Retinoids Regulate Adipogenesis Involving the TGFβ/SMAD and Wnt/β-Catenin Pathways in Human Bone Marrow Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
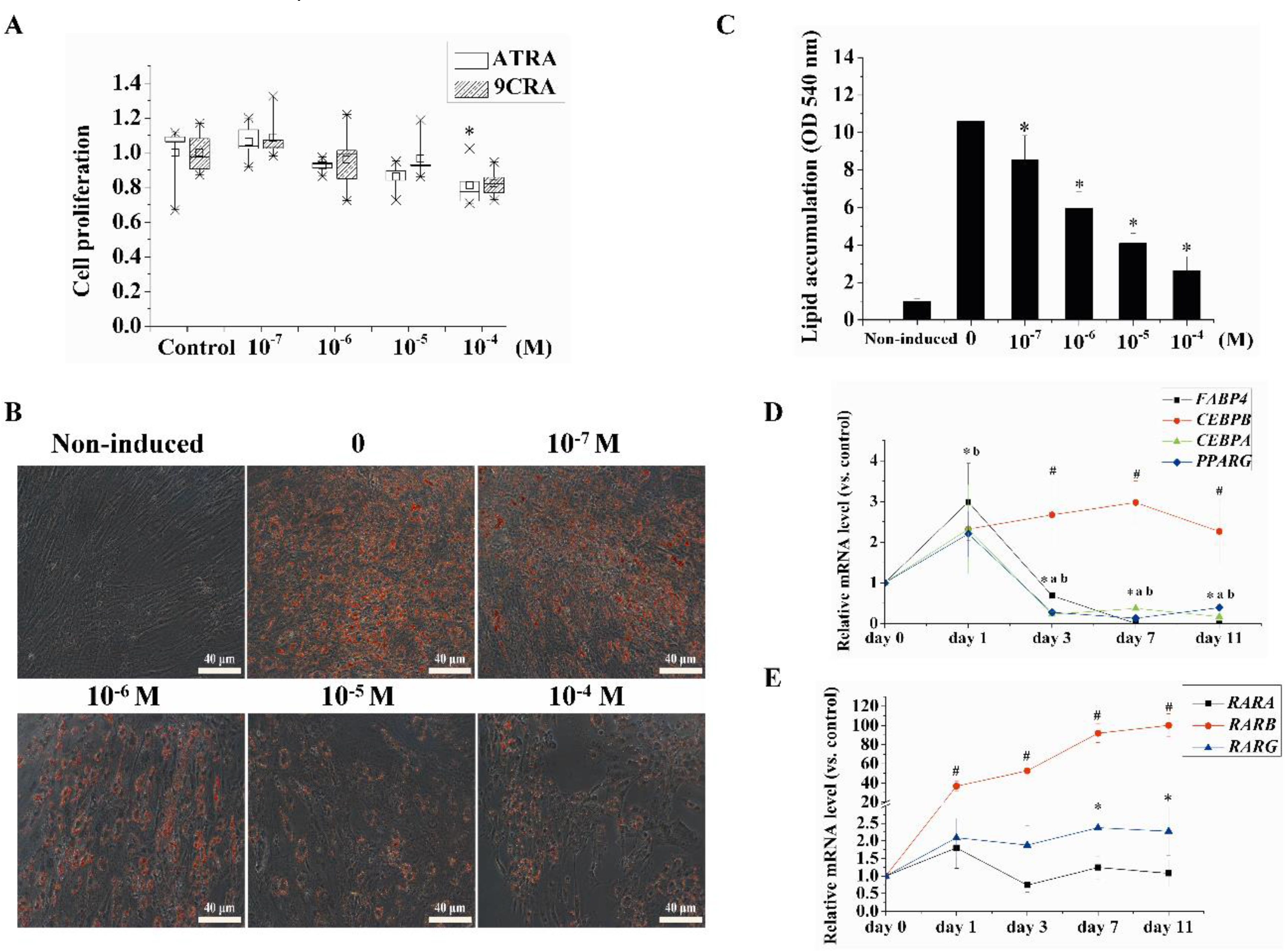
2.1. Retinoids Affected Proliferation and Adipogenic Differentiation in a Concentration- and Time-Dependent Manner in hBMSCs
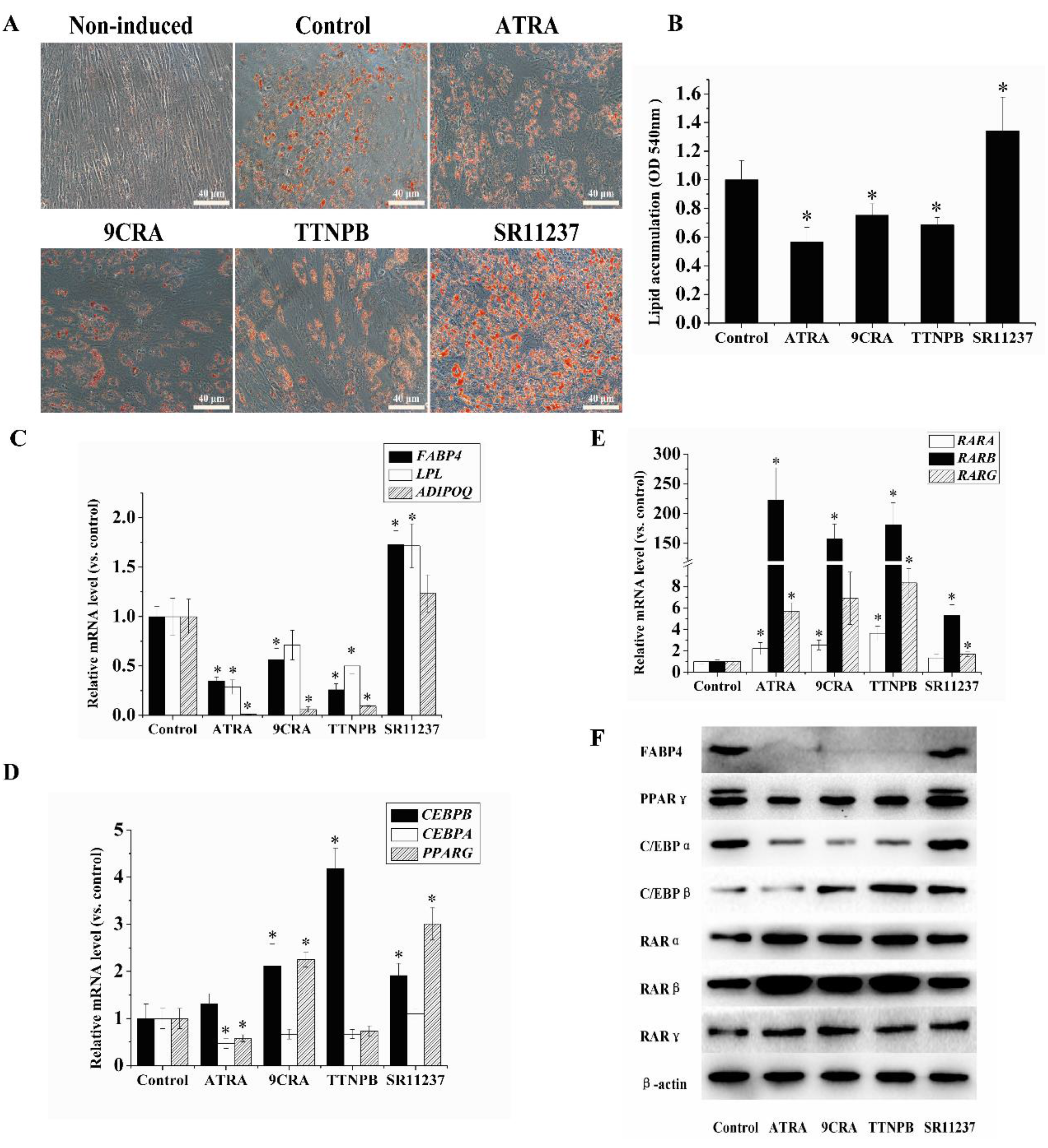
2.2. The Adipogenic Differentiation of hBMSCs Was Inhibited by RAR Agonists and Promoted by RXR Agonists
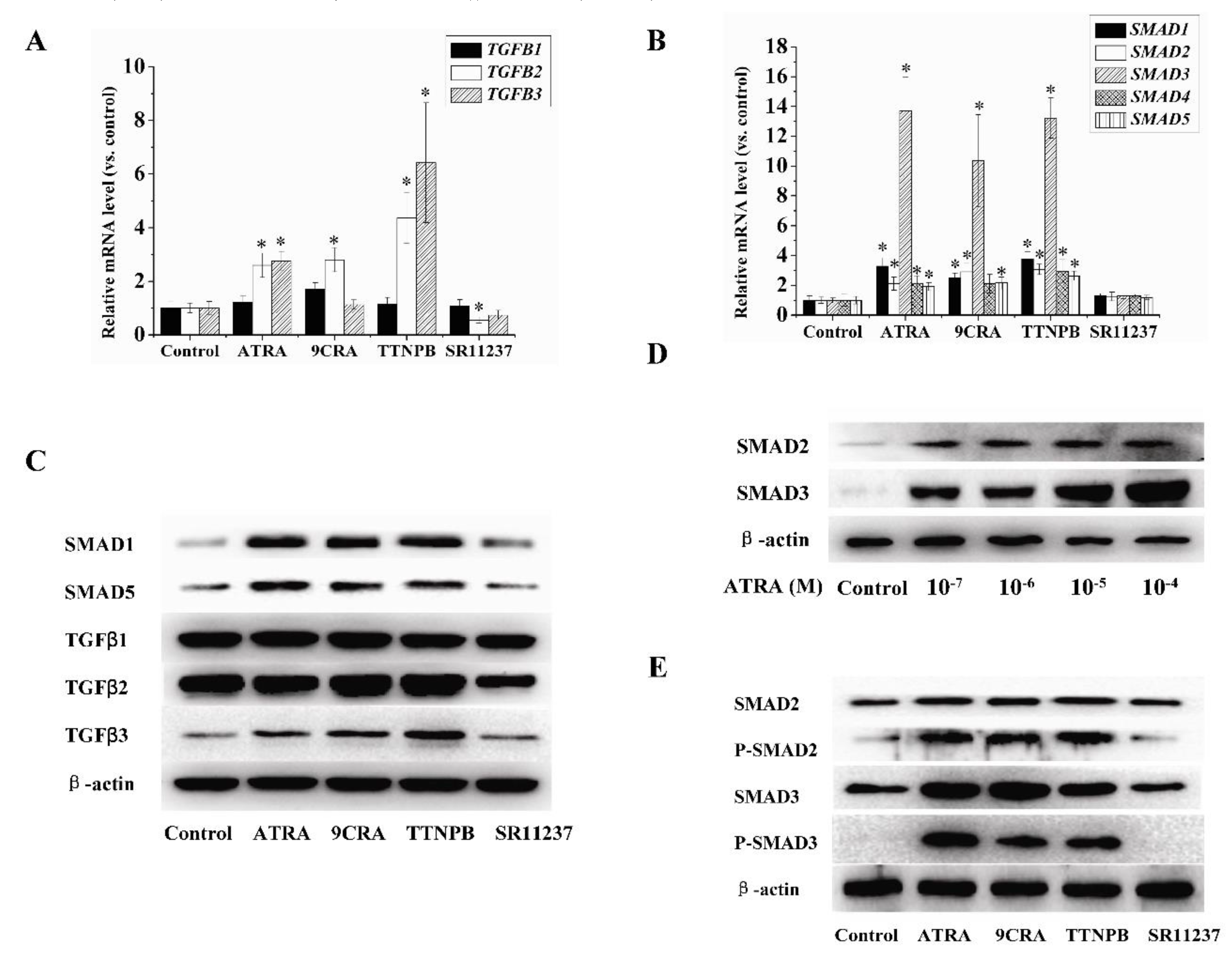
2.3. Effect of Retinoids on the Expression of TGFβ/SMAD Pathway-Related Genes in hBMSCs
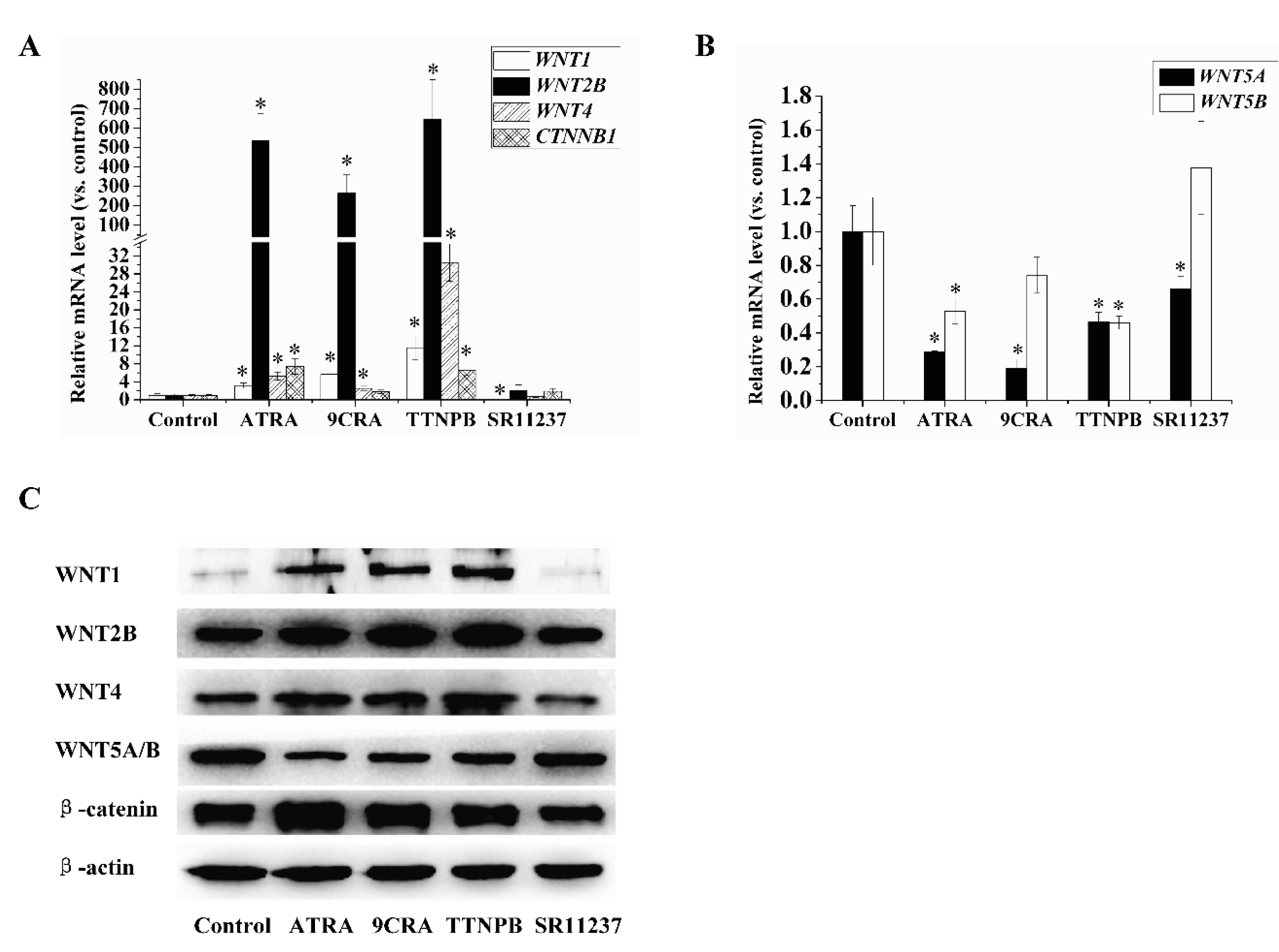
2.4. Effect of Retinoids on the Expression of Wnt Pathway-Related Genes in hBMSCs
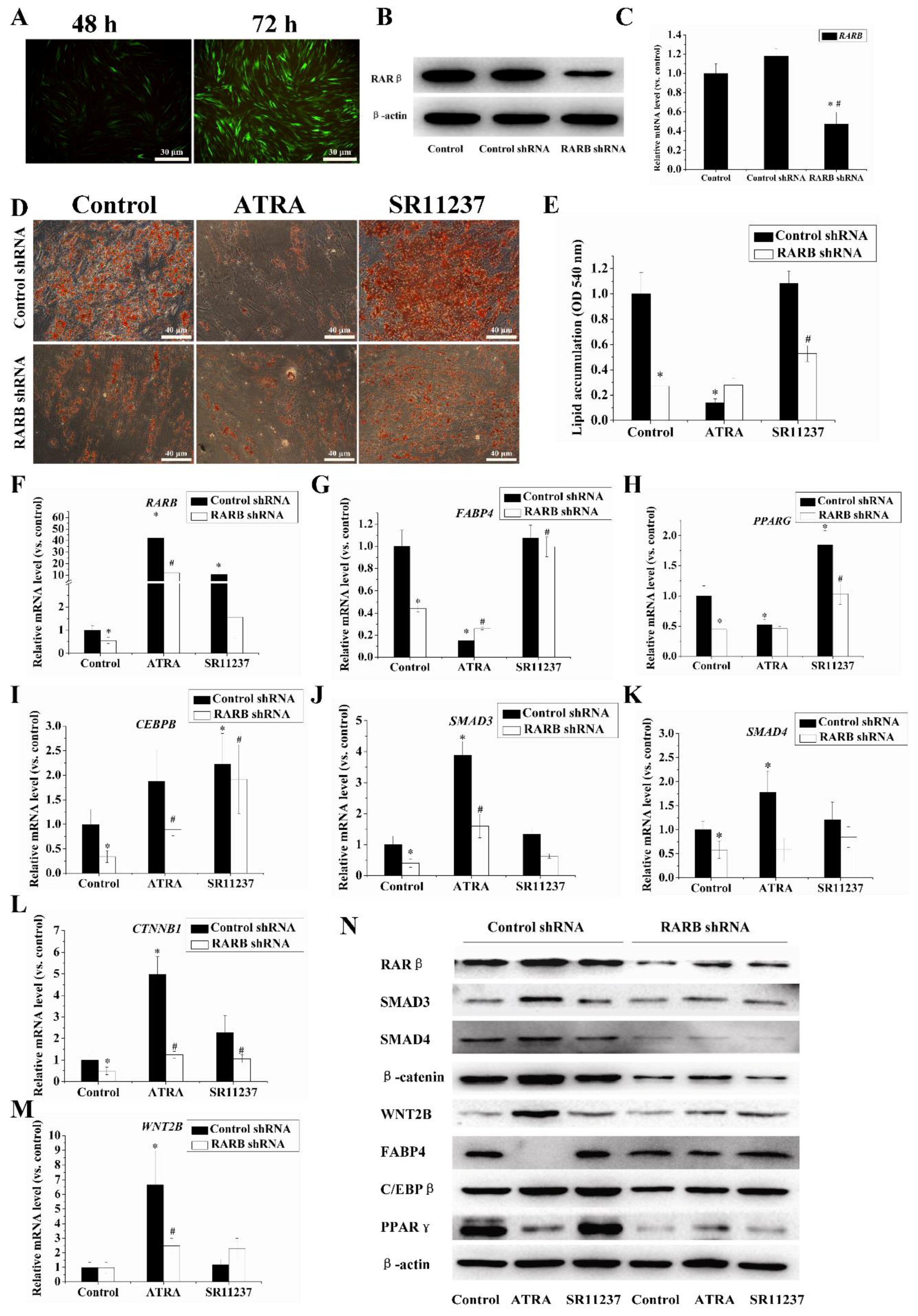
2.5. Knockdown of the RARB Gene Attenuated the Effect of Retinoids on Adipogenic Differentiation in hBMSCs
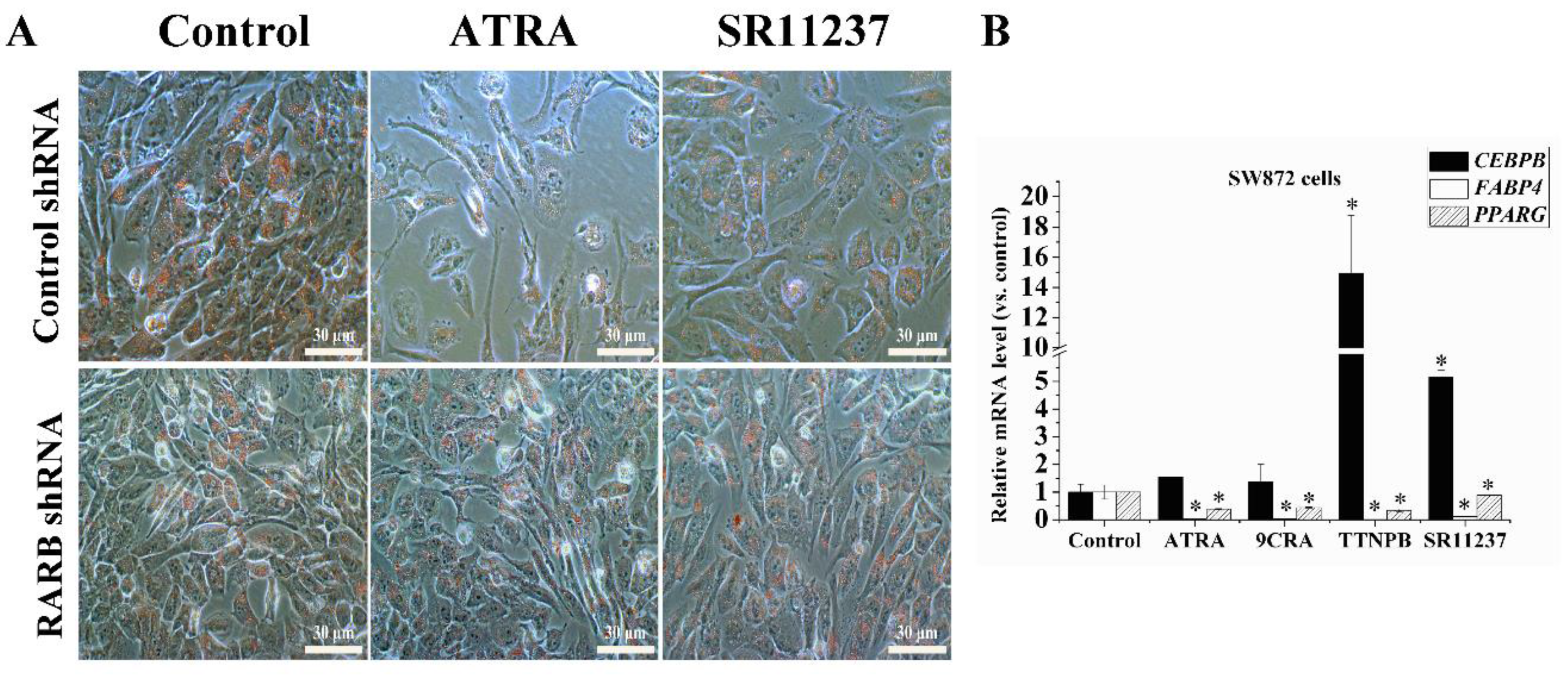
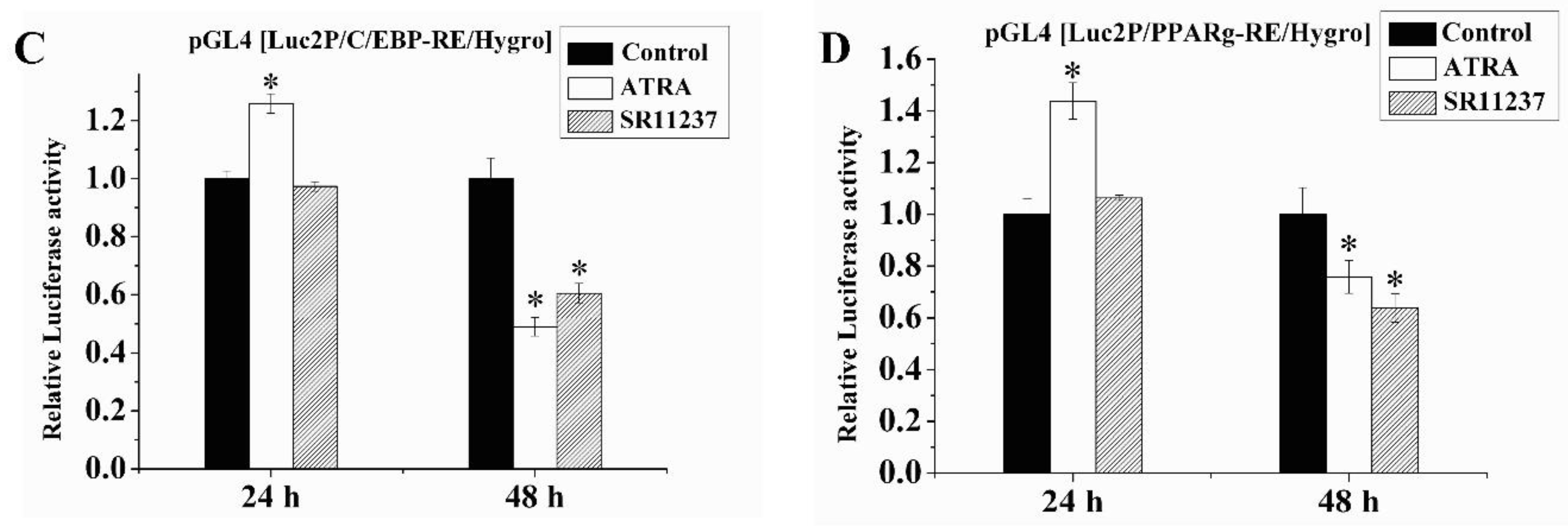
2.6. Both RAR Agonists and RXR Agonists Inhibited Adipogenic Differentiation and Blocked the Promoter Activity of C/EBPβ and PPARγ in SW872 Cells
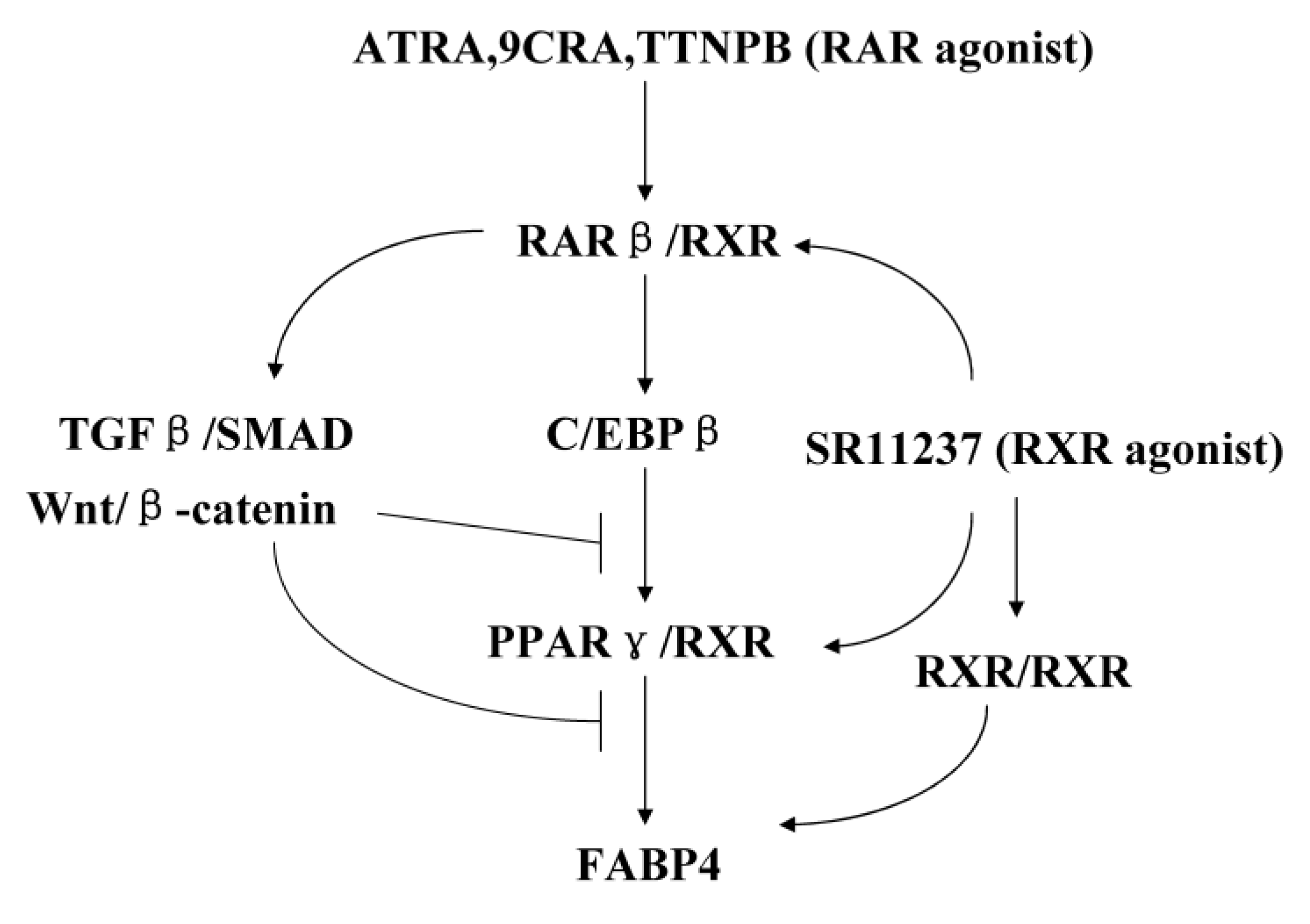
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Adipogenic Differentiation
4.2. Cell Proliferation Assays
4.3. Oil Red O Staining
4.4. Quantitative RT- PCR
4.5. Immunoblot Analysis
4.6. Luciferase Reporter Assay
4.7. Transient Transfection of shRNA
4.8. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gardner, O.F.; Alini, M.; Stoddart, M.J. Mesenchymal stem cells derived from human bone marrow. Methods Mol. Biol. 2015, 1340, 41–52. [Google Scholar] [PubMed]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem 2012, 81, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. Ppars are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J.; Wagner, J.A. Retinoids regulate stem cell differentiation. J. Cell. Physiol. 2011, 226, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Jong, L.; Fanjul, A.; Cameron, J.F.; Lu, X.P.; Haefner, P.; Dawson, M.I.; Pfahl, M. Retinoids selective for retinoid X receptor response pathways. Science 1992, 258, 1944–1946. [Google Scholar] [CrossRef] [PubMed]
- Szeles, L.; Poliska, S.; Nagy, G.; Szatmari, I.; Szanto, A.; Pap, A.; Lindstedt, M.; Santegoets, S.J.; Ruhl, R.; Dezso, B.; et al. Research resource: Transcriptome profiling of genes regulated by RXR and its permissive and nonpermissive partners in differentiating monocyte-derived dendritic cells. Mol. Endocrinol. 2010, 24, 2218–2231. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Kocovski, P.; Jovic, T.; Walia, M.K.; Chandraratna, R.A.; Martin, T.J.; Baker, E.K.; Purton, L.E. Retinoic acid receptor signalling directly regulates osteoblast and adipocyte differentiation from mesenchymal progenitor cells. Exp. Cell Res. 2017, 350, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Hiragun, A.; Mitsui, H. Preadipocytes possess cellular retinoid binding proteins and their differentiation is inhibited by retinoids. Biochem. Biophys. Res. Commun. 1980, 95, 1839–1845. [Google Scholar] [CrossRef]
- Mercader, J.; Madsen, L.; Felipe, F.; Palou, A.; Kristiansen, K.; Bonet, M.L. All-trans retinoic acid increases oxidative metabolism in mature adipocytes. Cell. Physiol. Biochem. 2007, 20, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; DeSantis, D.; Soltanian, H.; Croniger, C.M.; Noy, N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes 2012, 61, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- A, I.J.; Tan, N.S.; Gelman, L.; Kersten, S.; Seydoux, J.; Xu, J.; Metzger, D.; Canaple, L.; Chambon, P.; Wahli, W.; et al. In vivo activation of PPAR target genes by RXR homodimers. EMBO J 2004, 23, 2083–2091. [Google Scholar]
- Agarwal, V.R.; Bischoff, E.D.; Hermann, T.; Lamph, W.W. Induction of adipocyte-specific gene expression is correlated with mammary tumor regression by the retinoid X receptor-ligand lgd1069 (targretin). Cancer Res. 2000, 60, 6033–6038. [Google Scholar] [PubMed]
- Sato, M.; Yajima, Y.; Kawashima, S.; Tanaka, K.; Kagechika, H. Synergistic potentiation of thiazolidinedione-induced ST 13 preadipocyte differentiation by RAR synergists. Biochem. Biophys. Res. Commun. 2001, 280, 646–651. [Google Scholar] [CrossRef] [PubMed]
- van Zoelen, E.J.; Duarte, I.; Hendriks, J.M.; van der Woning, S.P. TGFβ-induced switch from adipogenic to osteogenic differentiation of human mesenchymal stem cells: Identification of drug targets for prevention of fat cell differentiation. Stem Cell. Res. Ther. 2016, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Petruschke, T.; Rohrig, K.; Hauner, H. Transforming growth factor β (TGFβ) inhibits the differentiation of human adipocyte precursor cells in primary culture. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 532–536. [Google Scholar] [PubMed]
- Choy, L.; Skillington, J.; Derynck, R. Roles of autocrine TGFβ receptor and SMAD signaling in adipocyte differentiation. J. Cell Biol. 2000, 149, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Santibanez, J.F.; Kocic, J. Transforming growth factor-beta superfamily, implications in development and differentiation of stem cells. Biomol Concepts 2012, 3, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Glowacki, J.; Zhou, S. Inhibition of adipocytogenesis by canonical Wnt signaling in human mesenchymal stem cells. Exp. Cell Res. 2011, 317, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Smith, U. Activation of canonical wingless-type MMTV integration site family (Wnt) signaling in mature adipocytes increases β-catenin levels and leads to cell dedifferentiation and insulin resistance. J. Biol. Chem. 2010, 285, 14031–14041. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, M.; Koyanagi, A.; Osada, S.; Imagawa, M. Wnt4 and Wnt5a promote adipocyte differentiation. FEBS Lett. 2008, 582, 3201–3205. [Google Scholar] [CrossRef] [PubMed]
- van Tienen, F.H.; Laeremans, H.; van der Kallen, C.J.; Smeets, H.J. Wnt5b stimulates adipogenesis by activating PPARγ, and inhibiting the β-catenin dependent Wnt signaling pathway together with Wnt5a. Biochem. Biophys. Res. Commun. 2009, 387, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Marchildon, F.; St-Louis, C.; Akter, R.; Roodman, V.; Wiper-Bergeron, N.L. Transcription factor smad3 is required for the inhibition of adipogenesis by retinoic acid. J. Biol. Chem. 2010, 285, 13274–13284. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.C.; Schwarz, E.J.; Chawla, A.; Lazar, M.A. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPARγ. Mol. Cell. Biol. 1996, 16, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rojas, P.; Antaramian, A.; Gonzalez-Davalos, L.; Villarroya, F.; Shimada, A.; Varela-Echavarria, A.; Mora, O. Induction of peroxisomal proliferator-activated receptor γ and peroxisomal proliferator-activated receptor γ coactivator 1 by unsaturated fatty acids, retinoic acid, and carotenoids in preadipocytes obtained from bovine white adipose tissue 1,2. J. Anim. Sci. 2010, 88, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, F.; Paquin, J. Differential effects of retinoids and inhibitors of ERK and p38 signaling on adipogenic and myogenic differentiation of p19 stem cells. Stem Cells Dev. 2013, 22, 2003–2016. [Google Scholar] [CrossRef] [PubMed]
- Sagara, C.; Takahashi, K.; Kagechika, H.; Takahashi, N. Molecular mechanism of 9-cis-retinoic acid inhibition of adipogenesis in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2013, 433, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.J.; Reginato, M.J.; Shao, D.; Krakow, S.L.; Lazar, M.A. Retinoic acid blocks adipogenesis by inhibiting C/EBPβ-mediated transcription. Mol. Cell. Biol 1997, 17, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Boruk, M.; Savory, J.G.; Hache, R.J. Af-2-dependent potentiation of ccaat enhancer binding protein beta-mediated transcriptional activation by glucocorticoid receptor. Mol. Endocrinol. 1998, 12, 1749–1763. [Google Scholar] [PubMed]
- Ayala-Sumuano, J.T.; Velez-DelValle, C.; Marsch-Moreno, M.; Beltran-Langarica, A.; Hernandez-Mosqueira, C.; Kuri-Harcuch, W. Retinoic acid inhibits adipogenesis modulating C/EBPβ phosphorylation and down regulating SREBF1A expression. J. Cell. Biochem. 2016, 117, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Nanduri, R.; Dkhar, H.K.; Bhagyaraj, E.; Rao, A.; Gupta, P. Nuclear MEK1 sequesters PPARγ and bisects MEK1/ERK signaling: A non-canonical pathway of retinoic acid inhibition of adipocyte differentiation. PLoS ONE 2014, 9, e100862. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.C.; Wdziekonski, B.; Villageois, P.; Vernochet, C.; Iehle, C.; Billon, N.; Dani, C. Commitment of mouse embryonic stem cells to the adipocyte lineage requires retinoic acid receptor beta and active GSK3. Stem Cells Dev. 2009, 18, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Choy, L.; Derynck, R. Transforming growth factor-beta inhibits adipocyte differentiation by smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 2003, 278, 9609–9619. [Google Scholar] [CrossRef] [PubMed]
- Zauberman, A.; Lapter, S.; Zipori, D. Smad proteins suppress ccaat/enhancer-binding protein (C/EBP) beta- and STAT3-mediated transcriptional activation of the haptoglobin promoter. J. Biol. Chem. 2001, 276, 24719–24725. [Google Scholar] [CrossRef] [PubMed]
- Osei-Sarfo, K.; Gudas, L.J. Retinoic acid suppresses the canonical Wnt signaling pathway in embryonic stem cells and activates the noncanonical Wnt signaling pathway. Stem Cells 2014, 32, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Choi, H.R.; Park, A.; Shin, S.M.; Bae, K.H.; Lee, S.C.; Kim, I.C.; Kim, W.K. Retinoic acid inhibits adipogenesis via activation of Wnt signaling pathway in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2013, 434, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, R.; Wang, X.; Huang, F.; Yan, Z.; Nie, M.; Huang, J.; Wang, Y.; Chen, L.; Yin, L.; et al. All-trans retinoic acid modulates bone morphogenic protein 9-induced osteogenesis and adipogenesis of preadipocytes through BMP/SMAD and Wnt/β-catenin signaling pathways. Int. J. Biochem. Cell Biol. 2014, 47, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, R.; Yuasa, T.; Williams, J.A.; Byers, S.W.; Shah, S.; Pacifici, M.; Iwamoto, M.; Enomoto-Iwamoto, M. Wnt/β-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J. Biol. Chem. 2010, 285, 317–327. [Google Scholar] [CrossRef] [PubMed]
- DiRenzo, D.M.; Chaudhary, M.A.; Shi, X.; Franco, S.R.; Zent, J.; Wang, K.; Guo, L.W.; Kent, K.C. A crosstalk between TGF-β/SMAD3 and wnt/β-catenin pathways promotes vascular smooth muscle cell proliferation. Cell Signal. 2016, 28, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ren, T.; Wu, J.; Niu, J. Small concentrations of tgf-beta1 promote proliferation of bone marrow-derived mesenchymal stem cells via activation of wnt/β-catenin pathway. Indian J. Exp. Biol. 2015, 53, 508–513. [Google Scholar] [PubMed]
- Colwell, A.S.; Krummel, T.M.; Longaker, M.T.; Lorenz, H.P. Wnt-4 expression is increased in fibroblasts after TGF-β1 stimulation and during fetal and postnatal wound repair. Plast. Reconstr. Surg. 2006, 117, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Demagny, H.; De Robertis, E.M. SMAD4/DPC4: A barrier against tumor progression driven by RTK/Ras/ERK and Wnt/GSK3 signaling. Mol. Cell. Oncol. 2016, 3, e989133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Eid, K.; Glowacki, J. Cooperation between TGF-β and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J. Bone Miner. Res. 2004, 19, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ward, M.G.; Adeola, O.; Ajuwon, K.M. Regulation of adipocyte differentiation and gene expression-crosstalk between TGFβ and Wnt signaling pathways. Mol. Biol. Rep. 2013, 40, 5237–5245. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Niu, M.; Zhai, X.; Zhou, Q.; Zhou, Y. Β-catenin pathway is required for TGF-β1 inhibition of PPARγ expression in cultured hepatic stellate cells. Pharmacol. Res. 2012, 66, 219–225. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| ACTB | 5′ GCGAGAAGATGACCCAGATCATGT 3′ | 5′ TACCCCTCGTAGATGGGCACA 3′ |
| ADIPOQ | 5′ CATCTCCTCCTCACTTCCATTCTG 3′ | 5′ GCCCTTGAGTCGTGGTTTCCT 3′ |
| FABP4 | 5′ GGATGATAAACTGGTGGTGGAATG 3′ | 5′ CAGAATGTTGTAGAGTTCAATGCGA 3′ |
| LPL | 5′ AGGAAAGGCACCTGCGGTAT 3′ | 5′ ACTTTTGAAACACCCCAAACACT 3′ |
| CEBPB | 5′ CAGGAGAAACTTTAGCGAGTCAGA 3′ | 5′ GGGTGGCCGCTATTAGTGAG 3′ |
| CEBPA | 5′ CCAAGAAGTCGGTGGACAAGAAC 3′ | 5′ CACCTTCTGCTGCGTCTCCA 3′ |
| PPARG | 5′ GGGATGTCTCATAATGCCATCAG 3′ | 5′ GCCCTCGCCTTTGCTTTG 3′ |
| RARA | 5′ GACCTGGTCTTTGCCTTCG 3′ | 5′ CTCCGCTTCCGCACGTAG 3′ |
| RARB | 5′ TGGATTGACCCAAACCGAA 3′ | 5′ GAGGGGGAGGAAGTGGAGAT 3′ |
| RARG | 5′ GCCATCTGCCTCATCTGCG 3′ | 5′ CGGAGGTCGGTGATTTTCATTA 3′ |
| TGFB1 | 5′ CCCACAACGAAATCTATGACAAG 3′ | 5′ GAGGTATCGCCAGGAATTGTTG 3′ |
| TGFB2 | 5′ GCATAAGGAGGCGGGAATC 3′ | 5′ TGGGTTACATCCAACACAAGG 3′ |
| TGFB3 | 5′ CCCCTGAGCCAACGGTGA 3′ | 5′ GTTCGTTGTGCTCCGCCA 3′ |
| SMAD1 | 5′ GCCGAATGCCTTAGTGACAG 3′ | 5′ CTCCCCAGCCCTTCACAA 3′ |
| SMAD2 | 5′ CTGGAGAATAACAGATGGGATGC 3′ | 5′ CCCTGGCTCCTCACTTGGC 3′ |
| SMAD3 | 5′ GTGCTACATAGGTGCTTTGGGC 3′ | 5′ GCGGTCGTGTTGACTAGGTGAA 3′ |
| SMAD4 | 5′ CTTCAGGGGCTTCTAAAACAG 3′ | 5′ TATCAGAGAGGGAAGAGACCAG 3′ |
| SMAD5 | 5′ TGGAGAGGTGTATGCGGAATG 3′ | 5′ TCCCCAACCCTTGACAAAACT 3′ |
| WNT1 | 5′ CGTCCCGTCCCACCGTC 3′ | 5′ AGGCAACAGCGCCCAGAG 3′ |
| WNT2B | 5′ GCTGTGGTCGCACGGCTG 3′ | 5′ ATAGCGTCGCCGCAGGTAAT 3′ |
| WNT4 | 5′ GCACCATGAGTCCCCGCTC 3′ | 5′ GATGCTCCCCACCGACGAC 3′ |
| WNT5A | 5′ TGAACCTGCACAACAACGAG 3′ | 5′ ATCACCCACCTTGCGGAA 3′ |
| WNT5B | 5′ CCAAGACTGGCATCAAGGAAT 3′ | 5′ GTCTCTCGGCTGCCTATCTG 3′ |
| CTNNB1 | 5′ AGGCTACTGTTGGATTGATTCG 3′ | 5′ CGCTGGGTATCCTGATGTGC 3′ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Ma, Y.; Yao, W.; Zhang, X.; Wu, D. Retinoids Regulate Adipogenesis Involving the TGFβ/SMAD and Wnt/β-Catenin Pathways in Human Bone Marrow Mesenchymal Stem Cells. Int. J. Mol. Sci. 2017, 18, 842. https://doi.org/10.3390/ijms18040842
Cao J, Ma Y, Yao W, Zhang X, Wu D. Retinoids Regulate Adipogenesis Involving the TGFβ/SMAD and Wnt/β-Catenin Pathways in Human Bone Marrow Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2017; 18(4):842. https://doi.org/10.3390/ijms18040842
Chicago/Turabian StyleCao, Jun, Yuhong Ma, Weiqi Yao, Xiaoye Zhang, and Dongcheng Wu. 2017. "Retinoids Regulate Adipogenesis Involving the TGFβ/SMAD and Wnt/β-Catenin Pathways in Human Bone Marrow Mesenchymal Stem Cells" International Journal of Molecular Sciences 18, no. 4: 842. https://doi.org/10.3390/ijms18040842





