Silk Fibroin-Alginate-Hydroxyapatite Composite Particles in Bone Tissue Engineering Applications In Vivo
Abstract
:1. Introduction
2. Results
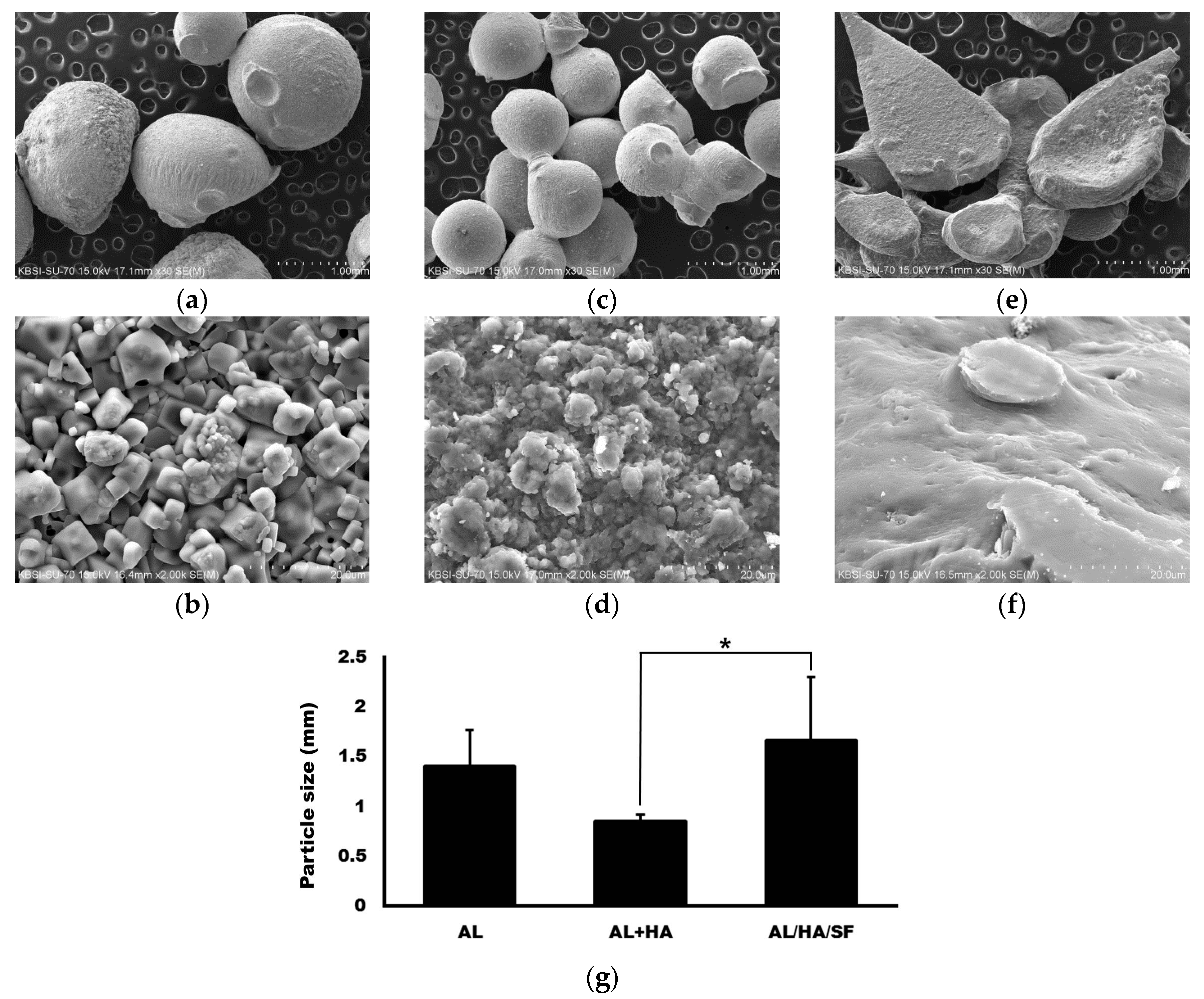
2.1. Morphology ofAlginate (AL), AL/Hydroxyapatite (HA), and AL/HA/Silk Fibroin (SF) Particles in Scanning Electron Microscope (SEM) Images
2.2. Attenuated Total Reflection Fourier Transform Infrared (ATR-FT-IR) Spectroscopy
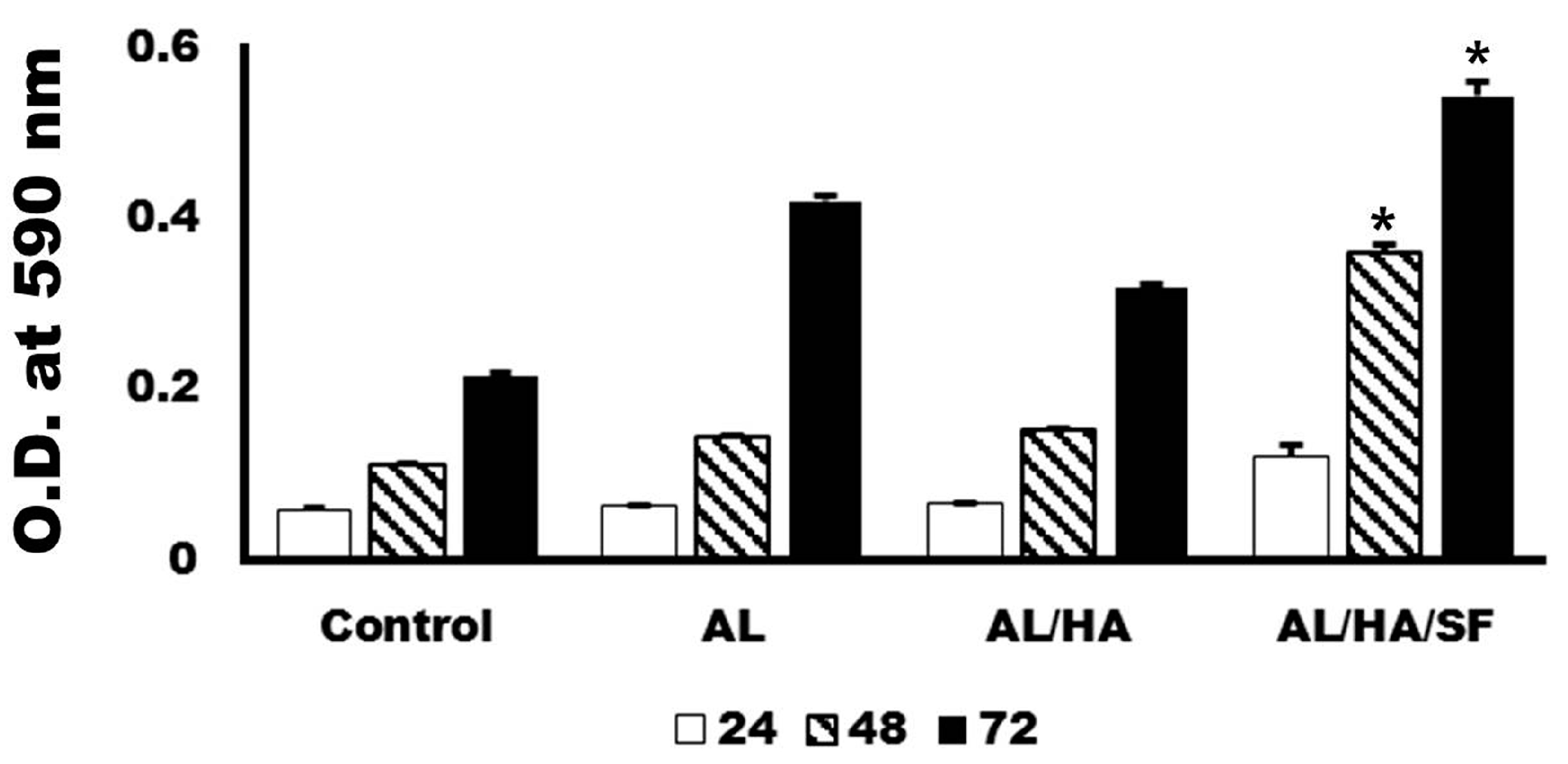
2.3. Analysis of Cytotoxicity
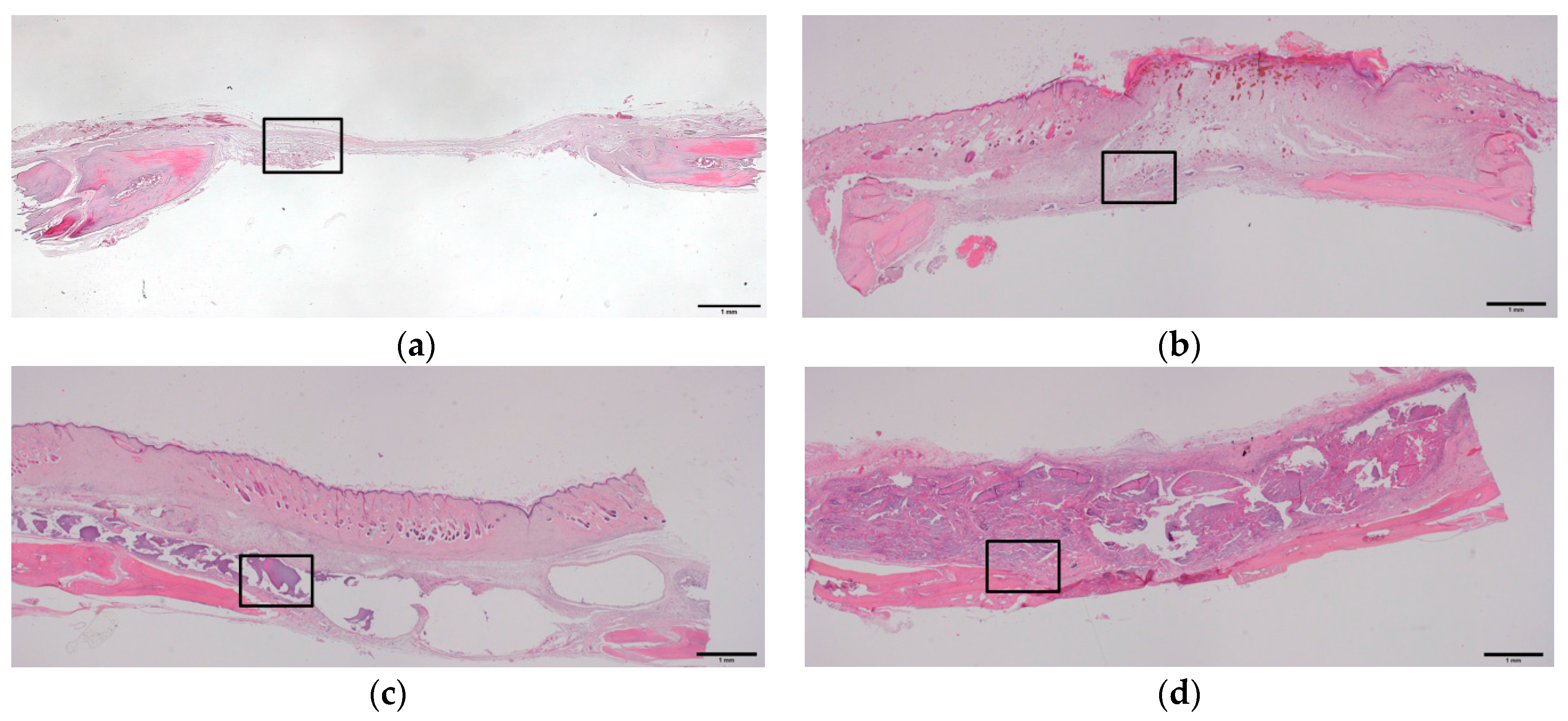
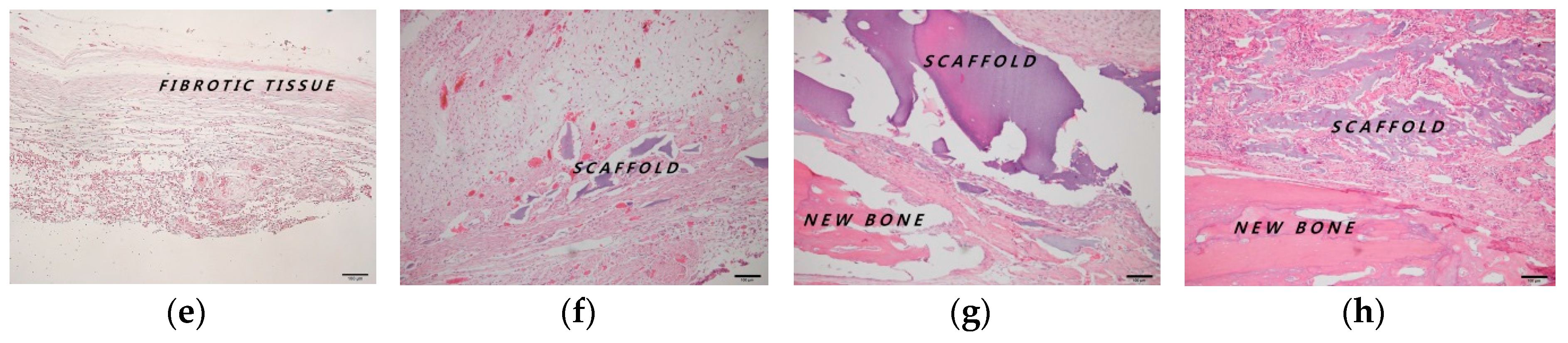
2.4. Histologic and Histomorphometric Evaluation Using Hematoxylin and Eosin Staining
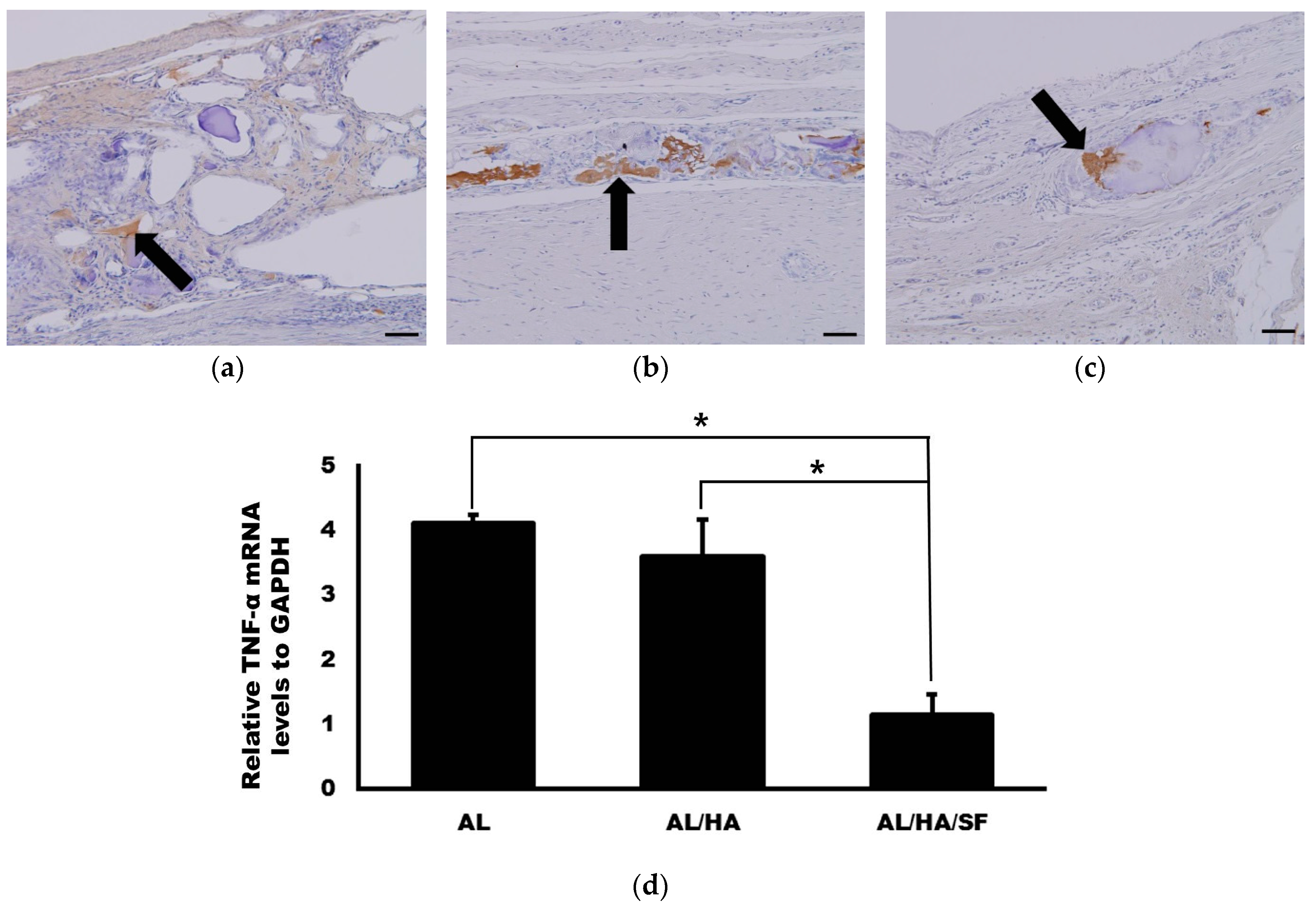
2.5. Analysis of TNF-α Expression
2.6. Analysis of Osteogenic Markers
3. Discussion
4. Materials and Methods
4.1. Preparation of AL, AL/HA, and AL/HA/SF Composites
4.2. Scanning Electron Microscopy (SEM)
4.3. ATR-FT-IR Spectroscopy
4.4. MTT Assay
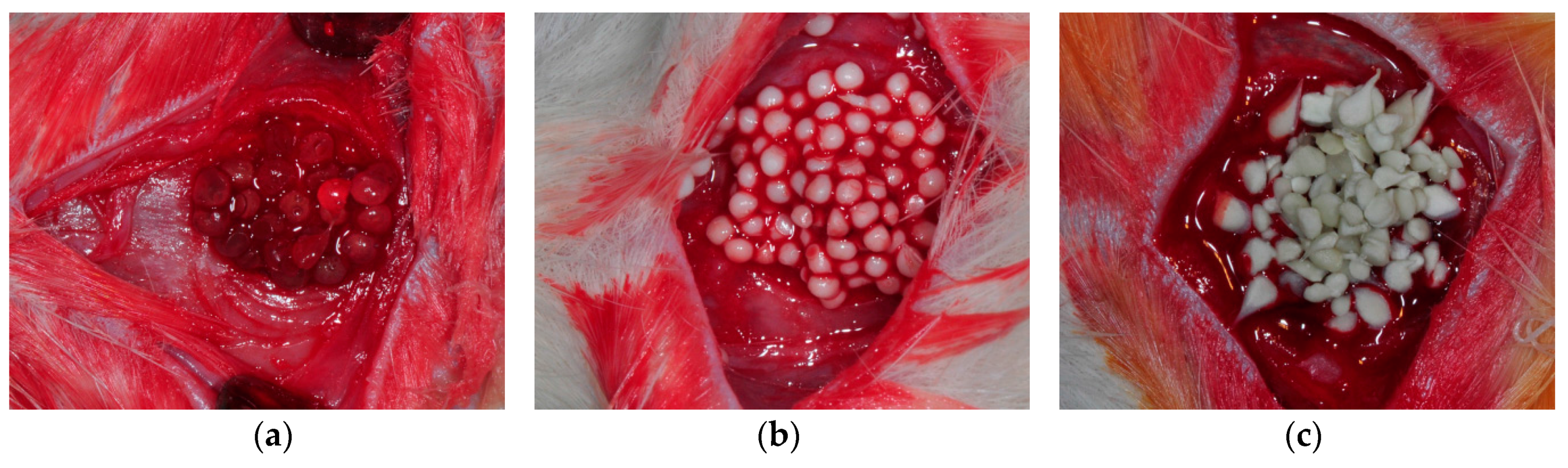
4.5. Animal Experiments
4.6. Histologic and Histomorphometric Evaluation
4.7. Immunohistochemical Evaluation of TNF-α Expression
4.8. qRT-PCR
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koch, J.C. The laws of bone architecture. Am. J. Anat. 1917, 21, 177–198. [Google Scholar] [CrossRef]
- Roesler, H. The history of some fundamental concepts in bone biomechanics. J. Biomech. 1987, 20, 1025–1034. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.G.; Balázsi, C.; Chae, W.S.; Lee, H.O. Comparative study of hydroxyapatite from eggshells and synthetic hydroxyapatite for bone regeneration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Asahina, I.; Watanabe, M.; Sakurai, N.; Mori, M.; Enomoto, S. Repair of bone defect in primate mandible using a bone morphogenetic protein (BMP)-hydroxyapatite-collagen composite. J. Med. Dent. Sci. 1997, 44, 63–70. [Google Scholar] [PubMed]
- Frohbergh, M.E.; Katsman, A.; Mondrinos, M.J.; Stabler, C.T.; Hankenson, K.D.; Oristaglio, J.T.; Lelkes, P.I. Osseointegrative properties of electrospun hydroxyapatite-containing nanofibrous chitosan scaffolds. Tissue Eng. Part A 2015, 21, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Winans, A.M.; Irvine, D.J. Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors. Acta Biomater. 2009, 5, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Tortelli, F; Cancedda, R. Three-dimensional cultures of osteogenic and chondrogenic cells: A tissue engineering approach to mimic bone and cartilage in vitro. Eur. Cells Mater. 2009, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Amirian, J.; Min, Y.K.; Lee, B.T. HAp granules encapsulated oxidized alginate–gelatin–biphasic calcium phosphate hydrogel for bone regeneration. Int. J. Biol. Macromol. 2015, 81, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Johansson, J.; Rising, A. Silk Spinning in Silkworms and Spiders. Int. J. Mol. Sci. 2016, 17, 1290. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Um, I.C.; Kim, S.G.; Cha, M.S. Evaluation of bone formation and membrane degradation in guided bone regeneration using a 4-hexylresorcinol-incorporated silk fabric membrane. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 32. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, M.K.; Kweon, H.; Jo, Y.Y.; Lee, K.G.; Lee, J.K. Comparison of unprocessed silk cocoon and silk cocoon middle layer membranes for guided bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 11. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the HL complex of silk fibroin produced by Bombyx mori. Insect Biochem. Mol. Biol. 1999, 29, 269–276. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6: 6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Che, L.; Ha, Y.; Ryu, W. Mechanically-reinforced electrospun composite silk fibroin nanofibers containing hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2014, 40, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, J.B.; Kim, H.H.; Ki, C.S.; Park, S.Y.; Kim, H.J.; Park, Y.H. Surface coating of hydroxyapatite on silk nanofiber through biomineralization using ten times concentrated simulated body fluid and the evaluation for bone regeneration. Macromol. Res. 2014, 22, 710–716. [Google Scholar] [CrossRef]
- He, P.; Sahoo, S.; Ng, K.S.; Chen, K.; Toh, S.L.; Goh, J.C.H. Enhanced osteoinductivity and osteoconductivity through hydroxyapatite coating of silk-based tissue-engineered ligament scaffold. J. Biomed. Mater. Res. A 2013, 101, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Kim, S.G.; Choi, J.Y. Inhibition of foreign body giant cell formation by 4-hexylresorcinol through suppression of diacylglycerol kinase delta gene expression. Biomaterials 2014, 35, 8576–8584. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.S.; Liu, M.; Zhao, W.; Coatney, D.D.; Li, F.; VanTubergen, E.A.; D’silva, N.J.; Kirkwood, K.L. Targeting mRNA stability arrests inflammatory bone loss. Mol. Ther. 2008, 16, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xia, Q.; Wu, Y.; Zhang, X.; Wen, F.; Chen, X.; Zhang, S.; Heng, B.C.; He, Y.; Ouyang, H.W. 3D-Printed Atsttrin-Incorporated Alginate/Hydroxyapatite Scaffold Promotes Bone Defect Regeneration with TNF/TNFR Signaling Involvement. Adv. Healthc. Mater. 2015, 4, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Hunter, I.; Nixon, G.F. Spatial compartmentalization of tumor necrosis factor (TNF) receptor 1-dependent signaling pathways in human airway smooth muscle cells. Lipid rafts are essential for TNF- α-mediated activation of RhoA but dispensable for the activation of the NF-kappaB and MAPK pathways. J. Biol. Chem. 2006, 281, 34705–34715. [Google Scholar] [PubMed]
- Bhattacharjee, P.; Naskar, D.; Maiti, T.K.; Bhattacharya, D.; Das, P.; Nandi, S.K.; Kundu, S.C. Potential of non-mulberry silk protein fibroin blended and grafted poly (Є-caprolactone) nanofibrous matrices for in vivo bone regeneration. Colloids Surf. B Biointerfaces 2016, 143, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Lamazza, L.; Guerra, F.; Pezza, M.; Messina, A.; Galluccio, A.; Spink, M.; De Biase, A. The use of etanercept as a non-surgical treatment for temporomandibular joint psoriatric arthritis: A case report. Aust. Dent. J. 2009, 54, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Gonciulea, A.; de Beur, S.J. The dynamic skeleton. Rev. Endocr. Metab. Discord. 2015, 16, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Arumugam, B.; Miranda, P.J.; Selvamurugan, N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int. J. Biol. Macromol. 2015, 78, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wenk, E.; Zhang, X.; Meinel, L.; Vunjak-Novakovic, G.; Kaplan, D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control. Release 2009, 134, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, F.; Ye, S.; Wu, Y.; Zhu, K.; Wang, D. Bioactive apatite incorporated alginate microspheres with sustained drug-delivery for bone regeneration application. Mater. Sci. Eng. C 2016, 62, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.G.; Ha, J.H.; Kim, S.G.; Chae, W.S. Spectroscopic determination of alkyl resorcinol concentration in hydroxyapatite composite. J. Anal. Sci. Technol. 2016, 7. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M. Adsorption studies of cationic dyes onto Ashoka (Saraca asoca) leaf powder. J. Taiwan Inst. Chem. 2012, 43, 604–613. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.K.; Jeon, J.Y.; Kim, Y.J.; Kim, S.G.; Hwang, K.G. Cell attachment and proliferation of osteoblast-like MG63 cells on silk fibroin membrane for guided bone regeneration. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 17. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, T.L.; de Molon, R.S.; Paim, P.R.; Marcantonio, E., Jr.; Faeda, R.S. Implant stability after sinus floor augmentation with deproteinized bovine bone mineral particles of different sizes: A prospective, randomized and controlled split-mouth clinical trial. Int. J. Oral Maxillofac. Surg. 2016, 45, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- McNamara, I.R.; Rayment, A.; Brooks, R.; Best, S.; Rushton, N. The effect of the addition of hydroxyapatite graft substitutes upon the hoop strain and subsequent subsidence of a femoral model during impaction bone grafting. J. Mech. Behav. Biomed. Mater. 2012, 5, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yang, Y.; Deng, Y.; Sun, Y.; Yang, H.; Wei, S. Peptide-incorporated 3D porous alginate scaffolds with enhanced osteogenesis for bone tissue engineering. Colloids Surf. B Biointerfaces 2016, 143, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Lee, S.W.; Hahn, B.D.; Lee, Y.C.; Kim, S.G. Hydroxyapatite and silk combination-coated dental implants result in superior bone formation in the peri-implant area compared with hydroxyapatite and collagen combination-coated implants. J. Oral Maxillofac. Surg. 2014, 72, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, J.Y.; Jeong, J.H.; Jang, E.S.; Kim, A.S.; Kim, S.G.; Kweon, H.Y.; Jo, Y.Y.; Yeo, J.H. Low molecular weight silk fibroin increases alkaline phosphatase and type I collagen expression in MG63 cells. BMB Rep. 2010, 43, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Wongputtaraksa, T.; Ratanavaraporn, J.; Pichyangkura, R.; Damrongsakkul, S. Surface modification of Thai silk fibroin scaffolds with gelatin and chitooligosaccharide for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.R.; Song, N.J.; Yang, D.K.; Cho, Y.J.; Kim, B.J.; Hong, J.W.; Yun, U.J.; Jo, D.G.; Lee, Y.M.; Choi, S.Y. Silk proteins stimulate osteoblast differentiation by suppressing the Notch signaling pathway in mesenchymal stem cells. Nutr. Res. 2013, 33, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Park, Y.T.; Kim, S.G.; Jin, H.J. The effect of silk fibroin particles coated with hydroxyapatites on bone regeneration in the rat calvarial defect model. Maxillofac. Plast. Reconstr. Surg. 2013, 35, 13–17. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, S.G.; Lee, J.W.; Chae, W.S.; Kweon, H.; Jo, Y.Y.; Lee, K.G.; Lee, Y.C.; Choi, J.Y.; Kim, J.Y. Accelerated healing with the use of a silk fibroin membrane for the guided bone regeneration technique. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, e26–e33. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.j.; Kim, J.H.; Song, J.M.; Yoon, S.Y.; Kim, H.S.; Shin, S.H. Chitin-fibroin-hydroxyapatite membrane for guided bone regeneration: Micro-computed tomography evaluation in a rat model. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Eum, W.S.; Jang, S.H.; Park, J.; Heo, D.H.; Sheen, S.H.; Lee, H.R.; Kweon, H.; Kang, S.W.; Lee, K.G. A transparent artificial dura mater made of silk fibroin as an inhibitor of inflammation in craniotomized rats: Laboratory investigation. J. Neurosurg. 2011, 114, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, C.; Luo, T.; Li, S.; Cheng, X.; Miron, R.J. Synthesis and inflammatory response of a novel silk fibroin scaffold containing BMP7 adenovirus for bone regeneration. Bone 2012, 51, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fan, J.-B.; Xu, D.W.; Zhang, J.; Cui, Z.M. Epigallocatechin-3-gallate protects against tumor necrosis factor α induced inhibition of osteogenesis of mesenchymal stem cells. Exp. Biol. Med. 2016, 241, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.T. The role of acquired immunity and periodontal disease progression. Crit. Rev. Oral Biol. Med. 2003, 14, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Algate, K.; Haynes, D.R.; Bartold, P.M.; Crotti, T.N.; Cantley, M.D. The effects of tumour necrosis factor-α on bone cells involved in periodontal alveolar bone loss; osteoclasts, osteoblasts and osteocytes. J. Periodontal Res. 2015, 51, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Noh, J.E.; Lee, M.J.; Chae, W.S.; Lee, S.Y.; Kim, S.G. The effect of 4-hexylresorcinol on xenograft degradation in a rat calvarial defect model. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 29. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Harding, K.; Moore, K. Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-α. Biomaterials 2000, 21, 1797–1802. [Google Scholar] [CrossRef]
- Acharya, C.; Ghosh, S.K.; Kundu, S. Silk fibroin protein from mulberry and non-mulberry silkworms: Cytotoxicity, biocompatibility and kinetics of L929 murine fibroblast adhesion. J. Mater. Sci. Mater. Med. 2008, 19, 2827–2836. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Lian, M.; Shen, Q.; Feng, G.; Huang, D.; Lu, X.; Gu, Z.; Li, L.; Zhang, J.; Huang, S. AGS3 is involved in TNF-α medicated osteogenic differentiation of human dental pulp stem cells. Differentiation 2015, 89, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, S.Y.; Lee, S.J.; Boo, Y.C.; Choi, J.Y.; Kim, J.E. Ucma, a direct transcriptional target of Runx2 and Osterix, promotes osteoblast differentiation and nodule formation. Osteoarthr. Cartil. 2015, 23, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RAN KL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Minamizaki, T.; Yoshiko, Y.; Yoshioka, H.; Tanne, K.; Aubin, J.E.; Maeda, N. 1 α,25-dihydroxyvitamin D3 acts predominately in mature osteoblasts under conditions of high extracellular phosphate to increase fibroblast growth factor 23 production in vitro. J. Endocrinol. 2010, 206, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Li, W.; Su, X.; Li, G.; Zhou, Y.; Kundu, S.C.; Yao, J.; Cai, Y. Degradation pattern of porous CaCO3 and hydroxyapatite microspheres in vitro and in vivo for potential application in bone tissue engineering. Colloids Surf. B Biointerfaces 2016, 143, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Kundu, S.C. Calcium alginate beads embedded in silk fibroin as 3D dual drug releasing scaffolds. Biomaterials 2009, 30, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- Peinnequin, A.; Mouret, C.; Birot, O.; Alonso, A.; Mathieu, J.; Clarençon, D.; Agay, D.; Chancerelle, Y.; Multon, E. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004, 5, 3. [Google Scholar] [CrossRef] [PubMed]



| Post-Implantation | 4 Weeks | |||
|---|---|---|---|---|
| Group | Control | AL | AL/HA | AL/HA/SF |
| Total new bone (%) | 13.87 ± 4.98 | 14.32 ± 6.39 | 28.60 ± 12.57 | 30.93 ± 11.05 |
| Residual graft (%) | 25.38 ± 13.55 | 31.85 ± 9.98 | 33.93 ± 16.45 | |
| Post-Implantation | 8 Weeks | |||
| Group | Control | AL | AL/HA | AL/HA/SF |
| Total new bone (%) | 23.85 ± 2.21 | 27.00 ± 11.59 | 33.25 ± 5.69 | 39.46 ± 12.92 |
| Residual graft (%) | 20.75 ± 11.70 | 29.25 ± 8.89 | 25.12 ± 0.83 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, Y.-Y.; Kim, S.-G.; Kwon, K.-J.; Kweon, H.; Chae, W.-S.; Yang, W.-G.; Lee, E.-Y.; Seok, H. Silk Fibroin-Alginate-Hydroxyapatite Composite Particles in Bone Tissue Engineering Applications In Vivo. Int. J. Mol. Sci. 2017, 18, 858. https://doi.org/10.3390/ijms18040858
Jo Y-Y, Kim S-G, Kwon K-J, Kweon H, Chae W-S, Yang W-G, Lee E-Y, Seok H. Silk Fibroin-Alginate-Hydroxyapatite Composite Particles in Bone Tissue Engineering Applications In Vivo. International Journal of Molecular Sciences. 2017; 18(4):858. https://doi.org/10.3390/ijms18040858
Chicago/Turabian StyleJo, You-Young, Seong-Gon Kim, Kwang-Jun Kwon, HaeYong Kweon, Weon-Sik Chae, Won-Geun Yang, Eun-Young Lee, and Hyun Seok. 2017. "Silk Fibroin-Alginate-Hydroxyapatite Composite Particles in Bone Tissue Engineering Applications In Vivo" International Journal of Molecular Sciences 18, no. 4: 858. https://doi.org/10.3390/ijms18040858






