cupric oxide. Environ. Sci. Technol. 2016, 50, 3975–3984. [Google Scholar] [CrossRef] [PubMed]
- Kaweeteerawat, C.; Chang, C.H.; Roy, K.R.; Liu, R.; Li, R.; Toso, D.; Fischer, H.; Ivask, A.; Ji, Z.; Zink, J.I.; et al. Cu nanoparticles have different impacts in Escherichia coli and Lactobacillus brevis than their microsized and ionic analogues. ACS Nano 2015, 9, 7215–7225. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and-negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kahru, A. Sub-toxic effects of CuO nanoparticles on bacteria: Kinetics, role of Cu ions and possible mechanisms of action. Environ. Pollut. 2012, 169, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.J.; Choi, J.W.; Lee, S.H.; Hong, S.W. Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: The importance of their dissolved fraction varying with preparation methods. J. Hazard. Mater. 2012, 227, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.N.; Yao, Y.; Chen, J. Evolution of size, morphology, and magnetic properties of CuO nanoparticles by thermal annealing. J. Appl. Phys. 2009, 105, 093901. [Google Scholar]
- Zhao, B.; Liu, P.; Zhuang, H.; Jiao, Z.; Fang, T.; Xu, W.; Lu, B.; Jiang, Y. Hierarchical self-assembly of microscale leaf-like CuO on graphene sheets for high-performance electrochemical capacitors. J. Mater. Chem. A. 2013, 1, 367–373. [Google Scholar] [CrossRef]
- Wan, L.; Cui, X.; Chen, H.; Shi, J. Synthesis of ordered mesoporous CuO/CeO2 composite via co-nanocasting replication method and its improved reactivity towards hydrogen. Mater. Lett. 2010, 64, 1379–1382. [Google Scholar] [CrossRef]
- Wu, R.; Ma, Z.; Gu, Z.; Yang, Y. Preparation and characterization of CuO nanoparticles with different morphology through a simple quick-precipitation method in DMAC-water mixed solvent. J. Alloy Compd. 2010, 504, 45–49. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S. Antimicrobial-resistant pathogens associated with healthcare-associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Skov, R.; Smyth, R.; Larsen, A.; Bolmstrôm, A.; Karlsson, A.; Mills, K.; Frimodt-Moller, N.; Kahlmeter, G. Phenotypic detection of methicillin resistance in Staphylococcus aureus by disk diffusion testing and Etest on Mueller-Hinton agar. J. Clin. Microbiol. 2006, 44, 4395–4399. [Google Scholar] [CrossRef] [PubMed]
- Juzwa, W.; Duber, A.; Myszka, K.; Białas, W.; Czaczyk, K. Identification of microbes from the surfaces of food-processing lines based on the flow cytometric evaluation of cellular metabolic activity combined with cell sorting. Biofouling 2016, 32, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Lytle, F.W. The EXAFS family tree: A personal history of the development of extended X-ray absorption fine structure. J. Synchrotron Radiat. 1999, 6, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA and ARTEMIS: Interactive graphical data analysis using IFEFFIT. Phys. Scr. 2005, 2005, 1007. [Google Scholar] [CrossRef]
- Ravel, B.; Hester, J.; Solé, V.; Newville, M. Towards data format standardization for X-ray absorption spectroscopy. J. Synchrotron Radiat. 2012, 19, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Cytotoxic origin of copper (II) oxide nanoparticles: Comparative studies with micron-sized particles, leachate, and metal salts. ACS Nano 2011, 5, 7214–7225. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef] [PubMed]



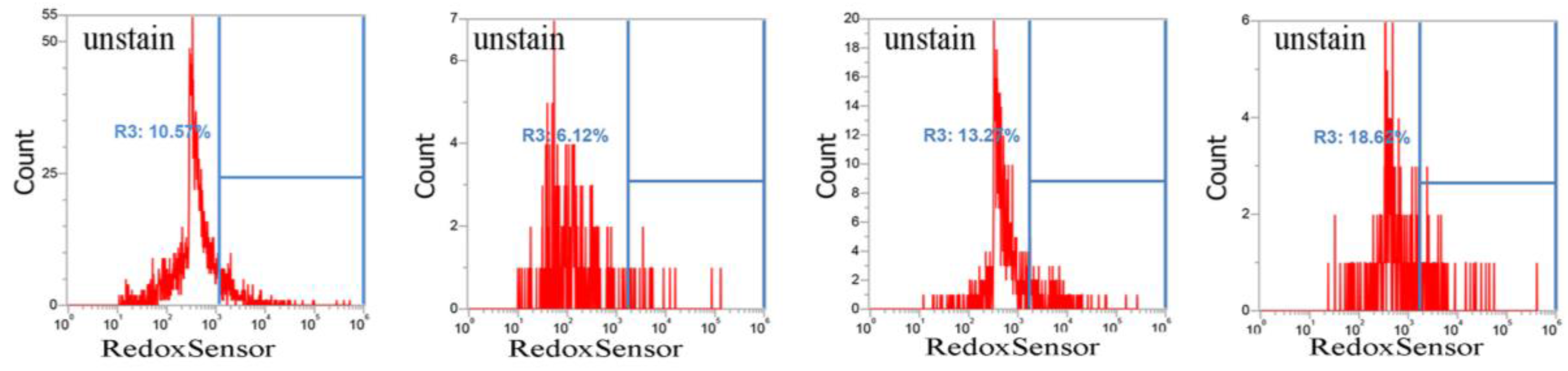
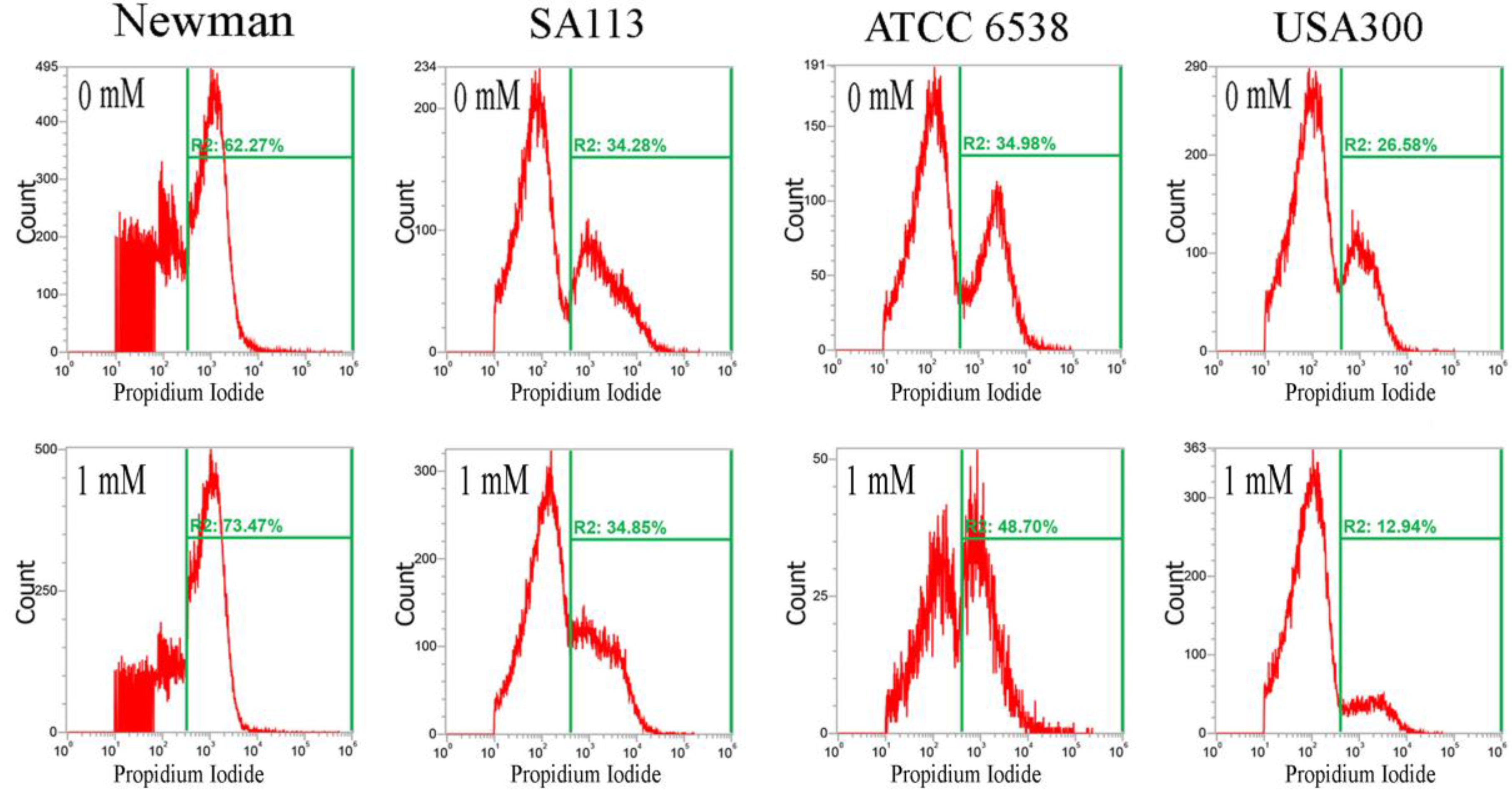
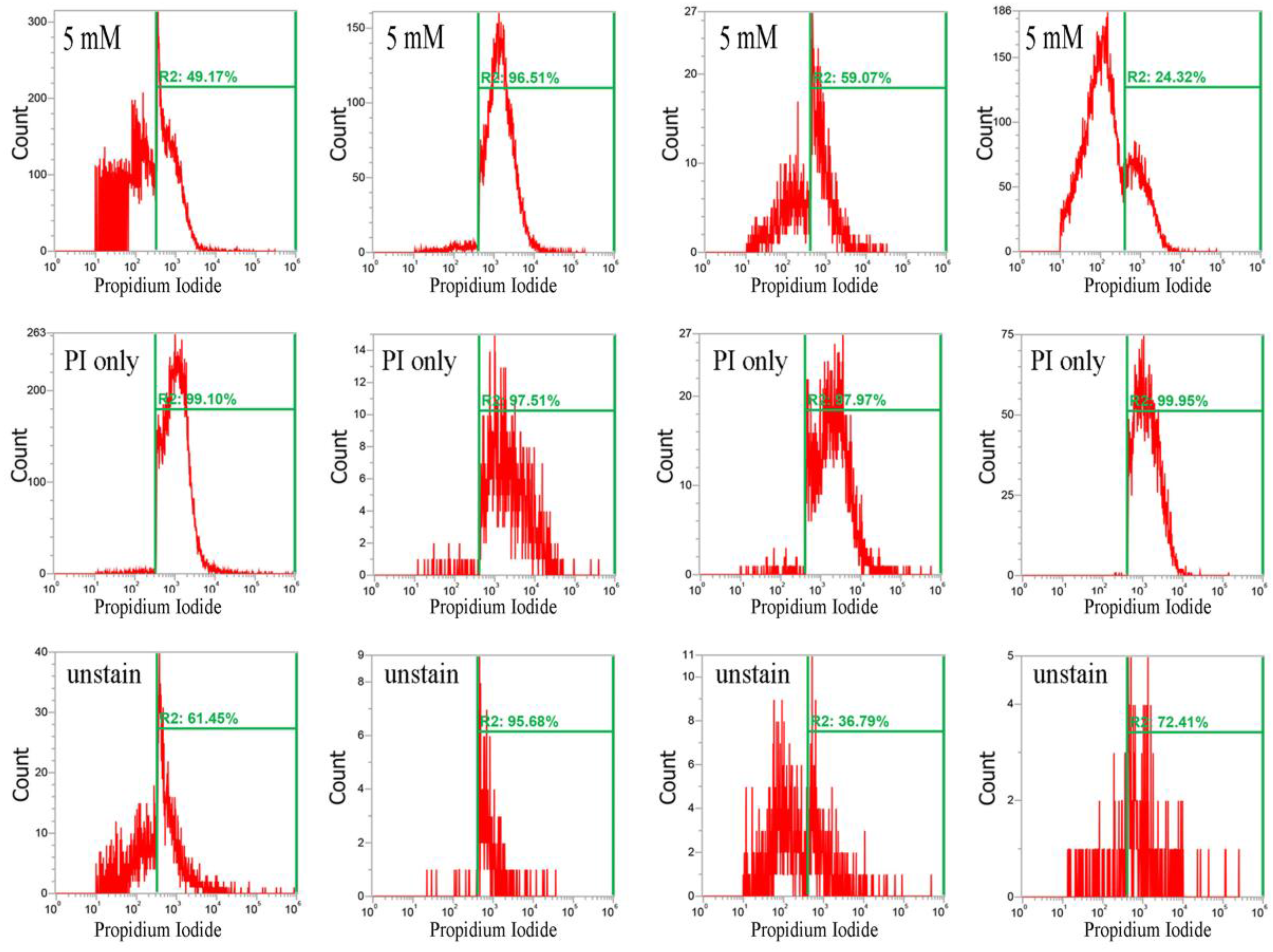
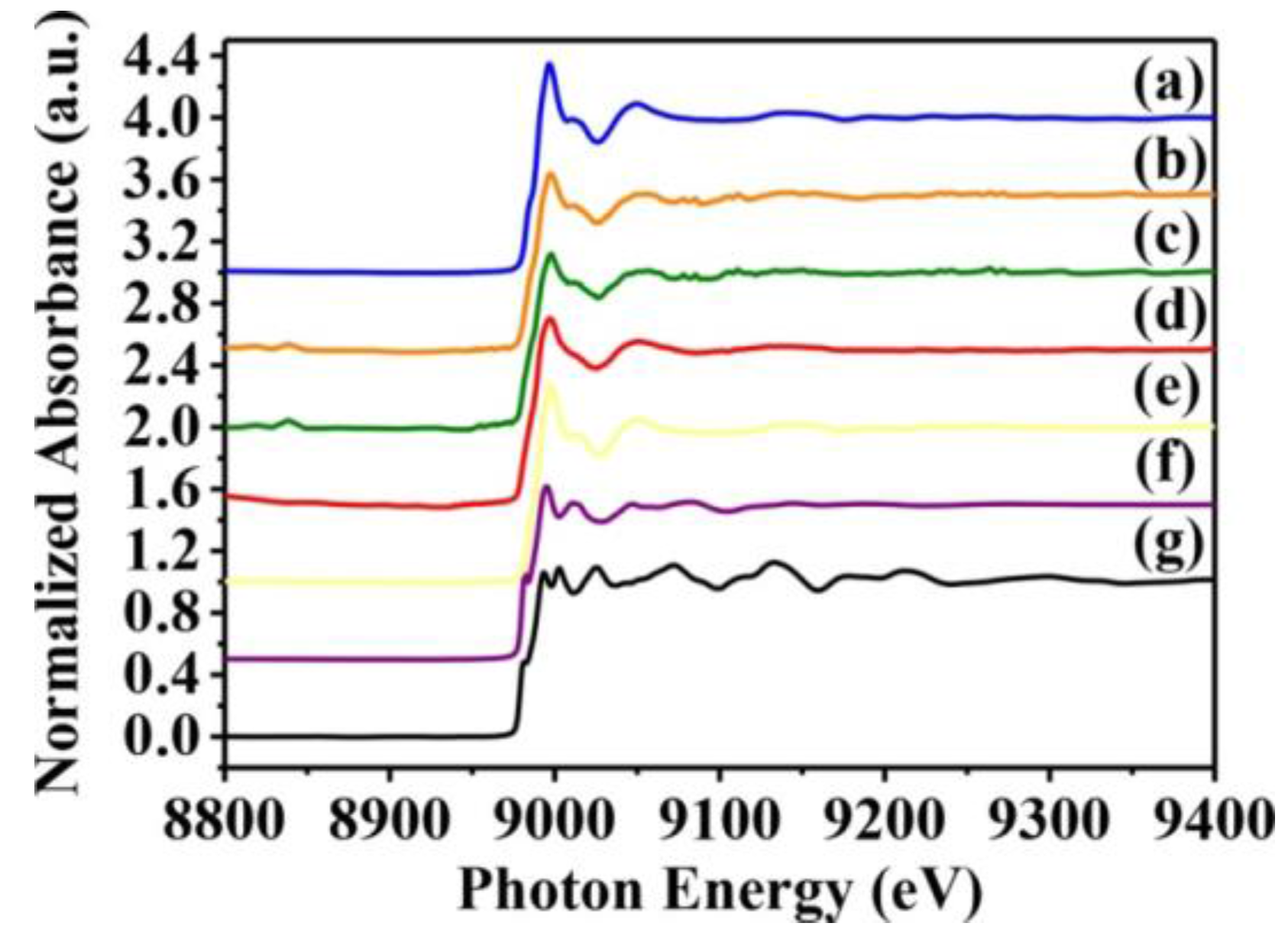
| Sample Name | pH = 5 | pH = 6 | pH = 7 |
|---|---|---|---|
| Average Concentration (mg/L) | 36.64 | 31.60 | 21.08 |
| Standard Deviation, SD (mg/L) | 0.20 | 0.04 | 0.16 |
| Average Concentration (mM) | 0.461 | 0.398 | 0.265 |
| Standard Deviation, SD (mM) | 2.5 × 10−4 | 5.0 × 10−7 | 2.0 × 10−3 |
| Sample | Shell (1st) | CN a (±0.05) | R (Å) b (±0.01 Å) | cΔ σ2(Å2) | R-Factor |
|---|---|---|---|---|---|
| Newman | Cu-O | 2.83 | 1.95 | 0.00183 | 0.00355 |
| Cu-S | 4.10 | 2.11 | 0.00983 | 0.05399 | |
| USA300 | Cu-O | 2.41 | 1.97 | 0.00659 | 0.00719 |
| Cu-S | 3.00 | 1.31 | 0.01731 | 0.30124 | |
| SA113 | Cu-O | 1.96 | 1.97 | 0.00065 | 0.03782 |
| Cu-S | 2.61 | 2.13 | 0.00725 | 0.04089 | |
| ATCC6538 | Cu-O | 1.74 | 1.97 | 0.0051 | 0.02559 |
| Cu-S | 2.34 | 2.13 | 0.00755 | 0.06644 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, Y.-H.; Tsai, P.-H.; Lin, K.-S. pH-Dependent Antimicrobial Properties of Copper Oxide Nanoparticles in Staphylococcus aureus. Int. J. Mol. Sci. 2017, 18, 793. https://doi.org/10.3390/ijms18040793
Hsueh Y-H, Tsai P-H, Lin K-S. pH-Dependent Antimicrobial Properties of Copper Oxide Nanoparticles in Staphylococcus aureus. International Journal of Molecular Sciences. 2017; 18(4):793. https://doi.org/10.3390/ijms18040793
Chicago/Turabian StyleHsueh, Yi-Huang, Ping-Han Tsai, and Kuen-Song Lin. 2017. "pH-Dependent Antimicrobial Properties of Copper Oxide Nanoparticles in Staphylococcus aureus" International Journal of Molecular Sciences 18, no. 4: 793. https://doi.org/10.3390/ijms18040793





