Down-Regulated Drebrin Aggravates Cognitive Impairments in a Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
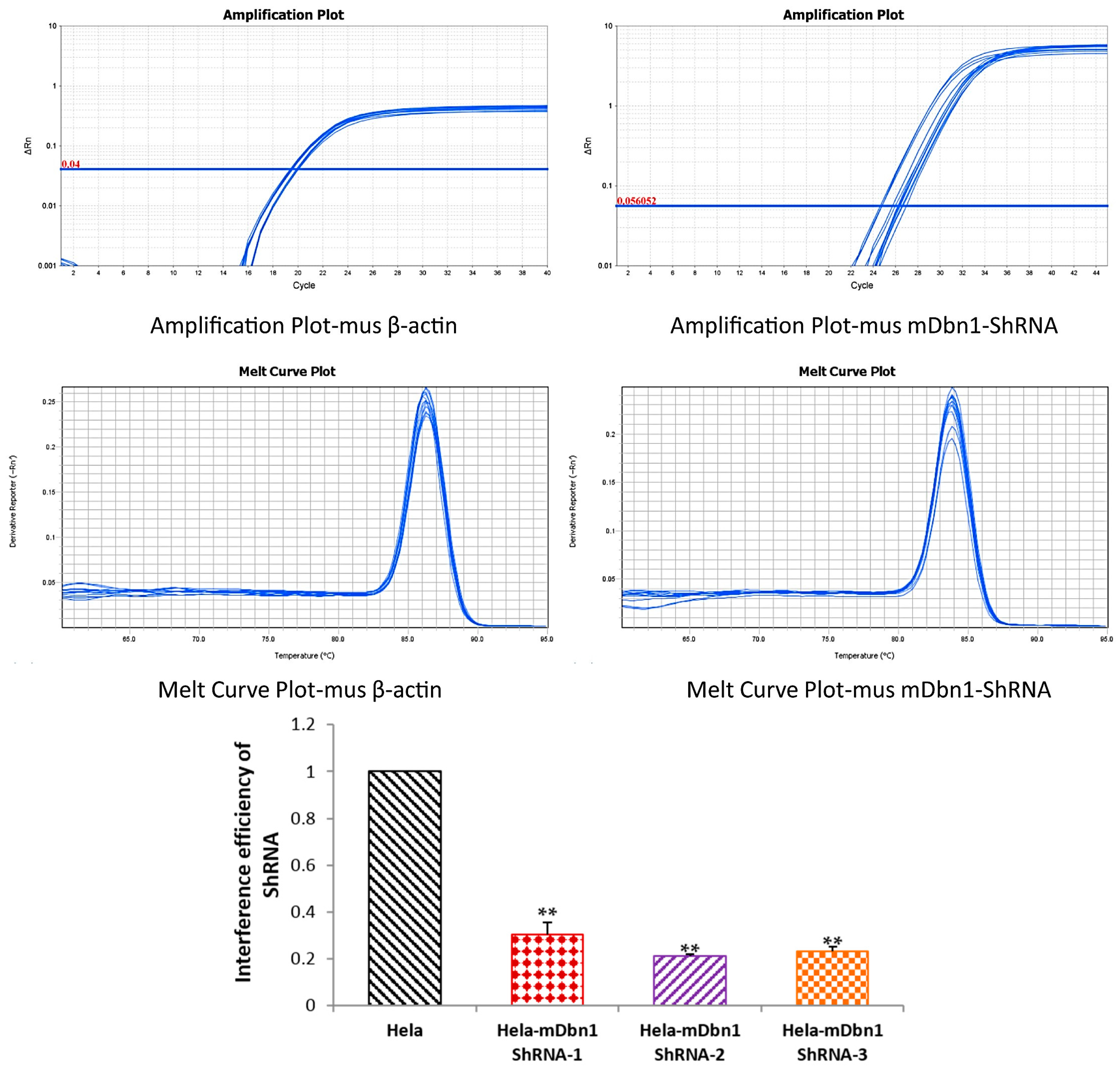
2.1. RAAV9-mDbn1 ShRNA Vector Produced and Injected into the Hippocampus of AD Mice
2.2. Cognition Changes in Mice
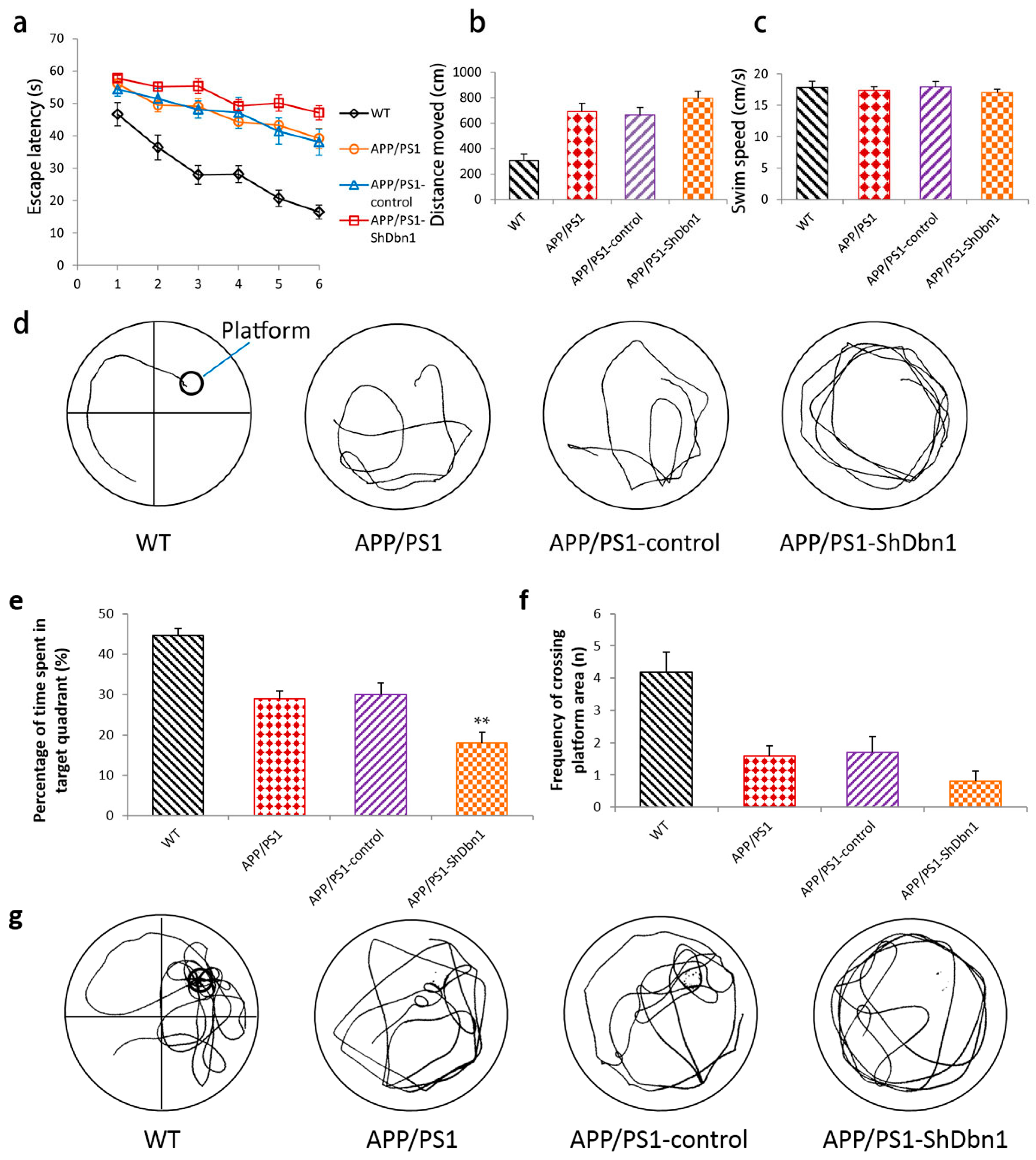
2.2.1. Morris Water Maze Test
2.2.2. Open Field Test
2.2.3. Novel Object Test
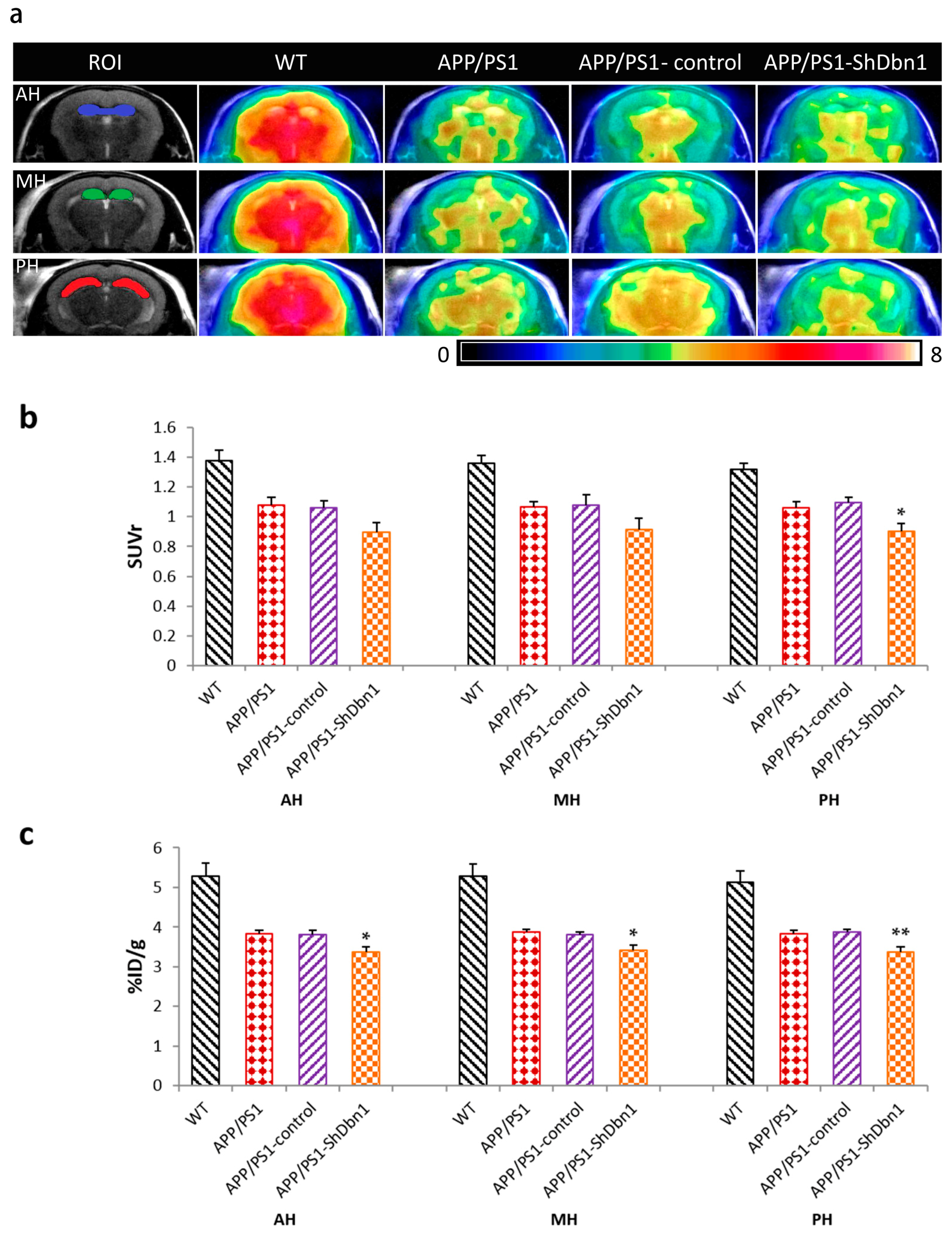
2.3. Glucose Metabolism Changes in Mice
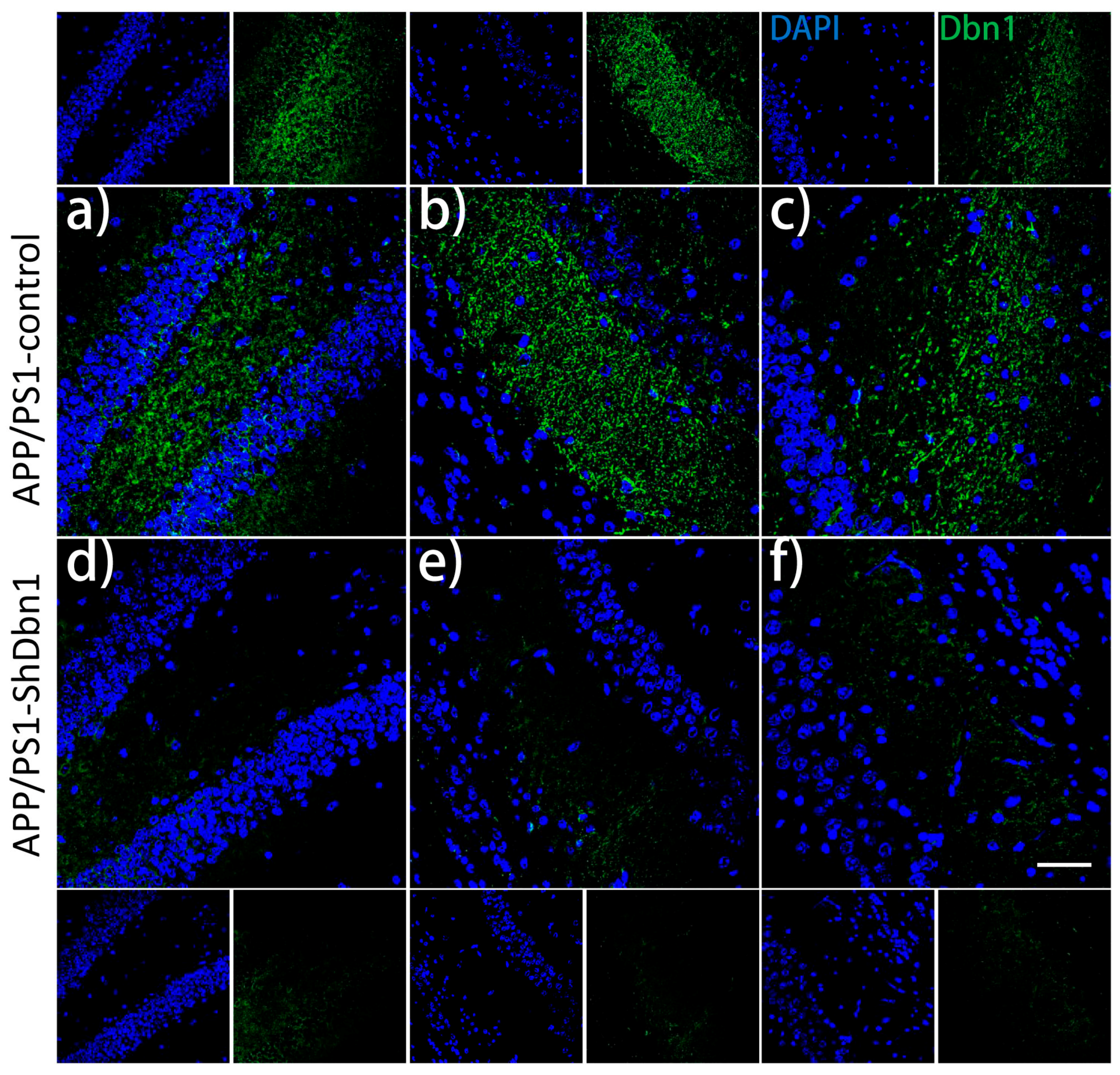
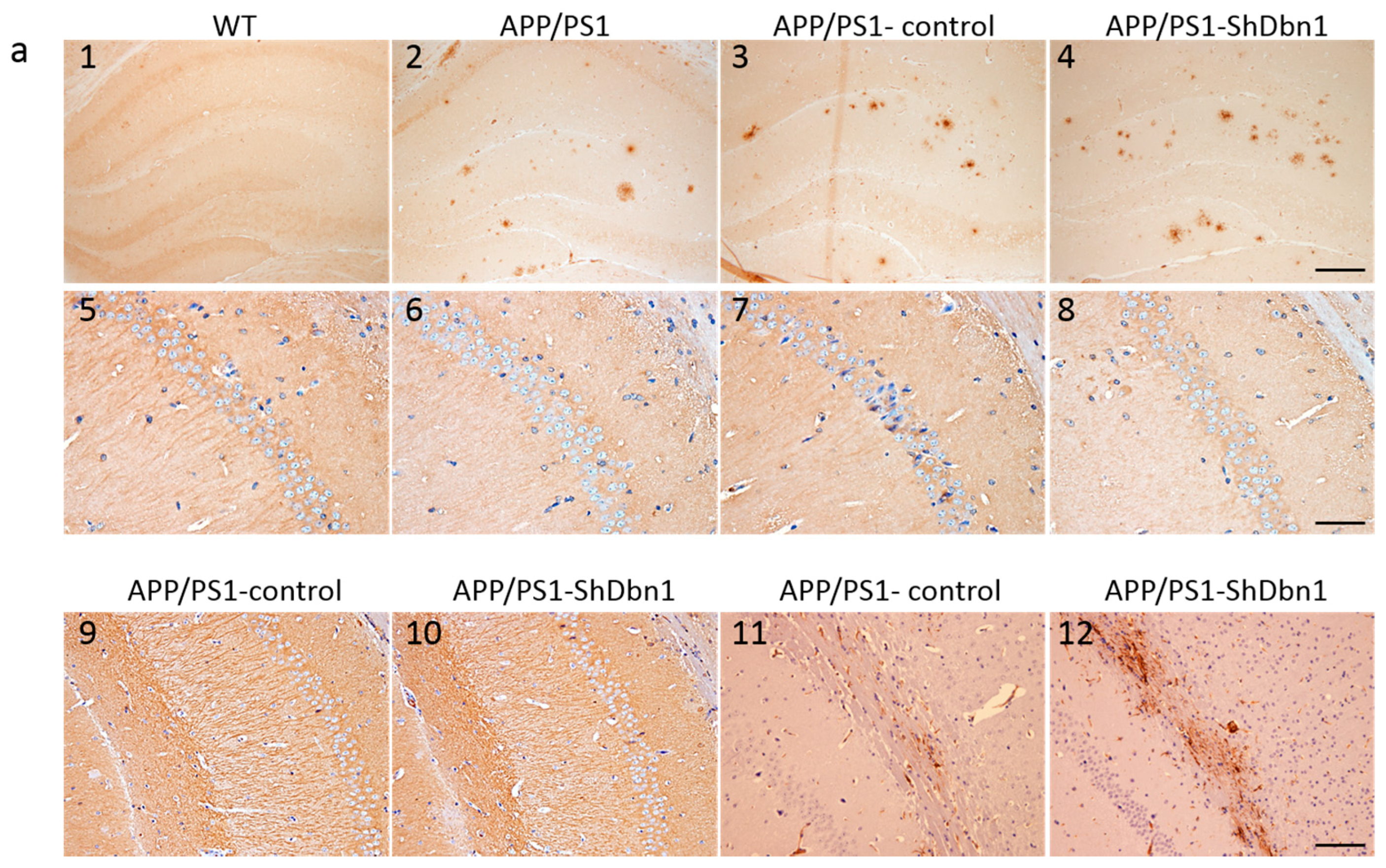
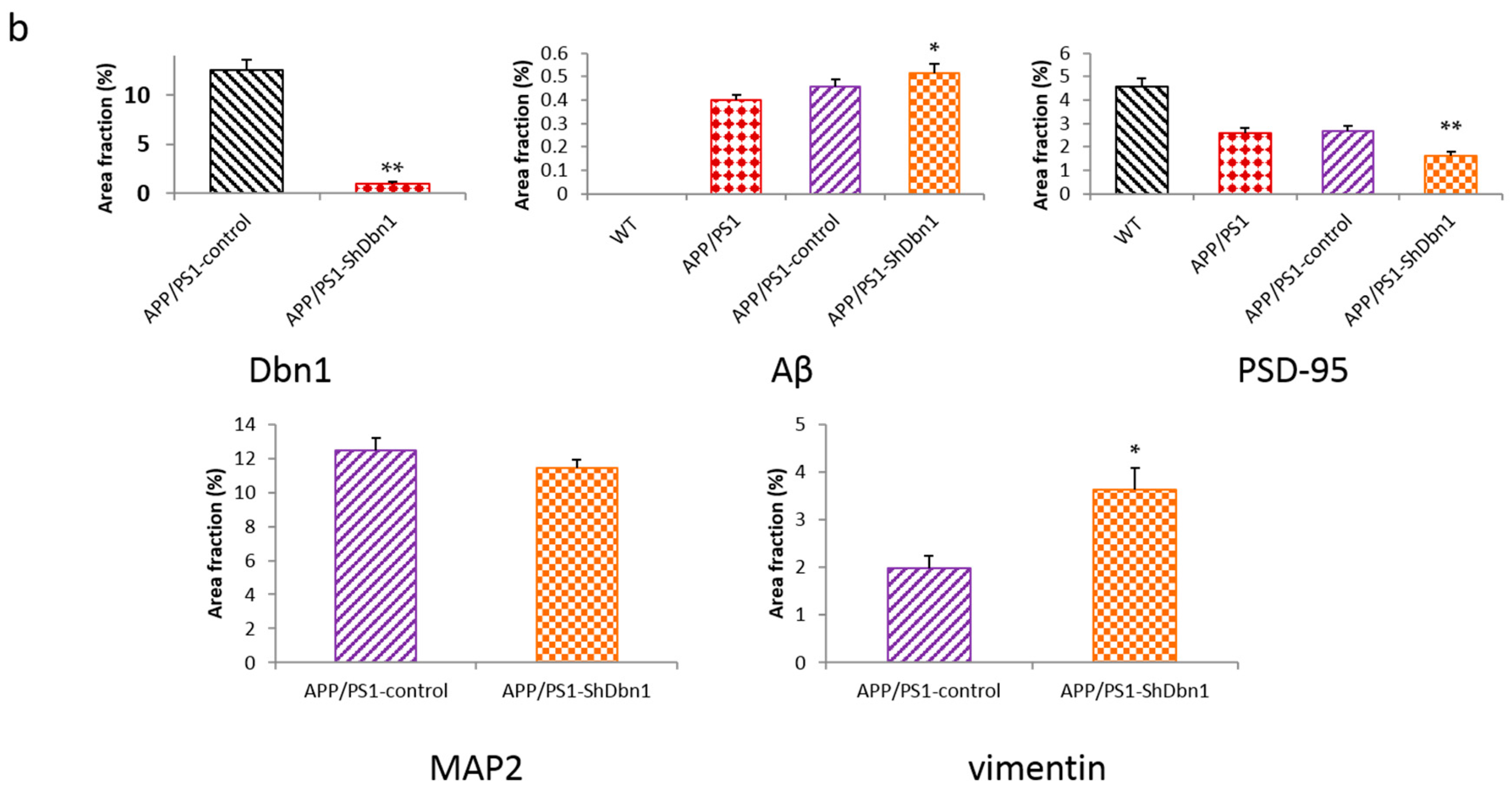
2.4. Dbn Protein Significantly Decreased in RAAV9-ShDbn1 Vector Injection Area of AD Mice
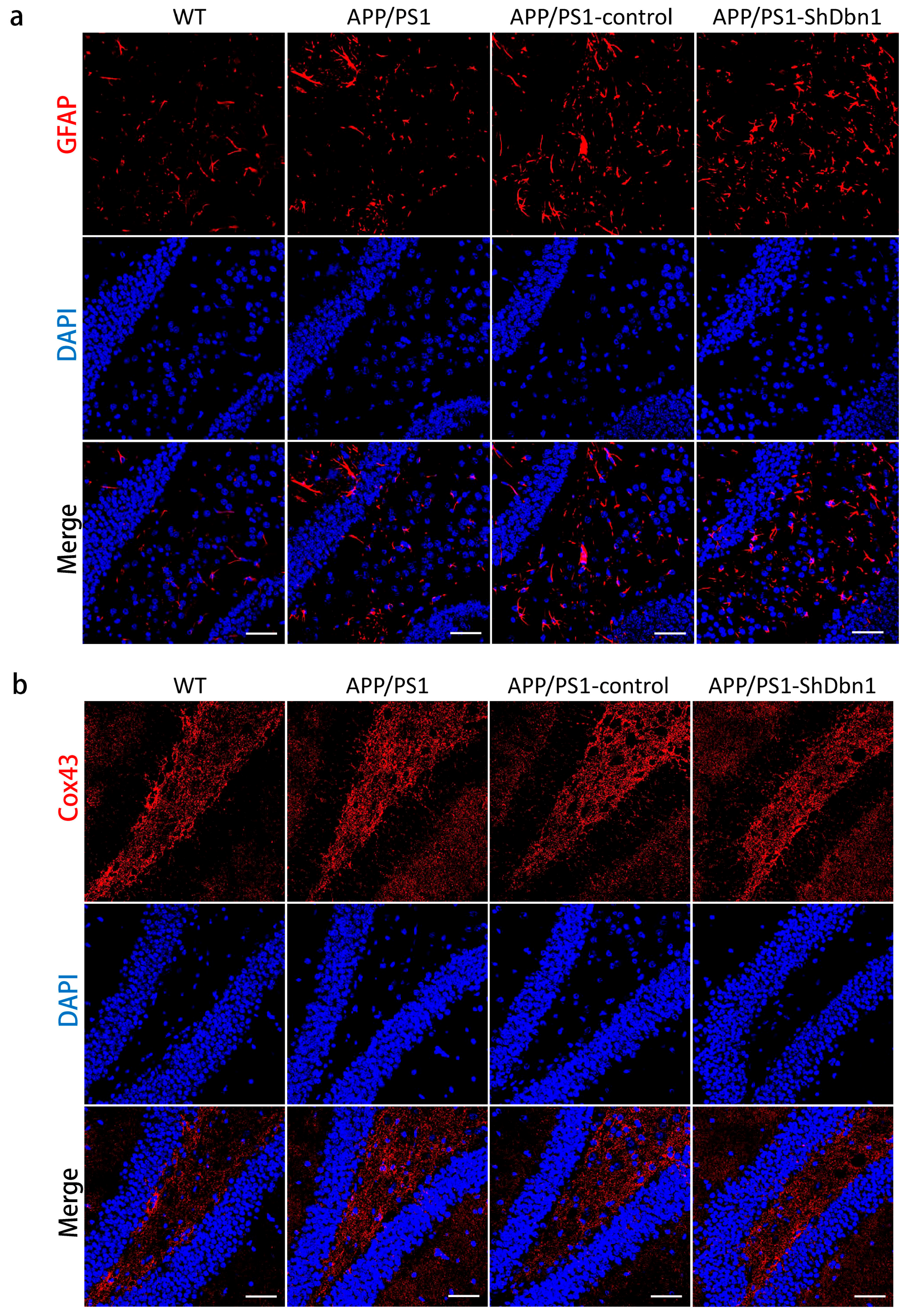
2.5. Aβ and GFAP Increased Within a Small Scale in Dbn1 Down-Regulated AD Mice
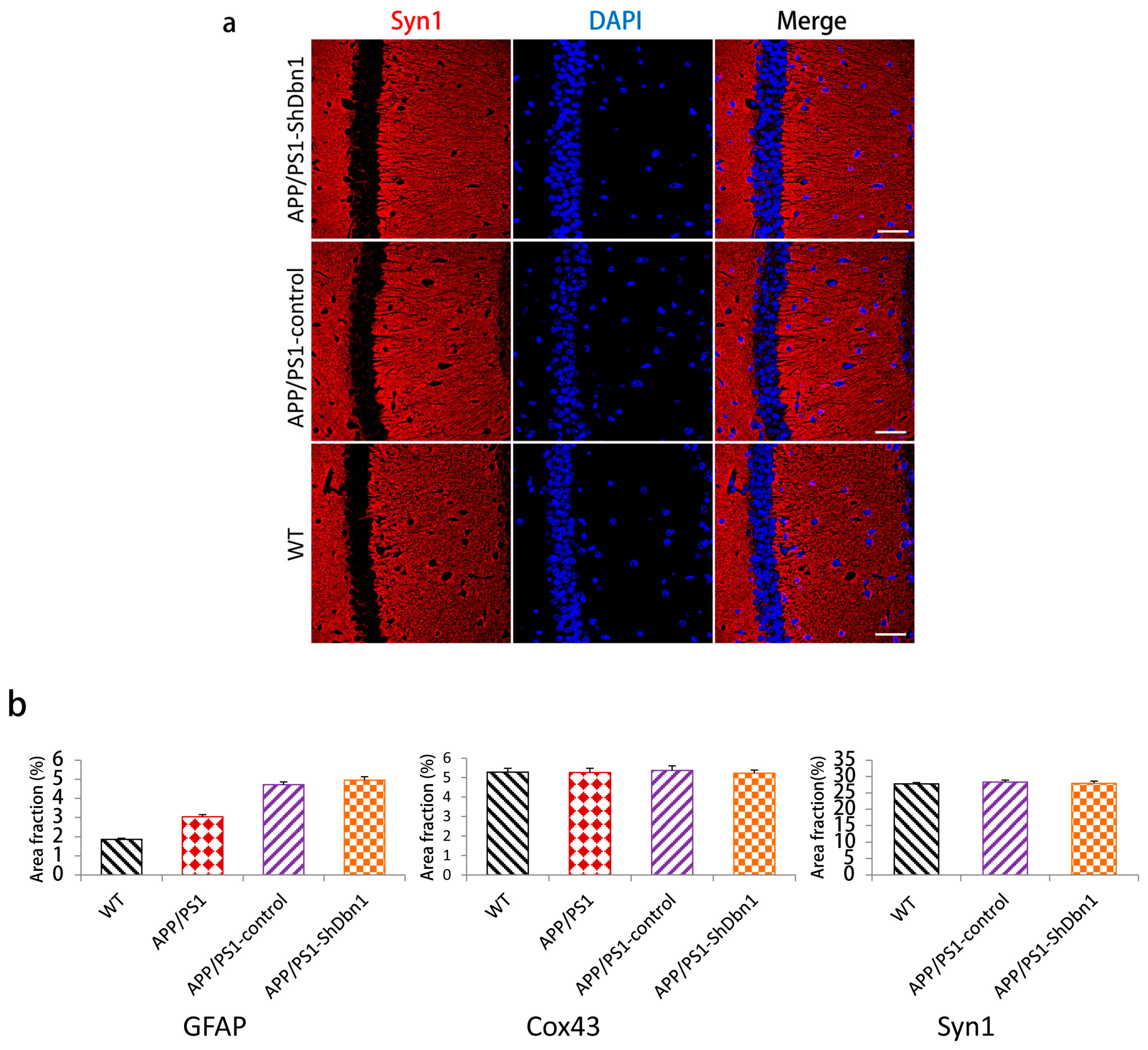
2.6. Expression Level of Relative Protein in Mice Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RAAV9-mDbn1 ShRNA Vector Production and Purification
4.3. Intrahippocampal Injection
4.4. Behavioural Tests
4.4.1. MWM Test
4.4.2. Open Field Test
4.4.3. Novel Object Test
4.5. Glucose Metabolism Detection
4.6. Immunohistochemical Staining
4.7. Immunofluorescence Staining
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Drebrin | Developmentally regulated brain protein |
| MWM | Morris water maze |
| ROI | Regions of interest |
| FDG | Fluorodeoxyglucose |
| %ID/g | Percentage injected dose per gram of 18F-FDG |
| SUV | Standardized uptake value |
| PSD-95 | Postsynaptic density-95 |
| Cox43 | Connexin43 |
| Syn1 | Synapsin1 |
| ANOVA | Analysis of variance |
References
- Giese, K.P.; Aziz, W.; Kraev, I.; Stewart, M.G. Generation of multi-innervated dendritic spines as a novel mechanism of long-term memory formation. Neurobiol. Learn. Mem. 2015, 124, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Kim, E.J.; Cicvaric, A.; Sase, S.; Gröger, M.; Höger, H.; Sialana, F.J.; Berger, J.; Monje, F.J.; Lubec, G. Drebrin depletion alters neurotransmitter receptor levels in protein complexes, dendritic spine morphogenesis and memory-related synaptic plasticity in the mouse the hippocampus. J. Neurochem. 2015, 134, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Mizui, T.; Kojima, N.; Yamazaki, H.; Katayama, M.; Hanamura, K.; Shirao, T. Drebrin E is involved in the regulation of axonal growth through actin-myosin interactions. J. Neurochem. 2009, 109, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Mizui, T.; Sekino, Y.; Yamazaki, H.; Ishizuka, Y.; Takahashi, H.; Kojima, N.; Kojima, M.; Shirao, T. Myosin II ATPase activity mediates the long-term potentiation-induced exodus of stable F-actin bound by drebrin A from dendritic spines. PLoS ONE 2014, 9, e85367. [Google Scholar] [CrossRef] [PubMed]
- Pollak, D.D.; Scharl, T.; Leisch, F.; Herkner, K.; Villar, S.R.; Hoeger, H.; Lubec, G. Strain-dependent regulation of plasticity-related proteins in the mouse the hippocampus. Behav. Brain Res. 2005, 165, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, H.; Hiroi, N.; Funahashi, A. Mathematical modeling of sustainable synaptogenesis by repetitive stimuli suggests signaling mechanisms in vivo. PLoS ONE 2012, 7, e51000. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Yamazaki, H.; Inaguma, Y.; Asada, A.; Kimura, T.; Takahashi, J.; Taoka, M.; Ohshima, T.; Furuichi, T.; Isobe, T.; et al. Phosphorylation of drebrin by cyclin-dependent kinase 5 and its role in neuronal migration. PLoS ONE 2014, 9, e92291. [Google Scholar] [CrossRef] [PubMed]
- Shirao, T.; González-Billault, C. Actin filaments and microtubules in dendritic spines. J. Neurochem. 2013, 126, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J.; Kulijewicz-Nawrot, M.; Rodrigez-Arellano, J.J.; Jendelova, P.; Sykova, E. Mesenchymal Stem Cells Preserve Working Memory in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2016, 17, E152. [Google Scholar] [CrossRef] [PubMed]
- Counts, S.E.; He, B.; Nadeem, M.; Wuu, J.; Scheff, S.W.; Mufson, E.J. Hippocampal drebrin loss in mild cognitive impairment. Neurodegener. Dis. 2012, 10, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.S.; Rapoport, S.I.; Kim, H.W. Altered neuroinflammatory, arachidonic acid cascade and synaptic markers in postmortem Alzheimer’s disease brain. Transl. Psychiatry 2011, 1, e31. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.S.; Pan, X.D.; Zhang, J.; Shen, H.; Collins, N.C.; Cole, A.M.; Koster, K.P.; Ben Aissa, M.; Dai, X.M.; Zhou, M.; et al. APOE4 enhances age-dependent decline in cognitive function by down-regulating an NMDA receptor pathway in EFAD-Tg mice. Mol. Neurodegener. 2015, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Rusanescu, G.; Mao, J. Peripheral nerve injury induces adult brain neurogenesis and remodelling. J. Cell. Mol. Med. 2017, 21, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, L.J.; Wang, J.; Li, D.; Ren, W.J.; Peng, J.; Wei, X.; Xu, T.; Xin, W.J.; Pang, R.P.; et al. TNF-α Differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J. Neurosci. 2017, 37, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Ashok, B.S.; Ajith, T.A.; Sivanesan, S. Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin. Exp. Pharmacol. Physiol. 2017, 44, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Ginestroni, A.; Bessi, V.; Toschi, N.; Padiglioni, S.; Ciulli, S.; Tessa, C.; Giannelli, M.; Bracco, L.; Diciotti, S. Regional analysis of the magnetization transfer ratio of the brain in mild Alzheimer disease and amnestic mild cognitive impairment. AJNR Am. J. Neuroradiol. 2013, 34, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Nishida, Y.; Sagare, A.P.; Rege, S.V.; Bell, R.D.; Perlmutter, D.; Sengillo, J.D.; Hillman, S.; Kong, P.; Nelson, A.R.; et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015, 18, 521–530. [Google Scholar] [CrossRef] [PubMed]
- AbdAlla, S.; Langer, A.; Fu, X.; Quitterer, U. ACE inhibition with captopril retards the development of signs of neurodegeneration in an animal model of Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 16917–16942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Giusti-Rodríguez, P.; Zhou, Y.; Rudenko, A.; Cho, S.; Ota, K.T.; Park, C.; Patzke, H.; Madabhushi, R.; Pan, L.; et al. Activity-dependent p25 generation regulates synaptic plasticity and Aβ-induced cognitive impairment. Cell 2014, 157, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; Barres, B.A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 2013, 14, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Helfand, B.T.; Chou, Y.H.; Shumaker, D.K.; Goldman, R.D. Intermediate filament proteins participate in signal transduction. Trends Cell Biol. 2005, 15, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Helfand, B.T.; Mendez, M.G.; Murthy, S.N.; Shumaker, D.K.; Grin, B.; Mahammad, S.; Aebi, U.; Wedig, T.; Wu, Y.I.; Hahn, K.M.; Inagaki, M.; Herrmann, H.; Goldman, R.D. Vimentin organization modulates the formation of lamellipodia. Mol. Biol. Cell 2011, 22, 1274–1289. [Google Scholar] [CrossRef] [PubMed]
- Butkevich, E.; Hülsmann, S.; Wenzel, D.; Shirao, T.; Duden, R.; Majoul, I. Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr. Biol. 2004, 14, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, C.; Ren, C.; Spagnol, G.; Cavin, G.; Cone, A.; Grintsevich, E.E.; Sosinsky, G.E.; Sorgen, P.L. Connexin43 Forms Supramolecular Complexes through Non-Overlapping Binding Sites for Drebrin, Tubulin, and ZO-1. PLoS ONE 2016, 11, e0157073. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Spagnol, G.; Naslavsky, N.; Caplan, S.; Sorgen, P.L. TC-PTP directly interacts with connexin43 to regulate gap junction intercellular communication. J. Cell Sci. 2014, 127, 3269–3279. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Price, D.A.; Ansari, M.A.; Roberts, K.N.; Schmitt, F.A.; Ikonomovic, M.D.; Mufson, E.J. Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 43, 1073–1090. [Google Scholar]
- Minter, M.R.; Moore, Z.; Zhang, M.; Brody, K.M.; Jones, N.C.; Shultz, S.R.; Taylor, J.M.; Crack, P.J. Deletion of the type-1 interferon receptor in APPSWE/PS1ΔE9 mice preserves cognitive function and alters glial phenotype. Acta Neuropathol. Commun. 2016, 4, 72. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Hwang, Y.J.; Ryu, H.; Kano, M.; Sakimura, K.; Cho, J. Conditional knockout of Cav2.1 disrupts the accuracy of spatial recognition of CA1 place cells and spatial/contextual recognition behavior. Front. Behav. Neurosci. 2016, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Verclytte, S.; Lopes, R.; Lenfant, P.; Rollin, A.; Semah, F.; Leclerc, X.; Pasquier, F.; Delmaire, C. Cerebral hypoperfusion and hypometabolism detected by arterial spin labeling MRI and FDG-PET in early-onset Alzheimer’s disease. J. Neuroimaging 2016, 26, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Murayama, N.; Ota, K.; Kasanuki, K.; Kondo, D.; Fujishiro, H.; Fukase, Y.; Tagaya, H.; Sato, K.; Iseki, E. Cognitive dysfunction in patients with very mild Alzheimer’s disease and amnestic mild cognitive impairment showing hemispheric asymmetries of hypometabolism on ¹⁸F-FDG PET. Int. J. Geriatr. Psychiatry 2016, 31, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhang, L.; Chen, W.; Zhu, H.; Deng, W.; Han, Y.; Guo, J.; Qin, C. Cyanidin 3-O-β-glucopyranoside activates peroxisome proliferator-activated receptor-γ and alleviates cognitive impairment in the APP(swe)/PS1(ΔE9) mouse model. Biochim. Biophys. Acta 2016, 1862, 1786–1800. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.Q.; Jiao, S.S.; Saadipour, K.; Zeng, F.; Wang, Q.H.; Zhu, C.; Shen, L.L.; Zeng, G.H.; Liang, C.R.; Wang, J.; et al. p75NTR ectodomain is a physiological neuroprotective molecule against amyloid-β toxicity in the brain of Alzheimer’s disease. Mol. Psychiatry 2015, 20, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Tang, Y.; Li, Y.; Gao, K.; Shi, X.; Li, Z. Behavioral changes and the hippocampus glucose metabolism in APP/PS1 transgenic mice via electro-acupuncture at governor vessel acupoints. Front. Aging Neurosci. 2017, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Desilva, S.; Abbruscato, T. The role of glucose transporters in brain disease: Diabetes and Alzheimer’s disease. Int. J. Mol. Sci. 2012, 13, 12629–12655. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; LaFerla, F.M. Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphony. Exp. Neurol. 2013, 239, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory response in the CNS: Friend or foe? Mol. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sajja, V.S.; Hlavac, N.; VandeVord, P.J. Role of Glia in Memory Deficits Following Traumatic Brain Injury: Biomarkers of Glia Dysfunction. Front. Integr. Neurosci. 2016, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. Local neuroinflammation and the progression of Alzheimer’s disease. J. Neurovirol. 2002, 8, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, L.; Lindholm, K.; Konishi, Y.; Yue, X.; Hampel, H.; Zhang, D.; Shen, Y. Tumor necrosis factor death receptor signaling cascade is required for amyloid-β protein-induced neuron death. J. Neurosci. 2004, 24, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Banks, W.A.; Butterfield, D.A. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in the hippocampus from subjects with amnestic mild cognitive impairment: Insights into their potential roles for loss of synapses and memory, accumulation of Aβ, and neurodegeneration in a prodromal stage of Alzheimer’s disease. J. Neurosci. Res. 2010, 88, 469–477. [Google Scholar] [PubMed]
- Wang, L.; Du, Y.; Wang, K.; Xu, G.; Luo, S.; He, G. Chronic cerebral hypoperfusion induces memory deficits and facilitates Aβ generation in C57BL/6J mice. Exp. Neurol. 2016, 283, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Valla, J.; Gonzalez-Lima, F.; Reiman, E.M. FDG autoradiography reveals developmental and pathological effects of mutant amyloid in PDAPP transgenic mice. Int. J. Dev. Neurosci. 2008, 26, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Y.; Chen, L.; Zhuang, G.; Zhang, J.; Li, M.; Meng, X.F. Contribution of single-minded 2 to hyperglycaemia-induced neurotoxicity. Neurotoxicology 2013, 35, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lebkuechner, I.; Wilhelmsson, U.; Möllerström, E.; Pekna, M.; Pekny, M. Heterogeneity of Notch signaling in astrocytes and the effects of GFAP and vimentin deficiency. J. Neurochem. 2015, 135, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.X.; Slinn, J.; Aylsworth, A.; Hou, S.T. Vimentin participates in microglia activation and neurotoxicity in cerebral ischemia. J. Neurochem. 2012, 122, 764–774. [Google Scholar] [CrossRef] [PubMed]
- George Paxinos, K.; Franklin, B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]

| Number | Direction | Oligonucleotide Sequence 5′–3′ |
|---|---|---|
| 1 | forward | GATCCGGGCAGTCTATCTTTGGTGACCACTCGAGTGGTCACCAAAGATAGACTGCTTTTTTAGATCTA |
| Reverse | AGCTTAGATCTAAAAAAGCAGTCTATCTTTGGTGACCACTCGAGTGGTCACCAAAGATAGACTGCCCG | |
| 2 | forward | GATCCGGGAGAACCAGAAAGTGATGTATCTCGAGATACATCACTTTCTGGTTCTCTTTTTTAGATCTA |
| Reverse | AGCTTAGATCTAAAAAAGAGAACCAGAAAGTGATGTATCTCGAGATACATCACTTTCTGGTTCTCCCG | |
| 3 | forward | GATCCGGCCAATGGAGAGACCACTCAAACTCGAGTTTGAGTGGTCTCTCCATTGGTTTTTTAGATCTA |
| Reverse | AGCTTAGATCTAAAAAACCAATGGAGAGACCACTCAAACTCGAGTTTGAGTGGTCTCTCCATTGGCCG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, Y.; Zhang, L.; Huang, L.; Yu, P.; Zhu, H.; Deng, W.; Qin, C. Down-Regulated Drebrin Aggravates Cognitive Impairments in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 800. https://doi.org/10.3390/ijms18040800
Liu Y, Xu Y, Zhang L, Huang L, Yu P, Zhu H, Deng W, Qin C. Down-Regulated Drebrin Aggravates Cognitive Impairments in a Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2017; 18(4):800. https://doi.org/10.3390/ijms18040800
Chicago/Turabian StyleLiu, Yan, Yanfeng Xu, Ling Zhang, Lan Huang, Pin Yu, Hua Zhu, Wei Deng, and Chuan Qin. 2017. "Down-Regulated Drebrin Aggravates Cognitive Impairments in a Mouse Model of Alzheimer’s Disease" International Journal of Molecular Sciences 18, no. 4: 800. https://doi.org/10.3390/ijms18040800






