Liposomal Encapsulation for Systemic Delivery of Propranolol via Transdermal Iontophoresis Improves Bone Microarchitecture in Ovariectomized Rats
Abstract
:1. Introduction
2. Results
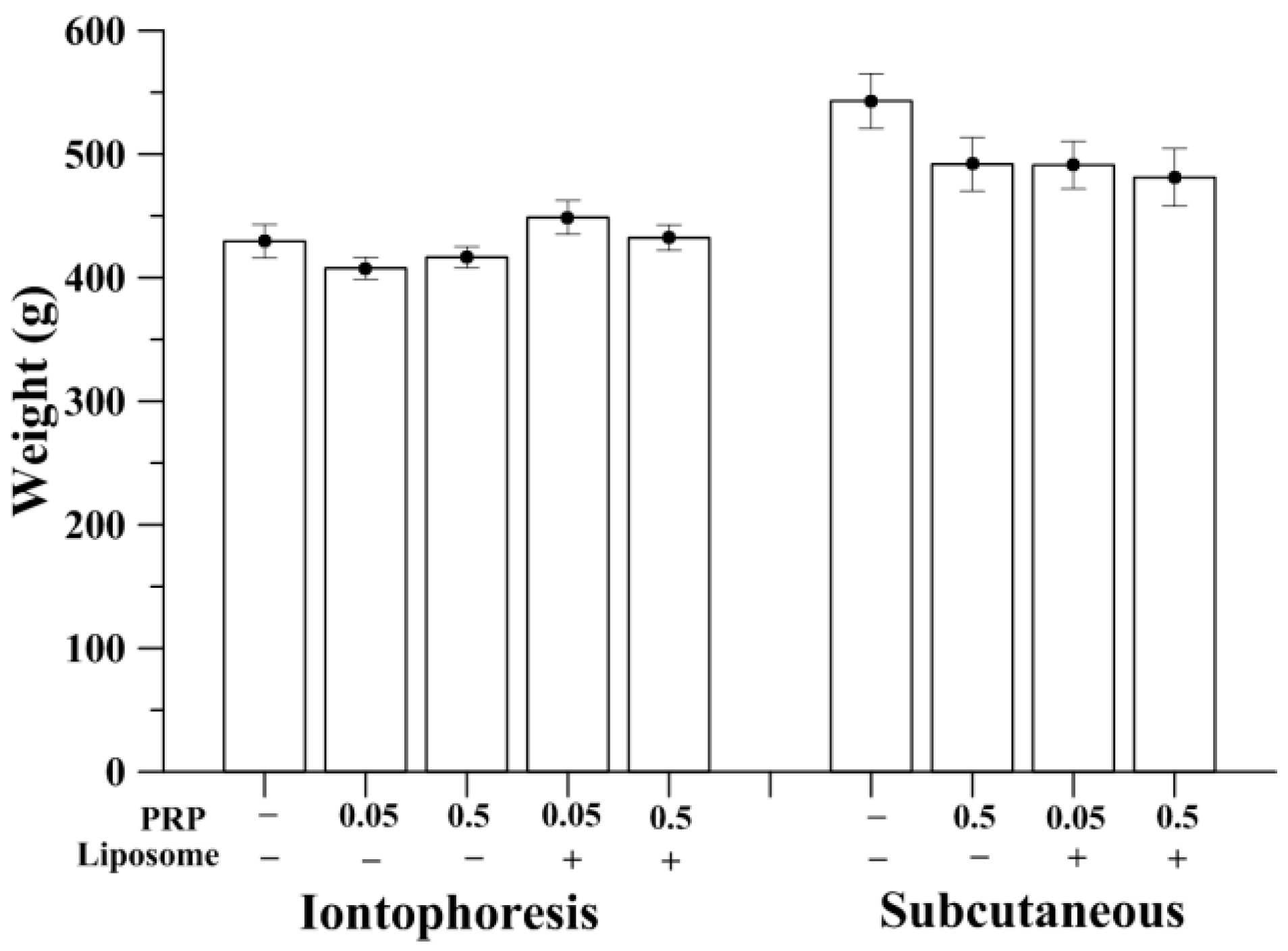
2.1. Measurement of Animal Weight
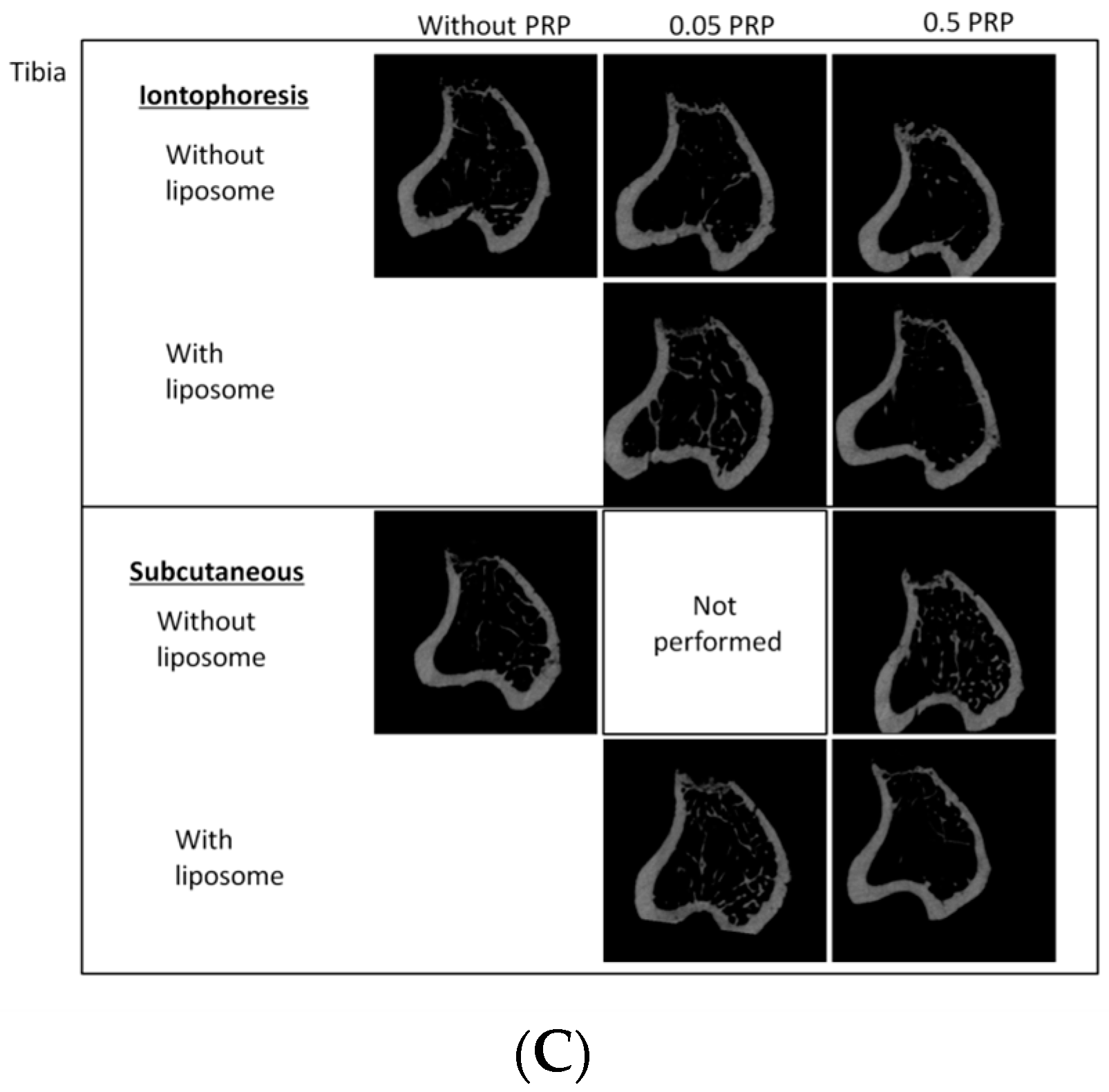
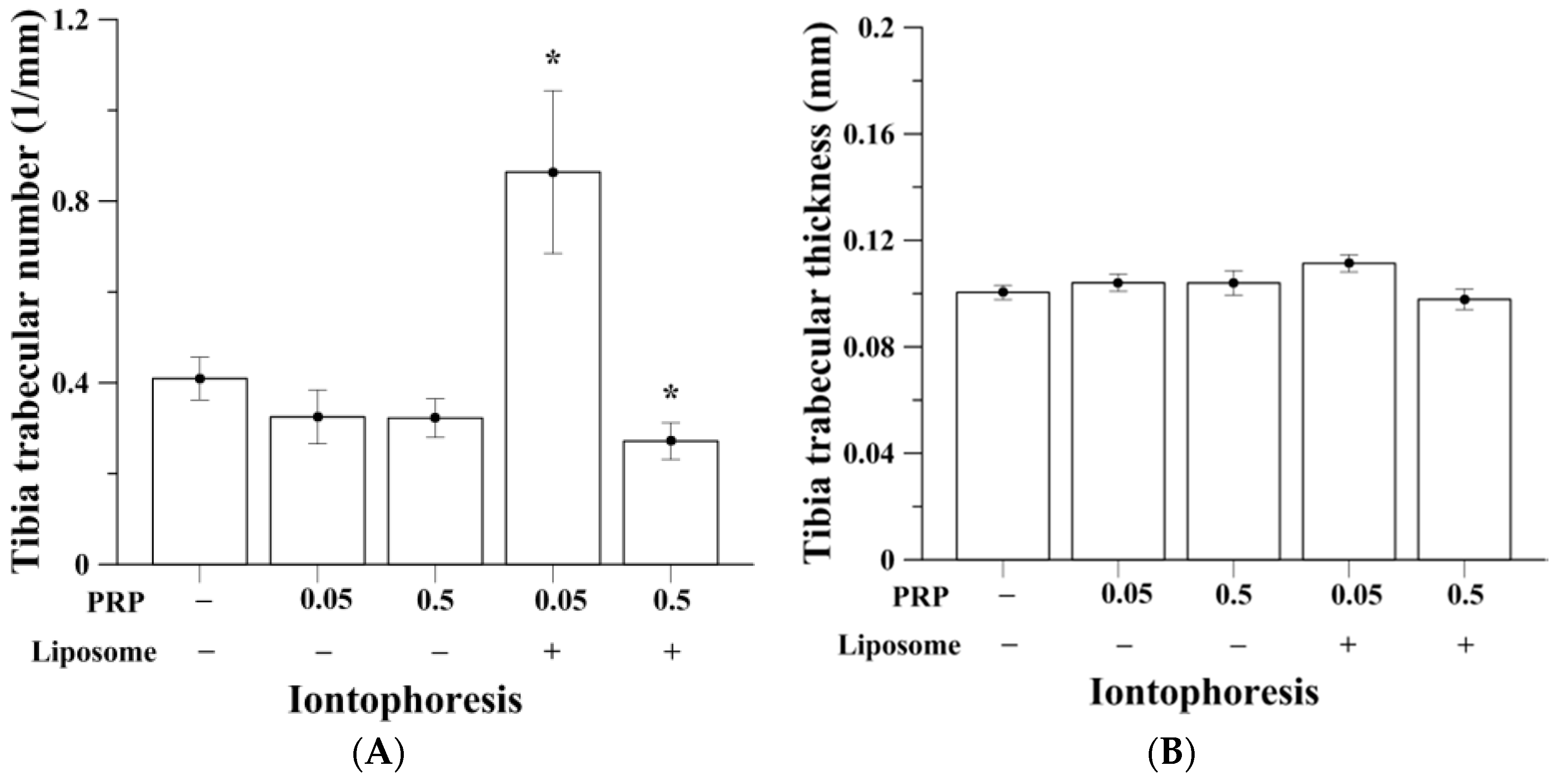
2.2. Trabecular Bone Analysis (Tibia)
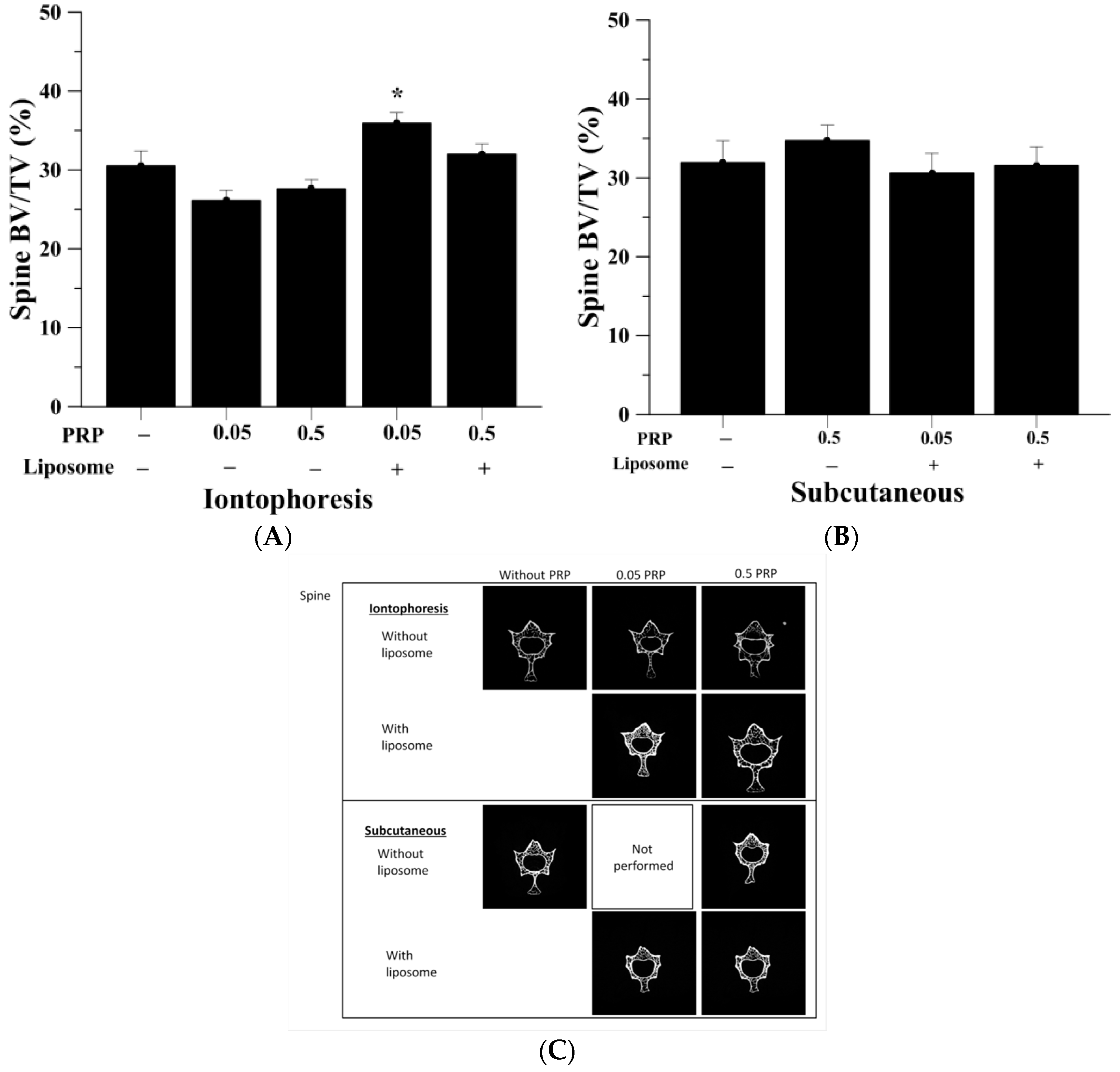
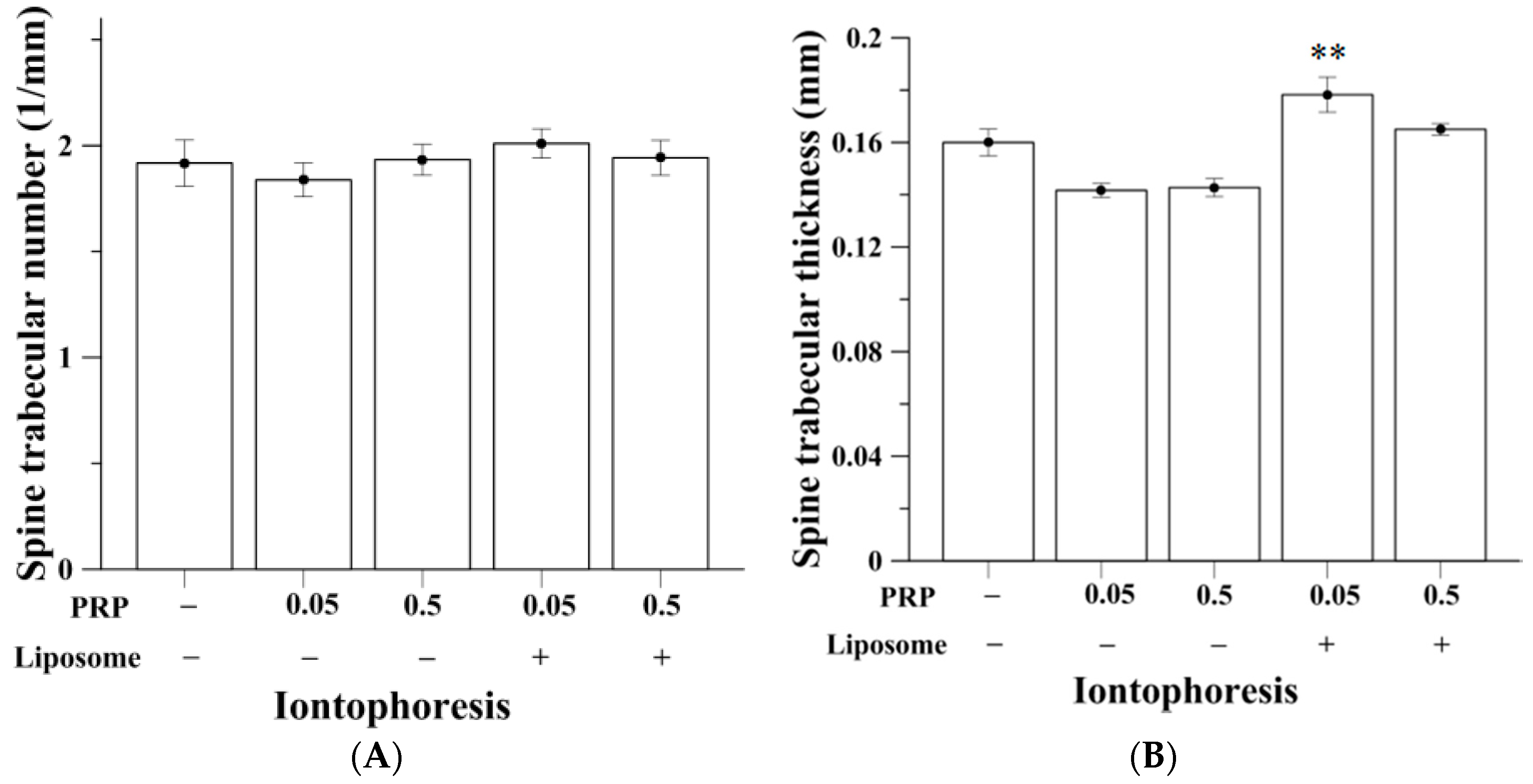
2.3. Trabecular Bone Analysis (Spine)
2.4. Effects on Serum Calcium, Phosphorous and Cholesterol Level
2.5. Effects on Liver and Kidney Function
3. Discussion
4. Methods
4.1. Materials
4.2. Production of DSPC Liposomes
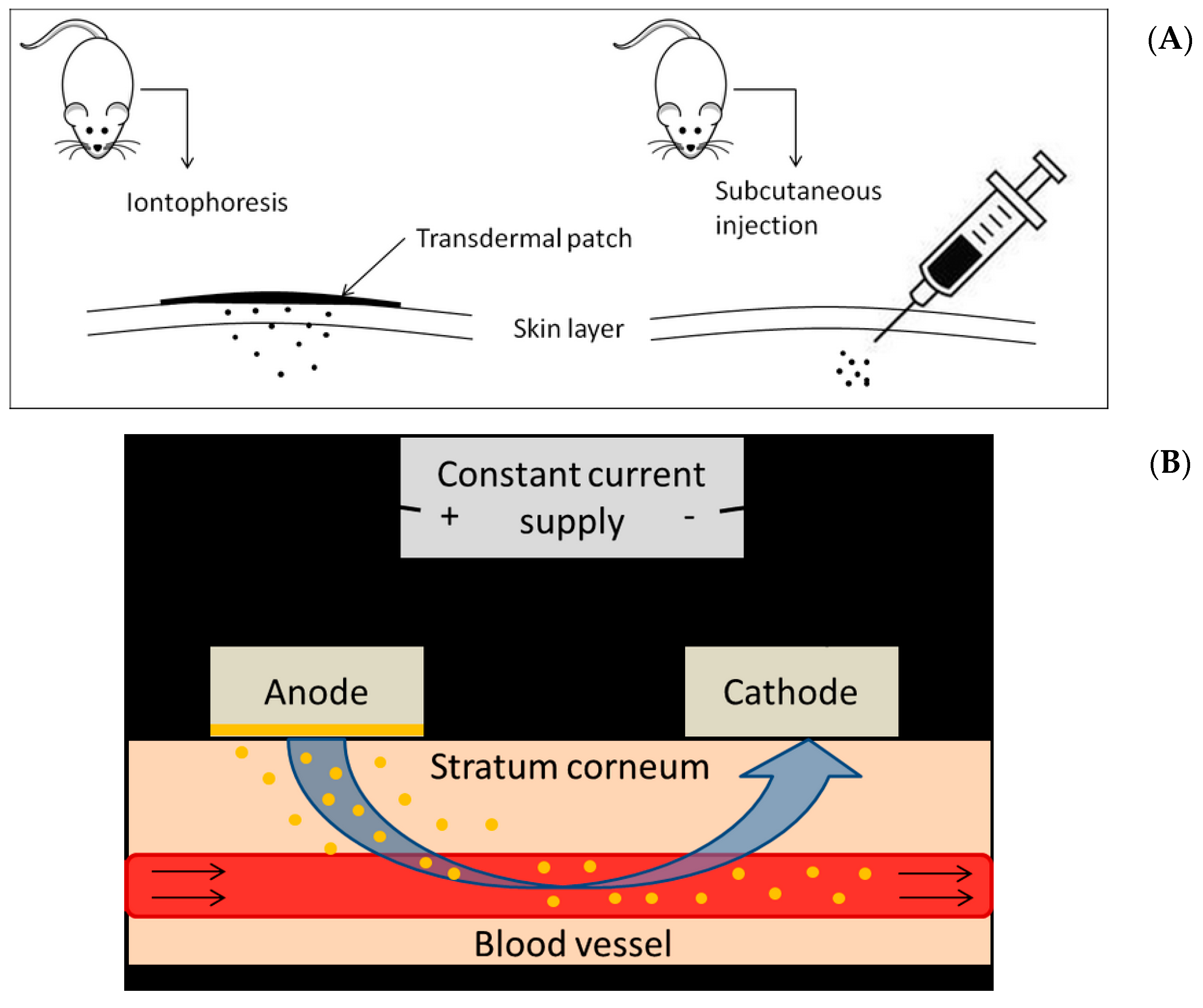
4.3. Experimental Animals and Pre-Clinical Study Design
4.4. Iontophoretic Parameters
4.5. Serum Biochemical Analysis
4.6. Measurement of Bone Porosity by Micro-Computed Tomography (Micro-CT)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Skeletal structural adaptations to mechanical usage (SATMU): 4. Mechanical influences on intact fibrous tissues. Anat. Rec. 1990, 226, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Additive effects of zoledronic acid and propranolol on bone density and biochemical markers of bone turnover in osteopenic ovariectomized rats. Rev. Bras. Reumatol. 2015, 55, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Kassem, M. Osteoblasts in osteoporosis: Past, emerging, and future anabolic targets. Eur. J. Endocrinol. 2011, 165, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.C.; Rybchyn, M.S.; Green, W.; Atwa, S.; Conigrave, A.D.; Mason, R.S. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br. J. Pharmacol. 2009, 157, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.M.; Prajapati, B.G.; Patel, H.V.; Patel, K.M. Mucoadhesive bilayer tablets of propranolol hydrochloride. AAPS Pharm. Sci. Tech. 2007, 8, E77. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.G.; Hill, S.J.; Summers, R.J. Evolution of β-blockers: From anti-anginal drugs to ligand-directed signalling. Trends Pharmacol. Sci. 2011, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Henry, M.J.; Sanders, K.M.; Kotowicz, M.A.; Seeman, E.; Nicholson, G.C.; Geelong Osteoporosis, S. β-Adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J. Bone Miner. Res. 2004, 19, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, N.; Laroche, N.; Vico, L.; Dolleans, E.; Benhamou, C.L.; Courteix, D. Dose effects of propranolol on cancellous and cortical bone in ovariectomized adult rats. J. Pharmacol. Exp. Ther. 2006, 318, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, N.; Gadois, C.; McCloskey, E.; Lemineur, G.; Lespessailles, E.; Courteix, D.; Benhamou, C.L. Protective effect of β blockers in postmenopausal women: Influence on fractures, bone density, micro and macroarchitecture. Bone 2007, 40, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Schlienger, R.G.; Kraenzlin, M.E.; Jick, S.S.; Meier, C.R. Use of β-blockers and risk of fractures. JAMA 2004, 292, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, W.F.; Madeira, M.F.; da Silva, T.A.; Clemente-Napimoga, J.T.; Miguel, C.B.; Dias-da-Silva, V.J.; Barbosa-Neto, O.; Lopes, A.H.; Napimoga, M.H. Low dose of propranolol down-modulates bone resorption by inhibiting inflammation and osteoclast differentiation. Br. J. Pharmacol. 2012, 165, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Brennan, T.C.; Muir, M.M.; Mason, R.S. Functional α1- and β2-adrenergic receptors in human osteoblasts. J. Cell. Physiol. 2009, 220, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Bonnet, N.; Benhamou, C.L.; Brunet-Imbault, B.; Arlettaz, A.; Horcajada, M.N.; Richard, O.; Vico, L.; Collomp, K.; Courteix, D. Severe bone alterations under β2 agonist treatments: Bone mass, microarchitecture and strength analyses in female rats. Bone 2005, 37, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Sultana, Y.; Ali, A. Transdermal delivery of β-blockers. Expert Opin. Drug Deliv. 2006, 3, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Negussie, A.H.; Miller, J.L.; Reddy, G.; Drake, S.K.; Wood, B.J.; Dreher, M.R. Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J. Control. Release 2010, 143, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kheadr, E.E.; Vuillemard, J.C.; El-Deeb, S.A. Impact of liposome-encapsulated enzyme cocktails on cheddar cheese ripening. Food Res. Int. 2003, 36, 241–252. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Lu, C.-T. Increasing the Entrapment of Protein-Loaded Liposomes with a Modified Freeze–Thaw Technique: A Preliminary Experimental Study. Drug Dev. Ind. Pharm. 2009, 35, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Touraki, M.; Rigas, P.; Kastritsis, C. Liposome mediated delivery of water soluble antibiotics to the larvae of aquatic animals. Aquaculture 1995, 136, 1–10. [Google Scholar] [CrossRef]
- Salem, I.I.; Flasher, D.L.; Düzgüneş, N. Liposome-Encapsulated Antibiotics. In Methods Enzymol; Nejat, D., Ed.; Academic Press: New York, NY, USA, 2005; Volume 391, pp. 261–291. [Google Scholar]
- De Logu, A.; Fadda, A.M.; Pellerano, M.L.; Diana, G.; Schivo, M.L. Prevention by l-α-phosphatidylcholine of antifungal activity in vitro of liposome-encapsulated imidazoles determined by using time-killing curves. Int. J. Antimicrob. Agents 2000, 15, 43–48. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Shashi, K.; Satinder, K.; Bharat, P. A complete review on: Liposomes. Int. Res. J. Pharm. 2012, 7, 10–16. [Google Scholar]
- Romberg, B.; Oussoren, C.; Snel, C.J.; Hennink, W.E.; Storm, G. Effect of liposome characteristics and dose on the pharmacokinetics of liposomes coated with poly(amino acid)s. Pharm. Res. 2007, 24, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Kotyńska, J.; Figaszewski, Z.A. Adsorption equilibria at interface separating electrolyte solution and phosphatidylcholine–stearylamine liposome membrane. Biophys. Chem. 2007, 127, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Teong, B.; Kuo, S.M.; Chen, C.H.; Chen, Y.K.; Cheng, Z.J.; Huang, H.H. Characterization and human osteoblastic proliferation- and differentiation-stimulatory effects of phosphatidylcholine liposomes-encapsulated propranolol hydrochloride. Biomed. Mater. Eng. 2014, 24, 1875–1887. [Google Scholar] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thakur, R.; Fan, Q.; Michniak, B. Transdermal iontophoresis: Combination strategies to improve transdermal iontophoretic drug delivery. Eur. J. Pharm. Biopharm. 2005, 60, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Liston, C.; Miller, M.M.; Goldwater, D.S.; Radley, J.J.; Rocher, A.B.; Hof, P.R.; Morrison, J.H.; McEwen, B.S. Stress-Induced Alterations in Prefrontal Cortical Dendritic Morphology Predict Selective Impairments in Perceptual Attentional Set-Shifting. J. Neurosci. 2006, 26, 7870–7874. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kang, L.; Lin, R.W.; Fu, Y.C.; Lin, Y.S.; Chang, J.K.; Chen, H.T.; Chen, C.H.; Lin, S.Y.; Wang, G.J.; Ho, M.L. (−)-Epigallocatechin-3-gallate improves bone microarchitecture in ovariectomized rats. Menopause 2013, 20, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Svedbom, A.; Hernlund, E.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A.; EU Review Panel of IOF. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 2013, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.I.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Turker, S.; Karatosun, V.; Gunal, I. β-Blockers increase bone mineral density. Clin. Orthop. Relat. Res. 2006, 443, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Henry, M.J.; Nicholson, G.C.; Schneider, H.G.; Kotowicz, M.A. β-Blockers reduce bone resorption marker in early postmenopausal women. Ann. Hum. Biol. 2005, 32, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Minkowitz, B.; Boskey, A.L.; Lane, J.M.; Pearlman, H.S.; Vigorita, V.J. Effects of propranolol on bone metabolism in the rat. J. Orthop. Res. 1991, 9, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Togari, A. In vivo stimulation of sympathetic nervous system modulates osteoblastic activity in mouse calvaria. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E661–E667. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, N.; Benhamou, C.L.; Malaval, L.; Goncalves, C.; Vico, L.; Eder, V.; Pichon, C.; Courteix, D. Low dose β-blocker prevents ovariectomy-induced bone loss in rats without affecting heart functions. J. Cell. Physiol. 2008, 217, 819–827. [Google Scholar] [CrossRef] [PubMed]
- D’Emanuele, A.; Staniforth, J.N. An electrically modulated drug delivery device. Pharm. Res. 1991, 8, 913–918. [Google Scholar]
- Azzi, M.; Charest, P.G.; Angers, S.; Rousseau, G.; Kohout, T.; Bouvier, M.; Pineyro, G. β-Arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 11406–11411. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.G.; Hall, I.P.; Hill, S.J. Agonist and inverse agonist actions of β-blockers at the human β 2-adrenoceptor provide evidence for agonist-directed signaling. Mol. Pharmacol. 2003, 64, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, H.; Chao, C. Effect of propranolol on calcium, phosphorus, and magnesium metabolic disorders in Graves’ disease. Metabolism 1992, 41, 552–555. [Google Scholar] [CrossRef]
- Manousakas, I.; Guan-Huei, L.; Shwu-Jen, C.; Shyh-Ming, K.; Yu-Chiuan, W. Application of Ultrasonic Extrusion in the Preparation of Liposomes. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- Tashiro, Y.; Sami, M.; Shichibe, S.; Kato, Y.; Hayakawa, E.; Itoh, K. Effect of lipophilicity on in vivo iontophoretic delivery. II. β-Blockers. Biol. Pharm. Bull. 2001, 24, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Conjeevaram, R.; Chaturvedula, A.; Betageri, G.V.; Sunkara, G.; Banga, A.K. Iontophoretic in vivo transdermal delivery of β-blockers in hairless rats and reduced skin irritation by liposomal formulation. Pharm. Res. 2003, 20, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kang, L.; Lo, H.C.; Hsu, T.H.; Lin, F.Y.; Lin, Y.S.; Wang, Z.J.; Chen, S.T.; Shen, C.L. Polysaccharides of Trametes versicolor Improve Bone Properties in Diabetic Rats. J. Agric. Food Chem. 2015, 63, 9232–9238. [Google Scholar] [CrossRef] [PubMed]


| Tibia | I-OVX | I-0.05PRP | I-0.5PRP | I-0.05PRP/L | I-0.5PRP/L | S-OVX | S-0.5PRP | S-0.05PRP/L | S-0.5PRP/L |
|---|---|---|---|---|---|---|---|---|---|
| BV/TV, % | 4.07 (0.45) | 3.41 (0.69) | 3.27 (0.41) | 9.00 a (2.04) | 2.61 (0.39) | 3.78 (0.80) | 5.65 (0.63) | 7.60 (2.01) | 4.77 (0.79) |
| TbTh, mm | 0.10 (0.00) | 0.10 (0.00) | 0.10 (0.01) | 0.11 (0.00) | 0.10 (0.00) | 0.11 (0.01) | 0.11 (0.00) | 0.12 (0.01) | 0.11 (0.01) |
| TbN, mm−1 | 0.41 (0.05) | 0.29 (0.05) | 0.32 (0.04) | 0.81 a (0.19) | 0.27 (0.04) | 0.41 (0.09) | 0.53 (0.07) | 0.68 (0.19) | 0.45 (0.08) |
| TbSp, mm | 1.10 (0.11) | 1.16 (0.07) | 1.22 (0.13) | 1.04 (0.14) | 1.26 (0.08) | 1.26 (0.22) | 1.09 (0.12) | 1.09 (0.22) | 1.35 (0.16) |
| Spine | I-OVX | I-0.05PRP | I-0.5PRP | I-0.05PRP/L | I-0.5PRP/L | S-OVX | S-0.5PRP | S-0.05PRP/L | S-0.5PRP/L |
|---|---|---|---|---|---|---|---|---|---|
| BV/TV, % | 30.51 (1.90) | 26.13 (1.29) | 27.60 (1.18) | 35.93 a (1.35) | 32.00 (1.31) | 31.93 (2.79) | 34.73 (1.95) | 30.61 (2.51) | 31.57 (2.37) |
| TbTh, mm | 0.16 (0.01) | 0.14 (0.00) | 0.14 (0.00) | 0.18 b (0.01) | 0.17 (0.00) | 0.18 (0.01) | 0.17 (0.01) | 0.16 (0.01) | 0.16 (0.01) |
| TbN, mm−1 | 1.92 (0.11) | 1.84 (0.08) | 1.93 (0.07) | 2.01 (0.07) | 1.94 (0.08) | 1.81 (0.16) | 2.01 (0.10) | 1.85 (0.08) | 1.93 (0.09) |
| TbSp, mm | 0.33 (0.03) | 0.34 (0.02) | 0.31 (0.01) | 0.29 (0.01) | 0.32 (0.02) | 0.32 (0.03) | 0.31 (0.01) | 0.31 (0.01) | 0.31 (0.02) |
| Groups | Calcium (mg/dL) | Phosphorous (mg/dL) | Cholesterol (mg/dL) |
|---|---|---|---|
| Iontophoresis | |||
| I-OVX | 11.34 ± 0.13 | 10.60 ± 0.54 | 122.92 ± 6.29 |
| I-0.05PRP | 11.01 ± 0.11 | 8.00 ± 0.29 c | 115.23 ± 4.54 |
| I-0.5PRP | 10.62 ± 0.40 | 11.72 ± 0.57 | 123.85 ± 5.98 |
| I-0.05PRP/L | 10.69 ± 0.13 b | 7.79 ± 0.55 b | 124.62 ± 6.25 |
| I-0.5PRP/L | 10.88 ± 0.10 b | 11.34 ± 0.57 | 125.31 ± 4.03 |
| Subcutaneous | |||
| S-OVX | 11.11 ± 0.13 | 13.95 ± 1.28 | 130.38 ± 7.34 |
| S-0.5PRP | 11.61 ± 0.23 | 13.10 ± 1.34 | 124.88 ± 4.54 |
| S-0.05PRP/L | 10.66 ± 0.08 a | 7.36 ± 0.36 c | 113.13 ± 5.74 |
| S-0.5PRP/L | 11.21 ± 0.13 | 15.76 ± 0.77 | 137.25 ± 8.17 |
| Groups | SGOT (U/L) | SGPT (U/L) | Creatinine (mg/dL) | BUN (mg/dL) |
|---|---|---|---|---|
| Iontophoresis | ||||
| I-OVX | 144.92 ± 13.80 | 66.85 ± 5.93 | 0.52 ± 0.01 | 17.81 ± 0.61 |
| I-0.05PRP | 134.38 ± 11.11 | 54.38 ± 2.64 | 0.45 ± 0.02 b | 17.97 ± 0.51 |
| I-0.5PRP | 163.08 ± 12.85 | 61.15 ± 4.47 | 0.47 ± 0.01 a | 19.34 ± 0.71 |
| I-0.05PRP/L | 106.85 ± 9.80 a | 58.00 ± 4.22 | 0.46 ± 0.01 b | 17.76 ± 0.54 |
| I-0.5PRP/L | 184.69 ± 10.52 a | 58.31 ± 3.05 | 0.45 ± 0.02 b | 19.65 ± 0.41 a |
| Subcutaneous | ||||
| S-OVX | 185.75 ± 19.16 | 71.00 ± 9.67 | 0.44 ± 0.01 | 16.80 ± 1.04 |
| S-0.5PRP | 137.00 ± 17.61 | 73.13 ± 4.75 | 0.48 ± 0.01 | 18.53 ± 0.52 |
| S-0.05PRP/L | 191.38 ± 17.11 | 69.50 ± 4.59 | 0.45 ± 0.01 | 16.84 ± 2.24 |
| S-0.5PRP/L | 182.38 ± 15.15 | 70.50 ± 3.65 | 0.44 ± 0.02 | 15.18 ± 1.39 |
| Treatment | Iontophoresis (n = 13) | Subcutaneous (n = 8) |
|---|---|---|
| OVX | I-OVX | S-OVX |
| OVX + PRP (0.05 mg/kg) | I-0.05PRP | NA |
| OVX + PRP (0.5 mg/kg) | I-0.5PRP | S-0.5PRP |
| OVX + PRP (0.05 mg/kg)/liposome | I-0.05PRP/L | S-0.05PRP/L |
| OVX + PRP (0.5 mg/kg)/liposome | I-0.5PRP/L | S-0.5PRP/L |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teong, B.; Kuo, S.M.; Tsai, W.-H.; Ho, M.-L.; Chen, C.-H.; Huang, H.H. Liposomal Encapsulation for Systemic Delivery of Propranolol via Transdermal Iontophoresis Improves Bone Microarchitecture in Ovariectomized Rats. Int. J. Mol. Sci. 2017, 18, 822. https://doi.org/10.3390/ijms18040822
Teong B, Kuo SM, Tsai W-H, Ho M-L, Chen C-H, Huang HH. Liposomal Encapsulation for Systemic Delivery of Propranolol via Transdermal Iontophoresis Improves Bone Microarchitecture in Ovariectomized Rats. International Journal of Molecular Sciences. 2017; 18(4):822. https://doi.org/10.3390/ijms18040822
Chicago/Turabian StyleTeong, Benjamin, Shyh Ming Kuo, Wei-Hsin Tsai, Mei-Ling Ho, Chung-Hwan Chen, and Han Hsiang Huang. 2017. "Liposomal Encapsulation for Systemic Delivery of Propranolol via Transdermal Iontophoresis Improves Bone Microarchitecture in Ovariectomized Rats" International Journal of Molecular Sciences 18, no. 4: 822. https://doi.org/10.3390/ijms18040822







