Studying Lactoferrin N-Glycosylation
Abstract
:1. Introduction
2. Characteristics of Lactoferrin
3. Biological Roles of Lactoferrin
4. Lactoferrin Sources
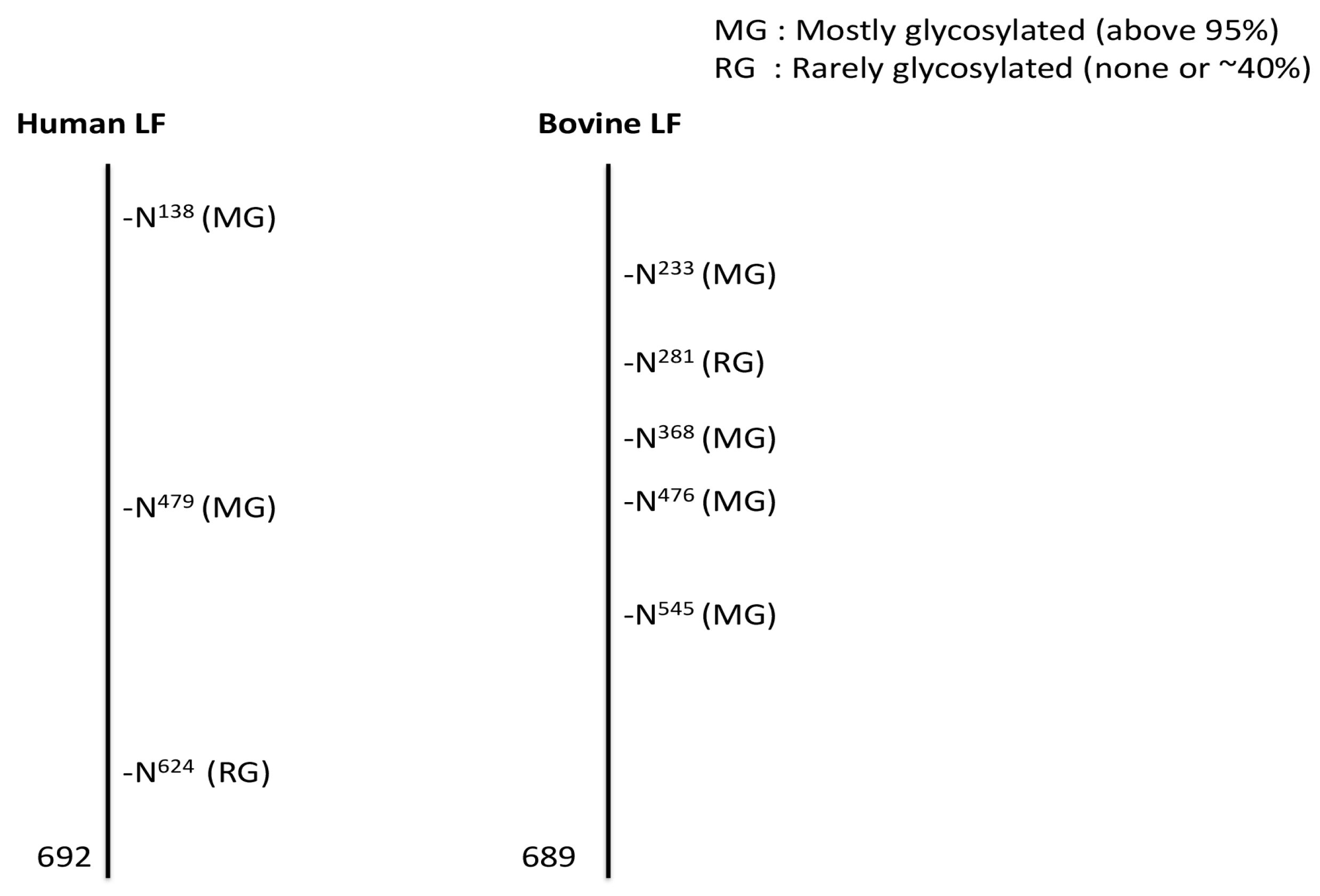
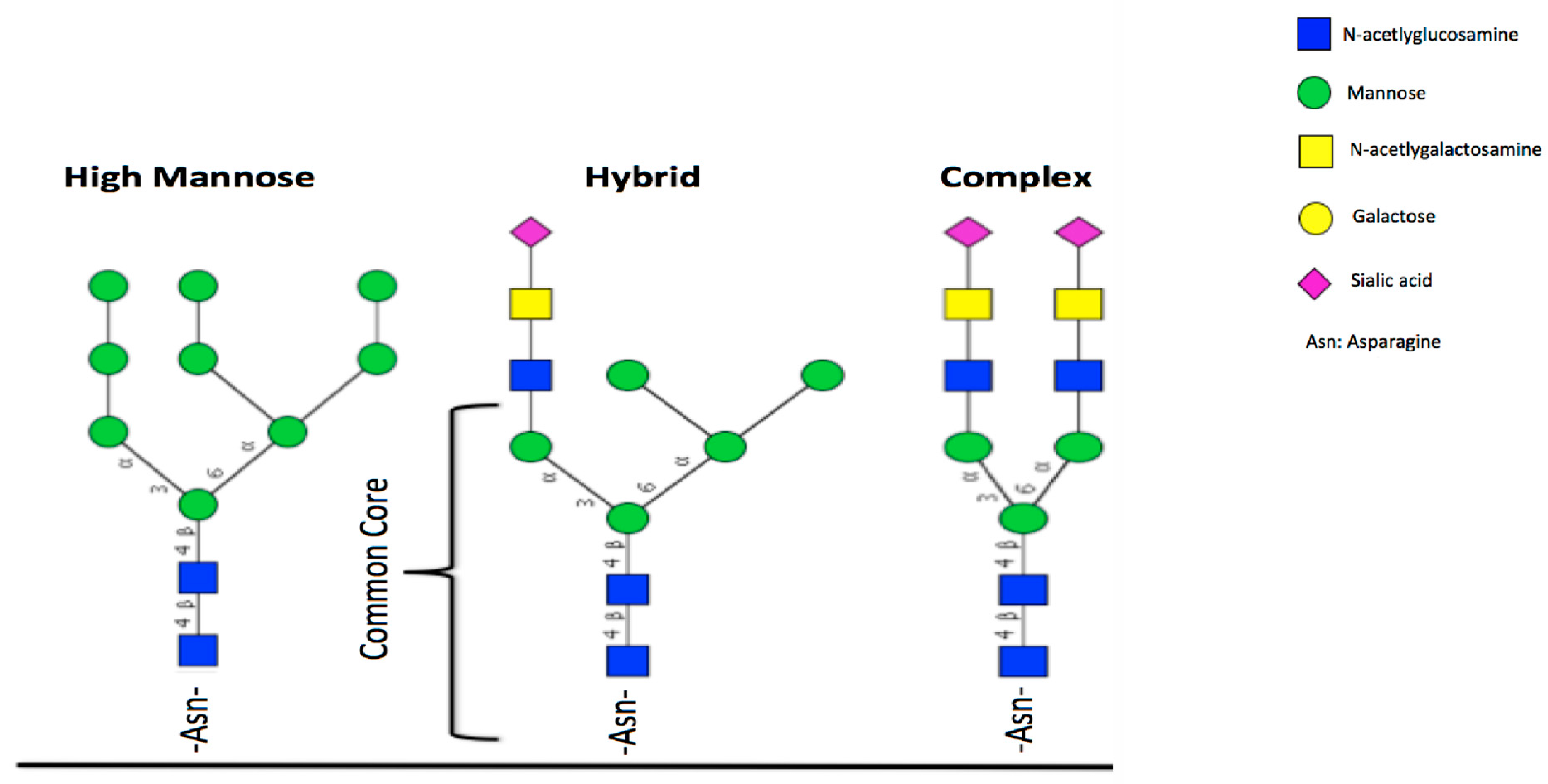
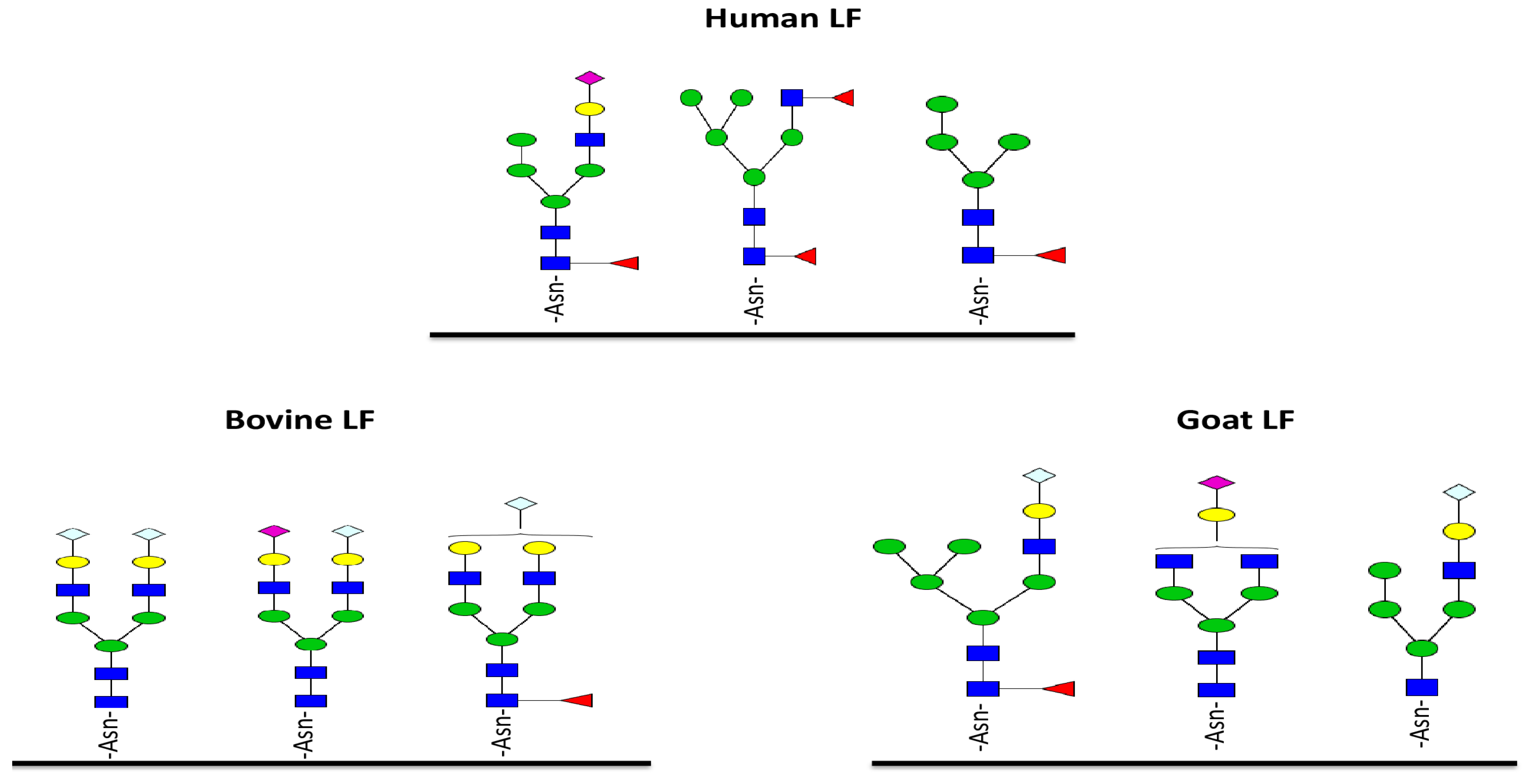
5. Glycosylation of Lactoferrin
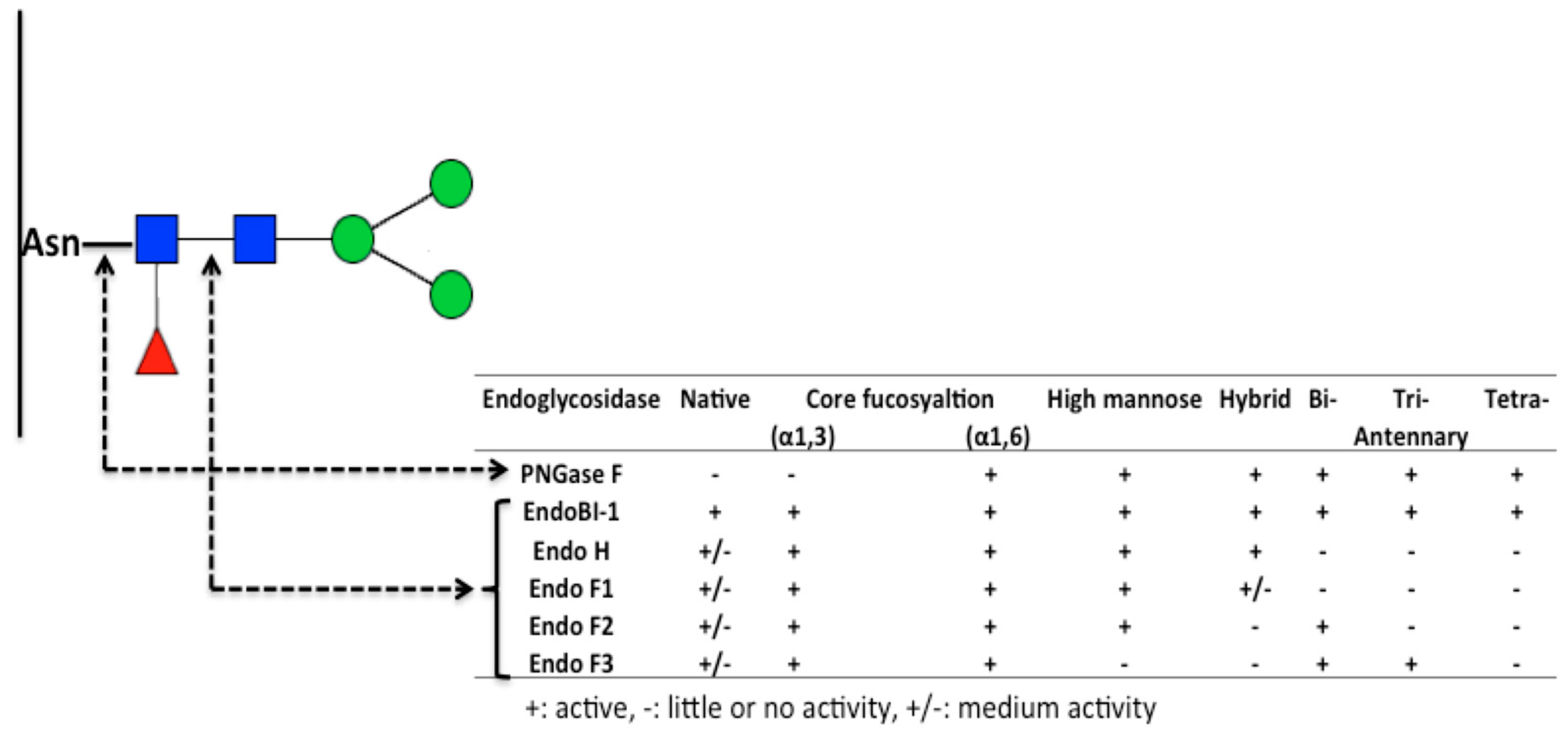
6. Deglycosylation Strategies to Study Protein Glycans
7. Analytical Characterization of N-Glycans by Mass Spectrometry
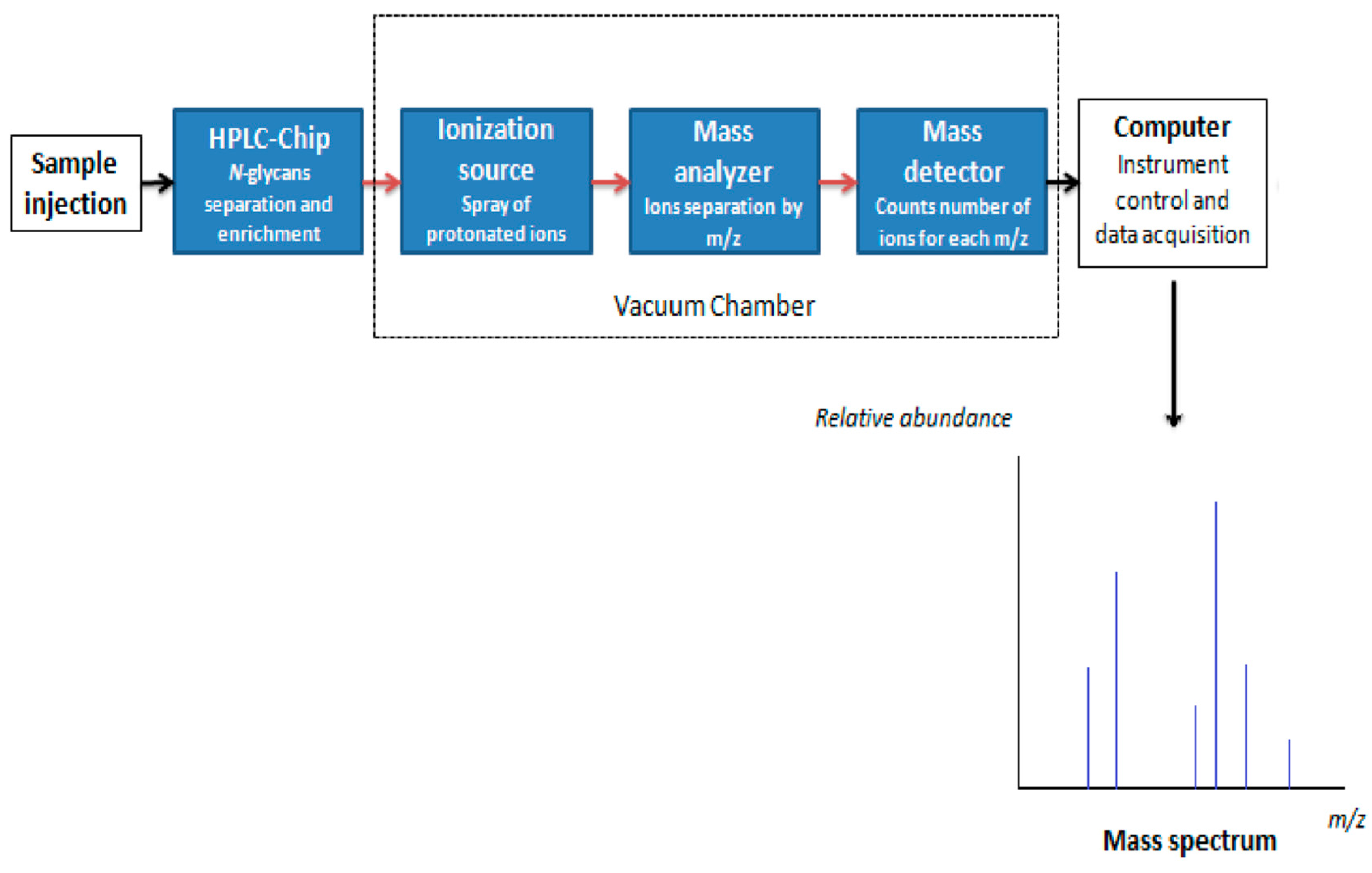
7.1. N-Glycan Separation Using HLPC
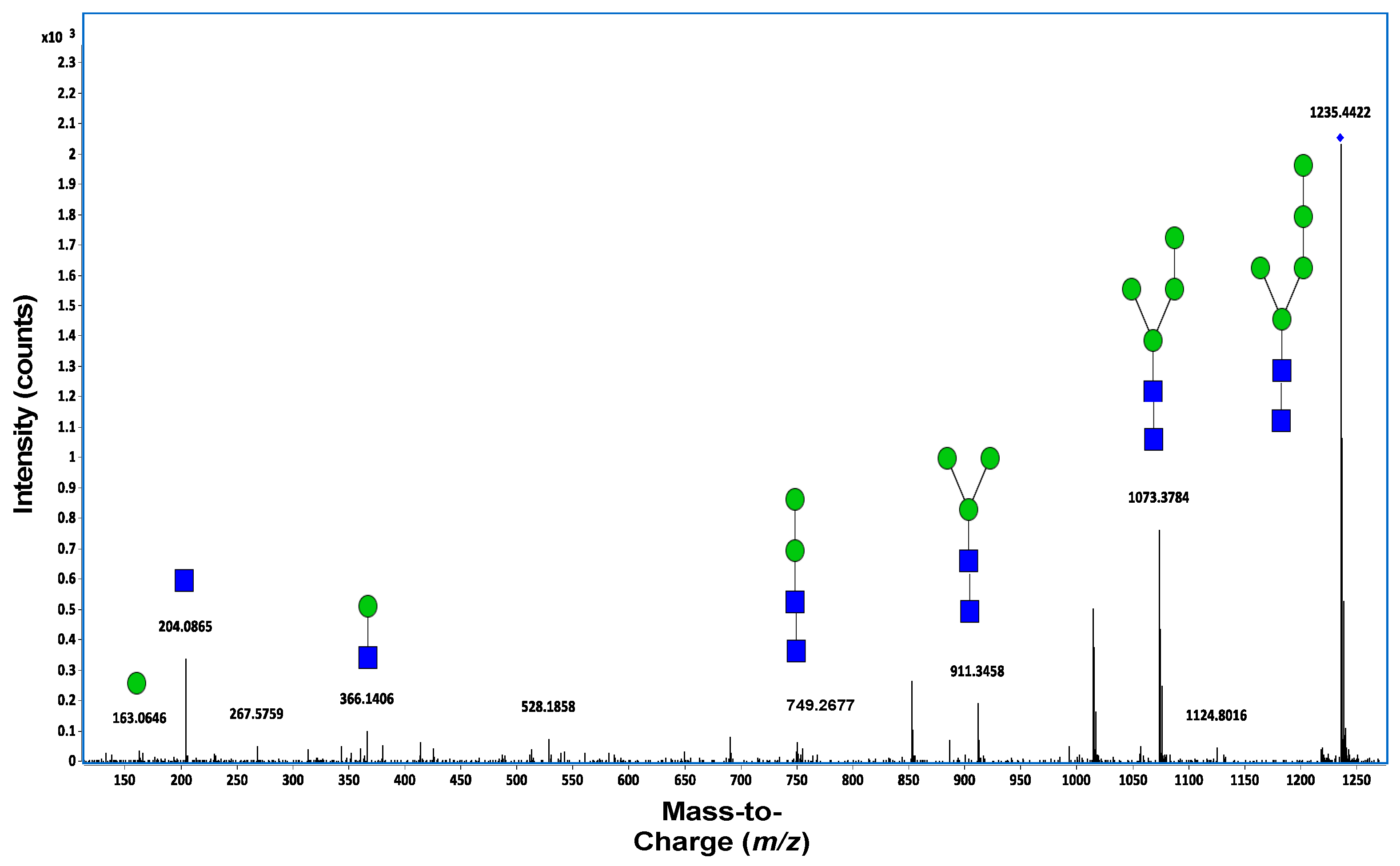
7.2. Mass Spectrometry
7.3. Tandem Mass Spectrometry (MS/MS)
8. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| Asn | Asparagine |
| bLF | Bovine lactoferrin |
| CID | Collision-induced dissociation |
| ESI | Electrospray ionization |
| Fuc | Fucose |
| GlcNAc | N-acetylglucosamine |
| Hex | Hexose |
| hLF | Human lactoferrin |
| HPLC-MS | High-pressure liquid chromatography coupled to mass spectrometry |
| MS | Mass spectrometry |
| MS | Mass spectrometry |
| NeuAC | Sialic acid |
| NeuGc | N-glycolylneuraminic acid |
| QTOF | Quadrupole time-of-flight |
| rhLF | Recombinant human lactoferrin |
References
- Sorensen, M.; Sorensen, S. The Proteins in Whey. In Compte rendu des Travaux du Laboratoire de Carlsberg; Hagerup in Komm: Copenhague, Denmark, 1939; Volume 23, pp. 55–99. [Google Scholar]
- Johanson, B. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Iyer, S.; Lonnerdal, B. Lactoferrin, lactoferrin receptors and iron metabolism. Eur. J. Clin. Nutr. 1993, 47, 232–241. [Google Scholar] [PubMed]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar] [PubMed]
- Steijns, J.M. Milk ingredients as nutraceuticals. Int. J. Dairy Technol. 2001, 54, 81–88. [Google Scholar] [CrossRef]
- Hirai, Y.; Kawakata, N.; Satoh, K.; Ikeda, Y.; Hisayasu, S.; Orimo, H.; Yoshino, Y. Concentrations of lactoferrin and iron in human milk at different stages of lactation. J. Nutr. Sci. Vitaminol. 1990, 36, 531. [Google Scholar] [CrossRef] [PubMed]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Heremans, J. Lactoferrin in milk from different species. Comp. Biochem. Physiol. Part B Comp. Biochem. 1971, 39, 119–129. [Google Scholar] [CrossRef]
- Tomita, M.; Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J. Dairy Sci. 1991, 74, 4137–4142. [Google Scholar] [CrossRef]
- Teraguchi, S.; Wakabayashi, H.; Kuwata, H.; Yamauchi, K.; Tamura, Y. Protection against infections by oral lactoferrin: Evaluation in animal models. Biometals 2004, 17, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Banno, Y.; Kato, Y.; Nozawa, Y.; Kawaguchi, M. Pepsin-digested bovine lactoferrin induces apoptotic cell death with JNK/SAPK activation in oral cancer cells. J. Pharmacol. Sci. 2005, 98, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Reghunathan, R.; Jayapal, M.; Hsu, L.-Y.; Chng, H.-H.; Tai, D.; Leung, B.P.; Melendez, A.J. Expression profile of immune response genes in patients with severe acute respiratory syndrome. BMC Immunol. 2005, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Medina, I.; Tombo, I.; Satué-Gracia, M.T.; German, J.B.; Frankel, E.N. Effects of natural phenolic compounds on the antioxidant activity of lactoferrin in liposomes and oil-in-water emulsions. J. Agric. Food Chem. 2002, 50, 2392–2399. [Google Scholar] [CrossRef] [PubMed]
- Adlerova, L.; Bartoskova, A.; Faldyna, M. Lactoferrin: A review. Vet. Med. 2008, 53, 457–468. [Google Scholar]
- Wei, Z.; Nishimura, T.; Yoshida, S. Characterization of glycans in a lactoferrin isoform, lactoferrin-a. J. Dairy Sci. 2001, 84, 2584–2590. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Totten, S.M.; Huang, J.; Grapov, D.; Durham, H.A.; Lammi-Keefe, C.J.; Lebrilla, C.; German, J.B. Human milk secretory immunoglobulin a and lactoferrin N-glycans are altered in women with gestational diabetes mellitus. J. Nutr. 2013, 143, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Zinger-Yosovich, K.D.; Sudakevitz, D.; Iluz, D.; Gilboa-Garber, N. Analyses of diverse mammals’ milk and lactoferrin glycans using five pathogenic bacterial lectins. Food Chem. 2011, 124, 1335–1342. [Google Scholar] [CrossRef]
- Spik, G.; Coddeville, B.; Mazurier, J.; Bourne, Y.; Cambillaut, C.; Montreuil, J. Primary and three-dimensional structure of lactotransferrin (lactoferrin) glycans. In Lactoferrin; Springer: Berlin, Germany, 1994; pp. 21–32. [Google Scholar]
- Thomassen, E.A.; van Veen, H.A.; van Berkel, P.H.; Nuijens, J.H.; Abrahams, J.P. The protein structure of recombinant human lactoferrin produced in the milk of transgenic cows closely matches the structure of human milk-derived lactoferrin. Transgenic Res. 2005, 14, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, G.; Slavov, S.; Gecheff, K.; Vlahova, M.; Atanassov, A. Expression of recombinant human lactoferrin in transgenic alfalfa plants. Biol. Plant. 2013, 57, 457–464. [Google Scholar] [CrossRef]
- Naidu, A. Activated lactoferrin—A new approach to meat safety. Food Technol. 2002, 56, 40–45. [Google Scholar]
- Marnila, P.; Korhonen, H. Lactoferrin for Human Health; Woodhead: Oxford, UK, 2009. [Google Scholar]
- Metz-Boutigue, M.H.; Jollés, J.; Mazurier, J.; Schoentgen, F.; Legrand, D.; Spik, G.; Montreuil, J.; Jollès, P. Human lactotransferrin: Amino acid sequence and structural comparisons with other transferrins. Eur. J. Biochem. 1984, 145, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Querinjean, P.; Masson, P.L.; Heremans, J.F. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur. J. Biochem. 1971, 20, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Bluard-Deconinck, J.-M.; Masson, P.L.; Osinski, P.A.; Heremans, J.F. Amino acid sequence of cysteic peptides of lactoferrin and demonstration of similarities between lactoferrin and transferrin. Biochim. Biophys. Acta BBA Protein Struct. 1974, 365, 311–317. [Google Scholar] [CrossRef]
- Furmanski, P.; Li, Z.; Fortuna, M.B.; Swamy, C.; Das, M.R. Multiple molecular forms of human lactoferrin. Identification of a class of lactoferrins that possess ribonuclease activity and lack iron-binding capacity. J. Exp. Med. 1989, 170, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Baker, E. Structure and reactivity of transferrins. Adv. Inorg. Chem. 1994, 41, 389–463. [Google Scholar]
- Brock, J.; Arzabe, F.; Lampreave, F.; Pineiro, A. The effect of trypsin on bovine transferrin and lactoferrin. Biochim. Biophys. Acta BBA Protein Struct. 1976, 446, 214–225. [Google Scholar] [CrossRef]
- Brines, R.; Brock, J. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum: Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim. Biophys. Acta BBA Gen. Subj. 1983, 759, 229–235. [Google Scholar] [CrossRef]
- Van Veen, H.A.; Geerts, M.E.; van Berkel, P.H.; Nuijens, J.H. The role of n-linked glycosylation in the protection of human and bovine lactoferrin against tryptic proteolysis. Eur. J. Biochem. 2004, 271, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Saito, H.; Miyakawa, H.; Tamura, Y.; Shimamura, S.; Nagao, E.; Tomita, M. Heat stability of bovine lactoferrin at acidic ph. J. Dairy Sci. 1991, 74, 65–71. [Google Scholar] [CrossRef]
- Oria, R.; Ismail, M.; Sánchez, L.; Calvo, M.; Brock, J.H. Effect of heat treatment and other milk proteins on the interaction of lactoferrin with monocytes. J. Dairy Res. 1993, 60, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Brisson, G.; Britten, M.; Pouliot, Y. Effect of iron saturation on the recovery of lactoferrin in rennet whey coming from heat-treated skim milk. J. Dairy Sci. 2007, 90, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hendrixson, D.R.; Baker, E.N.; Murphy, T.F.; Geme, J.W.S.; Plaut, A.G. Human milk lactoferrin inactivates two putative colonization factors expressed by haemophilus influenzae. Proc. Natl. Acad. Sci. USA 1998, 95, 12641–12646. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Interactions of lactoferrin with cells involved in immune function this paper is one of a selection of papers published in this special issue, entitled 7th international conference on lactoferrin: Structure, function, and applications, and has undergone the journal‘s usual peer review process. Biochem. Cell Biol. 2006, 84, 282–290. [Google Scholar] [PubMed]
- Kane, S.V.; Sandborn, W.J.; Rufo, P.A.; Zholudev, A.; Boone, J.; Lyerly, D.; Camilleri, M.; Hanauer, S.B. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am. J. Gastroenterol. 2003, 98, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Bezault, J.; Bhimani, R.; Wiprovnick, J.; Furmanski, P. Human lactoferrin inhibits growth of solid tumors and development of experimental metastases in mice. Cancer Res. 1994, 54, 2310–2312. [Google Scholar] [PubMed]
- Kirkpatrick, C.H.; Green, I.; Rich, R.R.; Schade, A.L. Inhibition of growth of candida albicans by iron-unsaturated lactoferrin: Relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infect. Dis. 1971, 124, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Bennett, S.H.; Hwang, F.F.; Yu, C. Neonatal small bowel epithelia: Enhancing anti-bacterial defense with lactoferrin and lactobacillus gg. Biometals 2004, 17, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Petschow, B.; Talbott, R.; Batema, R. Ability of lactoferrin to promote the growth of bifidobacterium spp. In vitro is independent of receptor binding capacity and iron saturation level. J. Med. Microbiol. 1999, 48, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; Le Parc, A.; de Moura, J.M.L.N.; Frese, S.A.; Kirmiz, N.; Block, D.E.; Barile, D.; Mills, D.A. Oligosaccharides released from milk glycoproteins are selective growth substrates for infant-associated bifidobacteria. Appl. Environ. Microbiol. 2016, 82, 3622–3630. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.H. The physiology of lactoferrin. Biochem. Cell. Biol. 2002, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, P.H.; Welling, M.M.; Geerts, M.; van Veen, H.A.; Ravensbergen, B.; Salaheddine, M.; Pauwels, E.K.; Pieper, F.; Nuijens, J.H.; Nibbering, P.H. Large scale production of recombinant human lactoferrin in the milk of transgenic cows. Nat. Biotechnol. 2002, 20, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, J.; Gong, G.; Sun, X.; Zhang, R.; Du, Z.; Liu, Y.; Li, R.; Ding, F.; Tang, B. Cattle mammary bioreactor generated by a novel procedure of transgenic cloning for large-scale production of functional human lactoferrin. PLoS ONE 2008, 3, e3453. [Google Scholar] [CrossRef] [PubMed]
- Conesa, C.; Calvo, M.; Sánchez, L. Recombinant human lactoferrin: A valuable protein for pharmaceutical products and functional foods. Biotechnol. Adv. 2010, 28, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, C.; Wang, J.; Hao, P.; Sui, S.; Chen, X.; Zhang, R.; Wang, P.; Yu, G.; Zhang, L. Comprehensive characterization of the site-specific N-glycosylation of wild-type and recombinant human lactoferrin expressed in the milk of transgenic cloned cattle. Glycobiology 2011, 21, 206–224. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.A.; Maga, E.A.; Murray, J.D. Production of human lactoferrin and lysozyme in the milk of transgenic dairy animals: Past, present, and future. Transgenic Res. 2015, 24, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, J.; Wang, J.; Yu, T.; Wang, J.; Li, N. Transgenic milk containing recombinant human lactoferrin modulates the intestinal flora in piglets 1. This article is part of a special issue entitled lactoferrin and has undergone the journal’s usual peer review process. Biochem. Cell Biol. 2012, 90, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Guo, C.; Sui, S.; Yu, T.; Wang, J.; Li, N. Comprehensive assessment of milk composition in transgenic cloned cattle. PLoS ONE 2012, 7, e49697. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Baker, H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Haridas, M.; Anderson, B.; Baker, E. Structure of human diferric lactoferrin refined at 2.2 Å resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 1995, 51, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional structure of diferric bovine lactoferrin at 2.8 Å resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Freeze, H.H. Glycans in acquired human diseases. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009. [Google Scholar]
- Coddeville, B.; Strecker, G.; Wieruszeski, J.-M.; Vliegenthart, J.F.; van Halbeek, H.; Peter-Katalinić, J.; Egge, H.; Spik, G. Heterogeneity of bovine lactotransferrin glycans. Characterization of α-d-galp-(1→3)-β-d-gal-and α-neuac-(2→6)-β-d-galpnac-(1→4)-β-d-glcnac-substituted N-linked glycans. Carbohydr. Res. 1992, 236, 145–164. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E.; Stanley, P.; Cummings, R.D. Structures common to different glycans. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2009. [Google Scholar]
- Chung, M. Structure and function of transferrin. Biochem. Educ. 1984, 12, 146–154. [Google Scholar] [CrossRef]
- Le Parc, A.; Dallas, D.C.; Duaut, S.; Leonil, J.; Martin, P.; Barile, D. Characterization of goat milk lactoferrin N-glycans and comparison with the N-glycomes of human and bovine milk. Electrophoresis 2014, 35, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Parc, A.L.; Karav, S.; Rouquié, C.; Maga, E.A.; Bunyatratchata, A.; Barile, D. Characterization of recombinant human lactoferrin N-glycans expressed in the milk of transgenic cows. PLoS ONE 2017, 12, e0171477. [Google Scholar] [CrossRef] [PubMed]
- Barboza, M.; Pinzon, J.; Wickramasinghe, S.; Froehlich, J.W.; Moeller, I.; Smilowitz, J.T.; Ruhaak, L.R.; Huang, J.; Lönnerdal, B.; German, J.B. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell. Proteom. 2012, 11, M111.015248. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, C.C.; Aldredge, D.L.; Lee, H.; Lerno, L.A.; Zivkovic, A.M.; German, J.B.; Lebrilla, C.B. Comparison of the human and bovine milk N-glycome via high-performance microfluidic chip liquid chromatography and tandem mass spectrometry. J. Proteome Res. 2012, 11, 2912–2924. [Google Scholar] [CrossRef] [PubMed]
- Kautto, L.; Nguyen-Khuong, T.; Everest-Dass, A.; Leong, A.; Zhao, Z.; Willcox, M.D.; Packer, N.H.; Peterson, R. Glycan involvement in the adhesion of pseudomonas aeruginosa to tears. Exp. Eye Res. 2016, 145, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Wormald, M.R.; Petrescu, A.J.; Pao, Y.-L.; Glithero, A.; Elliott, T.; Dwek, R.A. Conformational studies of oligosaccharides and glycopeptides: Complementarity of NMR, X-ray crystallography, and molecular modelling. Chem. Rev. 2002, 102, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Yehezkel, G.; Khalaila, I. Identification and quantification of protein glycosylation. Int. J. Carbohydr. Chem. 2012, 2012, 640923. [Google Scholar] [CrossRef]
- Turyan, I.; Hronowski, X.; Sosic, Z.; Lyubarskaya, Y. Comparison of two approaches for quantitative o-linked glycan analysis used in characterization of recombinant proteins. Anal. Biochem. 2014, 446, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Bruce, J.; Merry, A.; Bigge, C.; Wormald, M.; Parekh, R.; Jaques, A. Use of hydrazine to release in intact and unreduced form both N-and O-linked oligosaccharides from glycoproteins. Biochemistry 1993, 32, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, G.; Hansen, H. Human health perspective of environmental exposure to hydrazines: A review. Chemosphere 1998, 37, 801–843. [Google Scholar] [CrossRef]
- Altmann, F.; Schweiszer, S.; Weber, C. Kinetic comparison of peptide: N-glycosidases f and a reveals several differences in substrate specificity. Glycoconj. J. 1995, 12, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Jeong, H.N.; Dimapasoc, L.M.; Kang, I.; Han, C.; Choi, J.-S.; Lebrilla, C.B.; An, H.J. Isomer-specific LC/MS and LC/MS/MS profiling of the mouse serum N-glycome revealing a number of novel sialylated N-glycans. Anal. Chem. 2013, 85, 4636–4643. [Google Scholar] [CrossRef] [PubMed]
- Kronewitter, S.R.; An, H.J.; De Leoz, M.L.; Lebrilla, C.B.; Miyamoto, S.; Leiserowitz, G.S. The development of retrosynthetic glycan libraries to profile and classify the human serum N-linked glycome. Proteomics 2009, 9, 2986–2994. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. https://doi.org/10.3390/ijms18040870
Karav S, German JB, Rouquié C, Le Parc A, Barile D. Studying Lactoferrin N-Glycosylation. International Journal of Molecular Sciences. 2017; 18(4):870. https://doi.org/10.3390/ijms18040870
Chicago/Turabian StyleKarav, Sercan, J. Bruce German, Camille Rouquié, Annabelle Le Parc, and Daniela Barile. 2017. "Studying Lactoferrin N-Glycosylation" International Journal of Molecular Sciences 18, no. 4: 870. https://doi.org/10.3390/ijms18040870





