The Metalloproteinase ADAM28 Promotes Metabolic Dysfunction in Mice
Abstract
:1. Introduction
2. Results
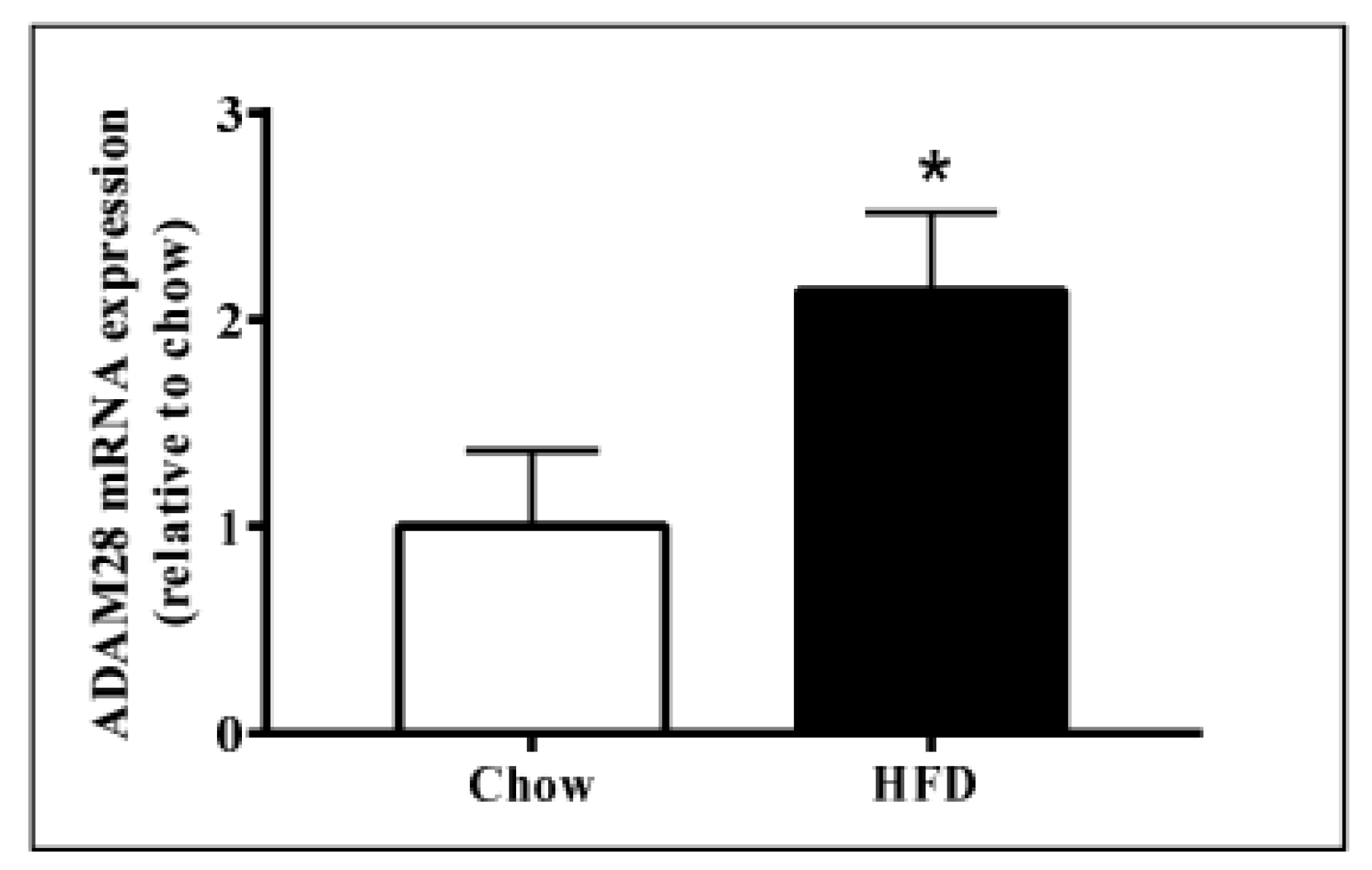
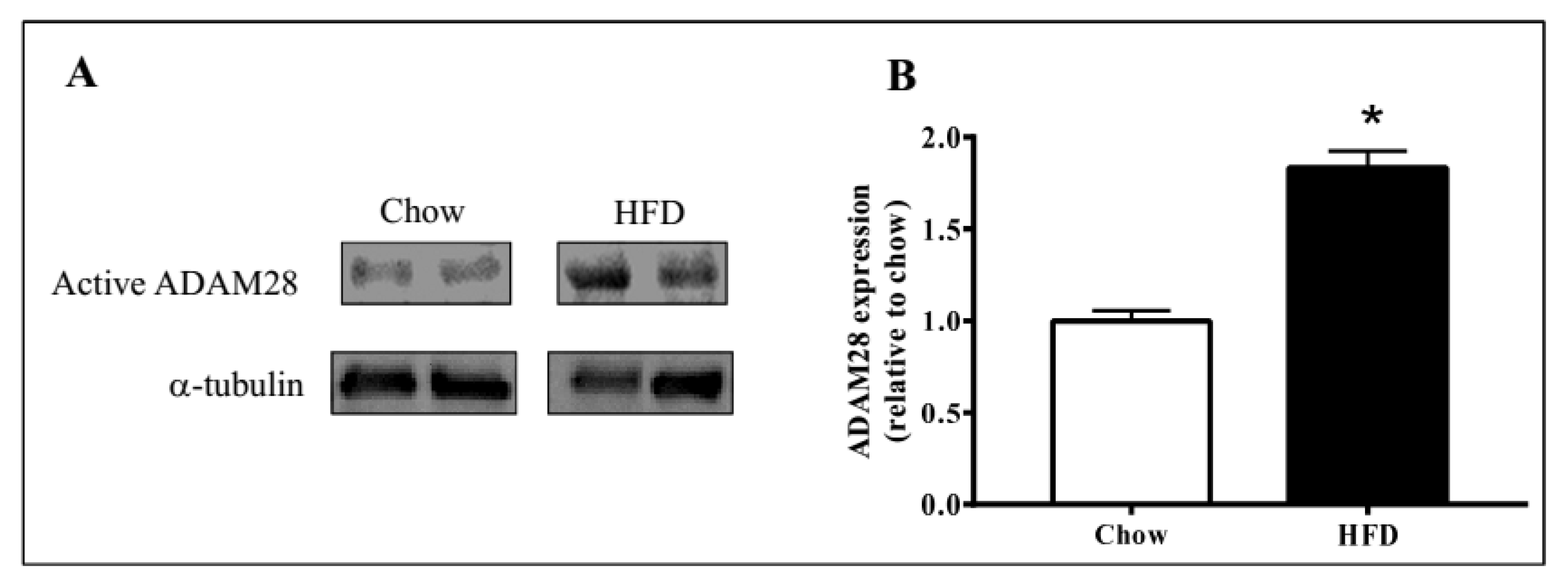
2.1. ADAM28 mRNA Expression Is Significantly Elevated in the Liver of High Fat Diet Fed Mice
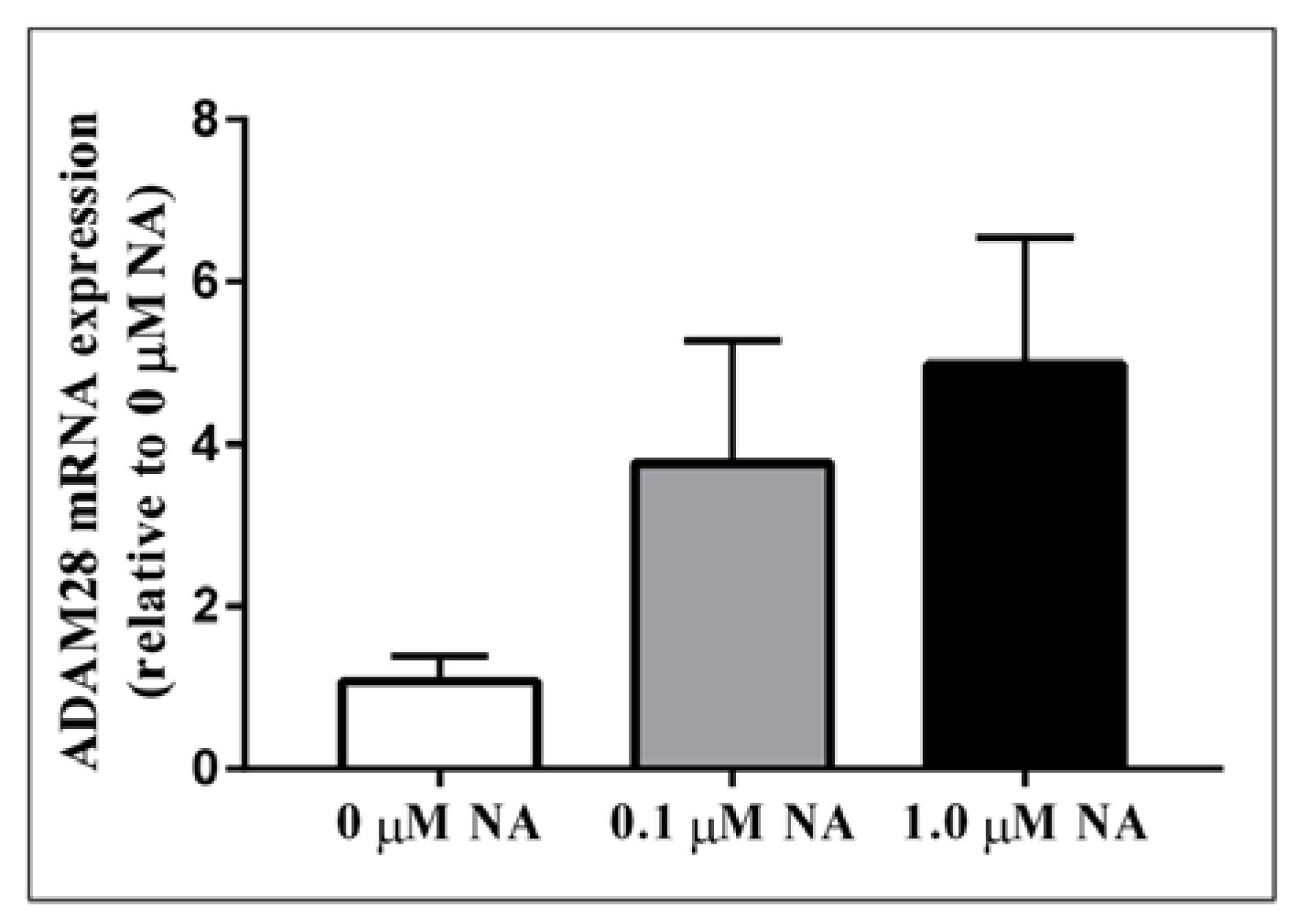
2.2. ADAM28 Expression Is Elevated in Human Monocytes Treated with Noradrenaline (NA)
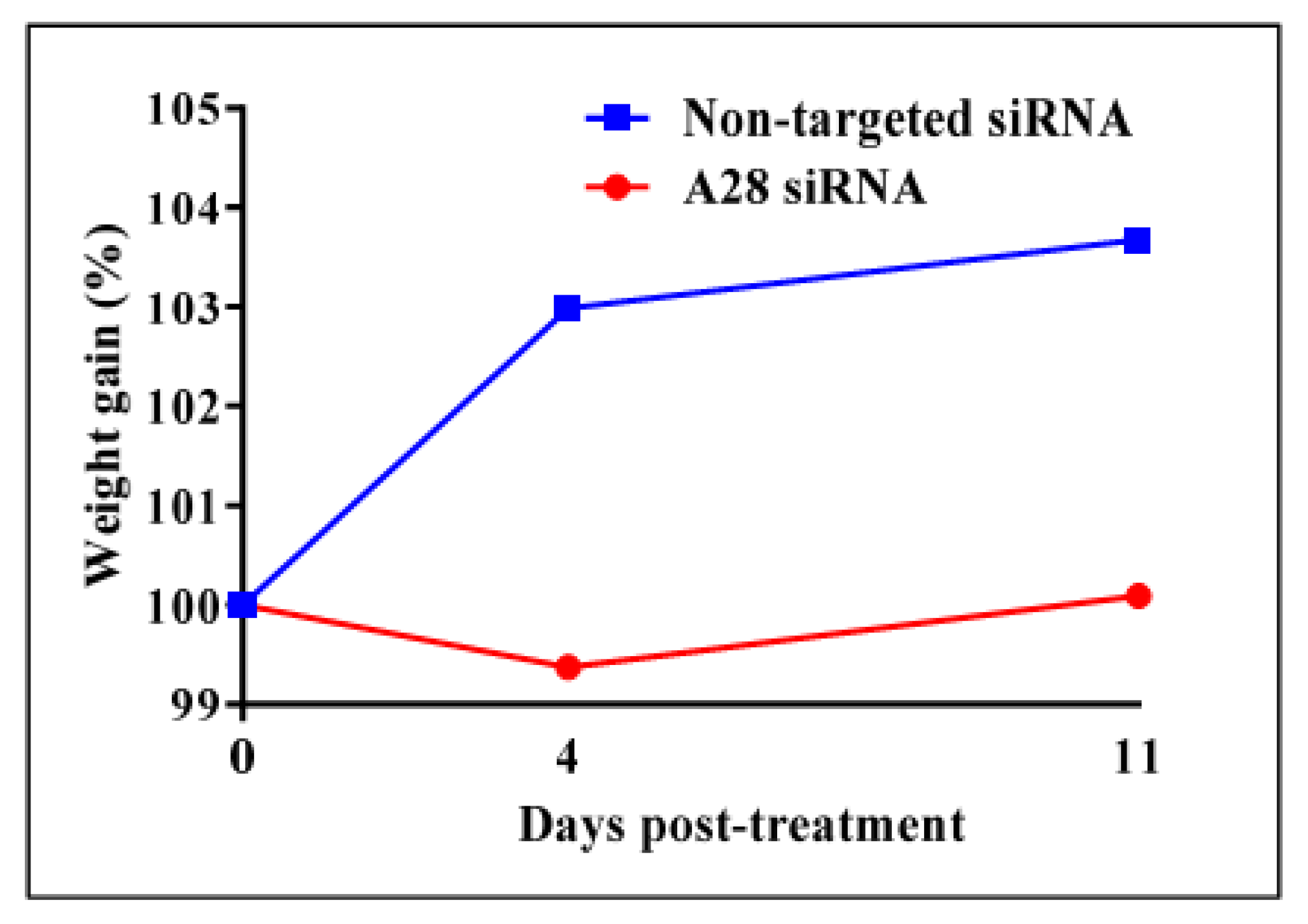
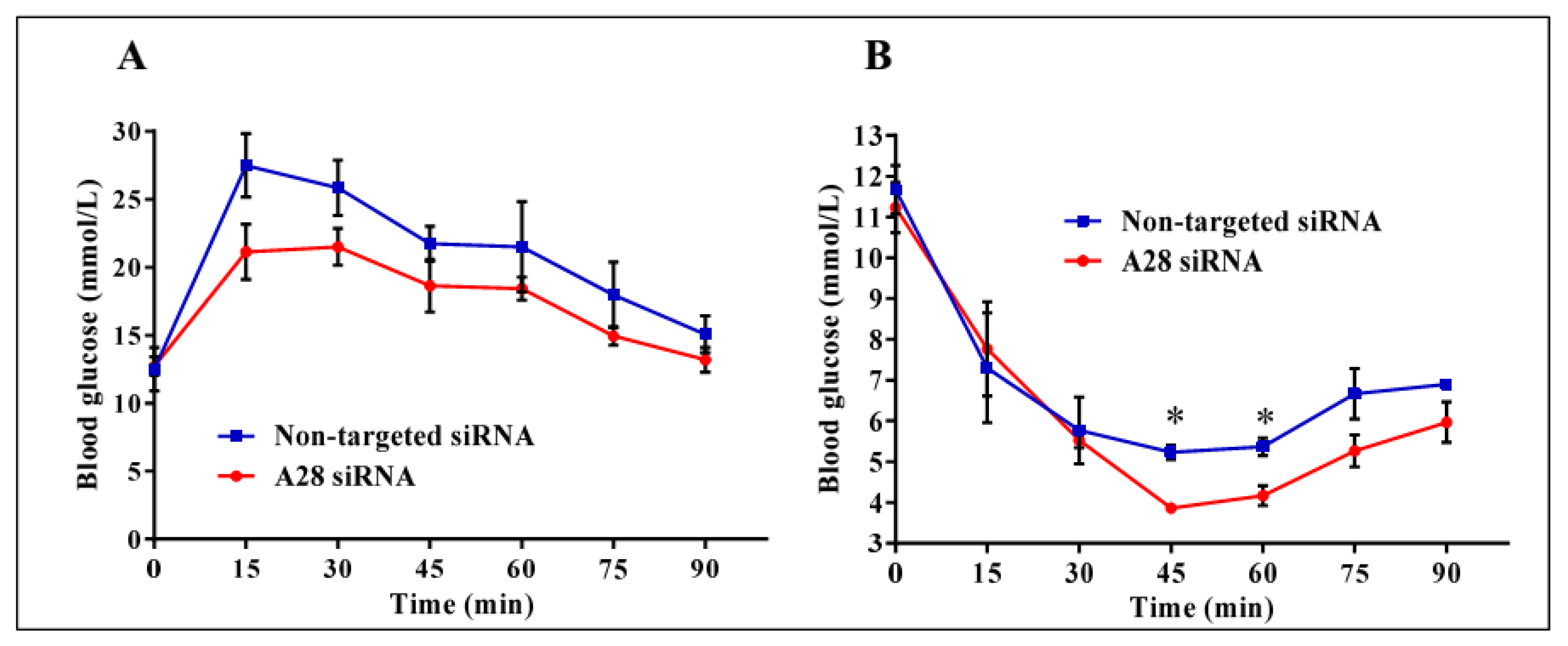
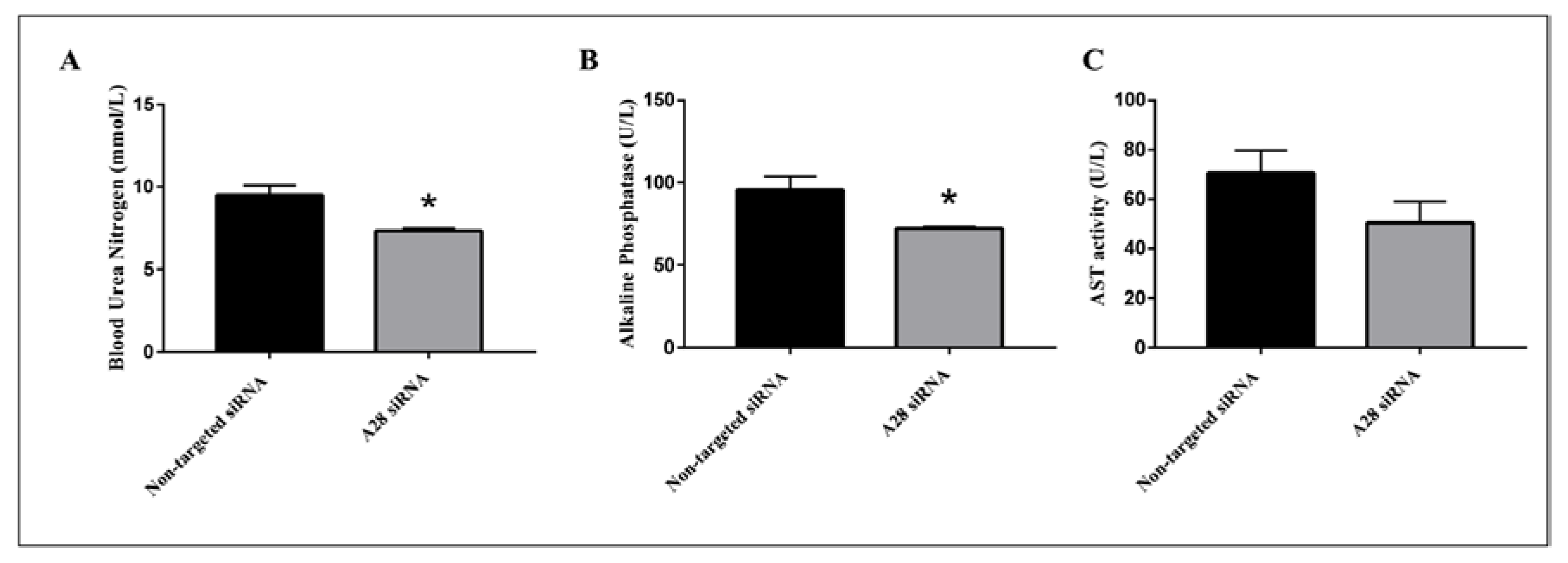
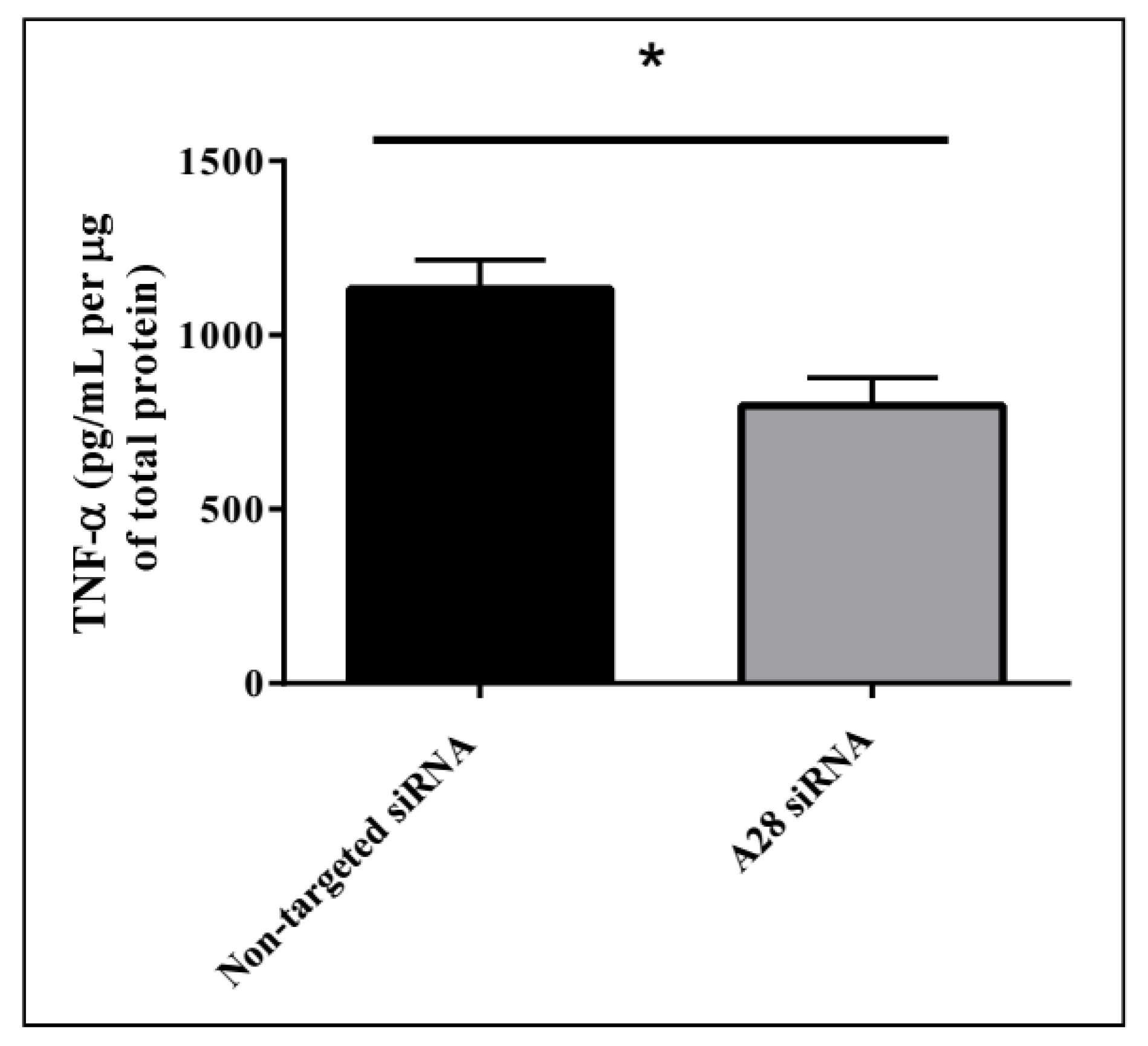
2.3. In Vivo Knock-Down of ADAM28 Ameliorated Parameters of the Metabolic Syndrome
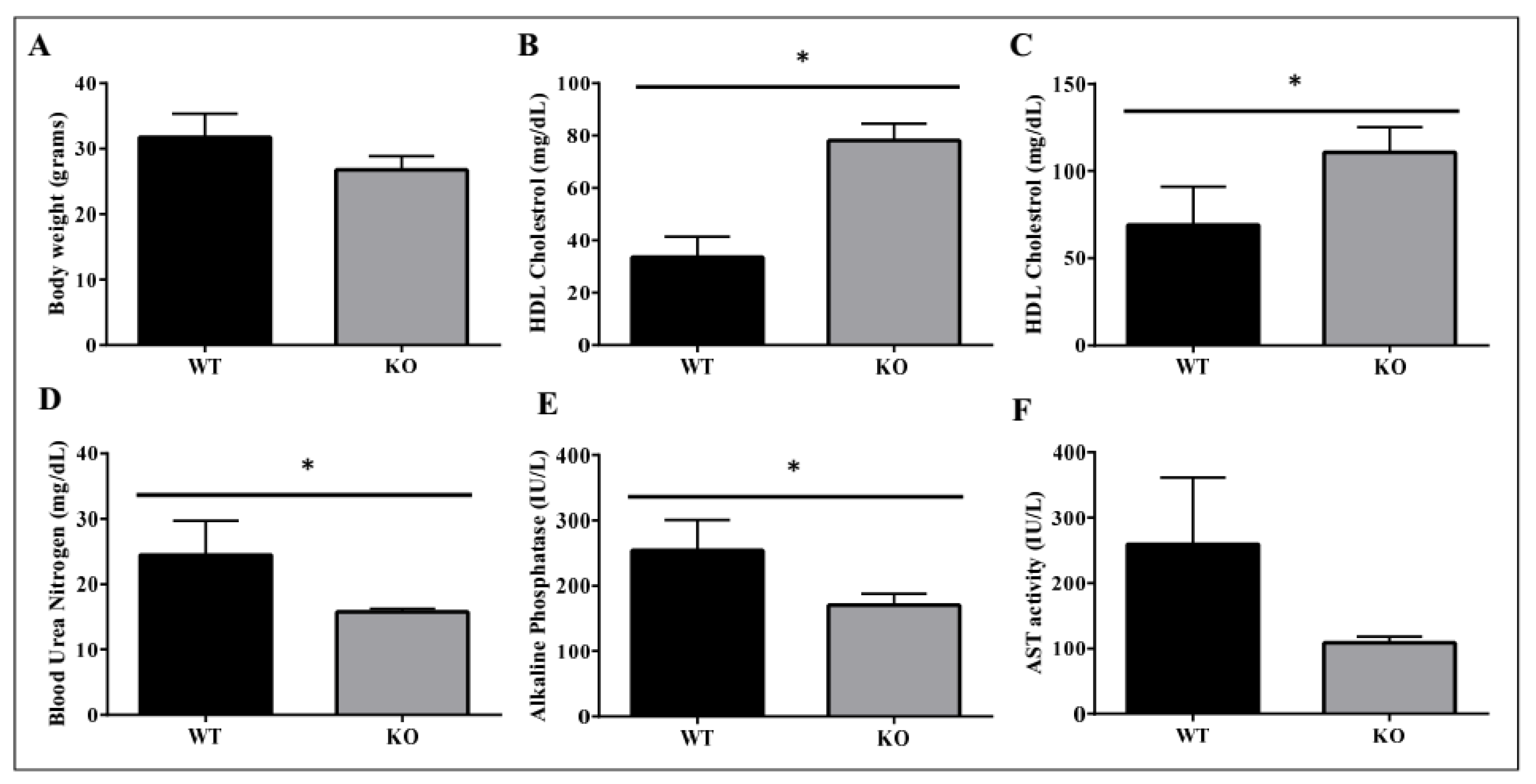
2.4. Metabolic Benefits in ADAM28 Knock-Out (KO) Mice
3. Discussion
4. Materials and Methods
4.1. Cell Culture Experiments
4.2. Animals
4.3. ADAM28 siSTABLE siRNA Treatment
4.4. Western Blotting
4.5. Adam28 mRNA Expression Studies in Human THP-1 Monocytes and Livers of Mice Fed Normal Chow or High Fat Diet (HFD)
4.6. ADAM28 KO Mice
4.7. TNF-α ELISA on Murine Liver Protein
4.8. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADAM28 | A Disintegrin And Metalloproteinase 28 |
| AST | Aspartate aminotransferase |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FCS | Foetal Calf Serum |
| HDL | High Density Lipoprotein |
| HFD | High fat diet |
| KO | Knock-out |
| mRNA | Messenger RNA |
| NA | Noradrenaline |
| SAFHS | San Antonio Family Heart Study |
| SEM | Standard error of the mean |
| siRNA | Small interfering RNA |
| SNS | Sympathetic nervous system |
| T2D | Type 2 Diabetes |
| TNF-α | tumour necrosis factor-α |
References
- Alwahsh, S.M.; Dwyer, B.J.; Forbes, S.; Thiel, D.H.; Lewis, P.J.; Ramadori, G. Insulin production and resistance in different models of diet-induced obesity and metabolic syndrome. Int. J. Mol. Sci. 2017, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, M.F.; Lazo, M.; Horska, A.; Bonekamp, S.; Lipkin, E.W.; Balasubramanyam, A.; Bantle, J.P.; Johnson, R.J.; Diehl, A.M.; Clark, J.M.; et al. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 2012, 56, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Xu, M.; Seyhan, H.A.; Ahmad, S.; Mihm, S.; Ramadori, G.; Schultze, F.C. Diet high in fructose leads to an overexpression of lipocalin-2 in rat fatty liver. World J. Gastroenterol. 2014, 20, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes?: Health be damned! Pour on the sugar. Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Evans, R.M. Exercise mimetics: Impact on health and performance. Cell Metab. 2017, 25, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.R.; Handsley, M.M.; Pennington, C.J. The ADAM metalloproteinases. Mol. Asp. Med. 2008, 29, 258–289. [Google Scholar] [CrossRef] [PubMed]
- Fourie, A.M.; Coles, F.; Moreno, V.; Karlsson, L. Catalytic activity of ADAM8, ADAM15, and MDC-l (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J. Biol. Chem. 2003, 278, 30469–30477. [Google Scholar] [CrossRef] [PubMed]
- Jowett, J.B.; Okada, Y.; Leedman, P.J.; Curran, J.E.; Johnson, M.P.; Moses, E.K.; Goring, H.H.; Mochizuki, S.; Blangero, J.; Stone, L.; et al. ADAM28 is elevated in humans with the metabolic syndrome and is a novel sheddase of human tumour necrosis factor-α. Immunol. Cell Biol. 2012, 90, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Allen, T.L.; Risis, S.; Chan, M.H.; Henstridge, D.C.; Watson, N.; Zaffino, L.A.; Babb, J.R.; Boon, J.; Meikle, P.J.; et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 2010, 53, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, M.; Straznicky, N.; Lambert, E.; Lambert, G. Metabolic syndrome: A sympathetic disease? Lancet Diabetes Endocrinol. 2015, 3, 148–157. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Davis, M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015, 14, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.; White, S.; Borodovsky, A.; Bettencourt, B.R.; Strahs, A.; Clausen, V.; Wijngaard, P.; Horton, J.D.; Taubel, J.; Brooks, A.; et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 2017, 376, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Schachar, R.A.; Nduaka, C.I.; Sperling, M.; Basile, A.S.; Klamerus, K.J.; Chi-Burris, K.; Yan, E.; Paggiarino, D.A.; Rosenblatt, I.; et al. Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study). Investig. Ophthalmol. Vis. Sci. 2012, 53, 7666–7674. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Oka, T.; Chow, F.L.; Cooper, S.B.; Odenbach, J.; Lopaschuk, G.D.; Kassiri, Z.; Fernandez-Patron, C. Tumor necrosis factor-α-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension 2009, 54, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Weerasekera, L.; Rudnicka, C.; Sang, Q.-X.; Curran, J.E.; Johnson, M.P.; Moses, E.K.; Göring, H.H.H.; Blangero, J.; Hricova, J.; Schlaich, M.; et al. ADAM19: A novel target for metabolic syndrome in humans and mice. Mediat. Inflamm. 2017, 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Baurakiades, E.; Costa, V.H., Jr.; Raboni, S.M.; de Almeida, V.R.; Larsen, K.S.; Kohler, J.N.; Gozzo Pdo, C.; Klassen, G.; Manica, G.C.; de Noronha, L. The roles of ADAM33, ADAM28, IL-13 and IL-4 in the development of lung injuries in children with lethal non-pandemic acute infectious pneumonia. J. Clin. Virol. 2014, 61, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, Y.; Mochizuki, S.; Kodama, T.; Shimoda, M.; Ohtsuka, T.; Shiomi, T.; Chijiiwa, M.; Ikeda, T.; Kitajima, M.; Okada, Y. ADAM28 is overexpressed in human breast carcinomas: Implications for carcinoma cell proliferation through cleavage of insulin-like growth factor binding protein-3. Cancer Res. 2006, 66, 9913–9920. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska-Zajdel, E.; Mazurek, U.; Wierzgon, J.; Kokot, T.; Fatyga, E.; Ziolko, E.; Klakla, K.; Blazelonis, A.; Waniczek, D.; Glogowski, L.; et al. Expression of ADAM28 and IGFBP-3 genes in patients with colorectal cancer—A preliminary report. Int. J. Immunopathol. Pharmacol. 2013, 26, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, C.; Mochizuki, S.; Okada, Y.; McLaughlin, C.; Leedman, P.J.; Stuart, L.; Epis, M.; Hoyne, G.; Boulos, S.; Johnson, L.; et al. Overexpression and knock-down studies highlight that a disintegrin and metalloproteinase 28 controls proliferation and migration in human prostate cancer. Medicine 2016, 95, e5085. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Wang, Y.; Wang, L.; Yan, X.; Zhang, D.; Ma, X.; Du, Y.; Liu, X.; Yang, Y. MicroRNA-552 enhances metastatic capacity of colorectal cancer cells by targeting a disintegrin and metalloprotease 28. Oncotarget 2016, 7, 70194–70210. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Zhu, F.; Khatri, I.; Huo, Q.; Spaner, D.E.; Gorczynski, R.M. Characterization of CD200 ectodomain shedding. PLoS ONE 2016, 11, e0152073. [Google Scholar] [CrossRef] [PubMed]
- Wood, O.; Woo, J.; Seumois, G.; Savelyeva, N.; McCann, K.J.; Singh, D.; Jones, T.; Peel, L.; Breen, M.S.; Ward, M.; et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget 2016, 7, 56781–56797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Wang, C.C.; Jiang, Q.; Yang, S.M.; Jiang, H.; Lu, J.; Wang, Q.M.; Feng, F.E.; Zhu, X.L.; Zhao, T.; et al. ADAM28 overexpression regulated via the PI3K/AKT pathway is associated with relapse in de novo adult B-cell acute lymphoblastic leukemia. Leuk. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- McGinn, O.J.; English, W.R.; Roberts, S.; Ager, A.; Newham, P.; Murphy, G. Modulation of integrin α4β1 by ADAM28 promotes lymphocyte adhesion and transendothelial migration. Cell Biol. Int. 2011, 35, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Bret, C.; Hose, D.; Reme, T.; Kassambara, A.; Seckinger, A.; Meissner, T.; Schved, J.F.; Kanouni, T.; Goldschmidt, H.; Klein, B. Gene expression profile of adams and adamtss metalloproteinases in normal and malignant plasma cells and in the bone marrow environment. Exp. Hematol. 2011, 39, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Masaki, M.; Kurisaki, T.; Shirakawa, K.; Sehara-Fujisawa, A. Role of meltrin α(ADAM12) in obesity induced by high-fat diet. Endocrinology 2005, 146, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Menghini, R.; Fiorentino, L.; Amoruso, R.; Mauriello, A.; Lauro, D.; Sbraccia, P.; Hribal, M.L.; Lauro, R.; Federici, M. Mice heterozygous for tumor necrosis factor-α converting enzyme are protected from obesity-induced insulin resistance and diabetes. Diabetes 2007, 56, 2541–2546. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Shimoda, M.; Shiomi, T.; Fujii, Y.; Okada, Y. ADAM28 is activated by MMP-7 (matrilysin-1) and cleaves insulin-like growth factor binding protein-3. Biochem. Biophys. Res. Commun. 2004, 315, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Maier, I.B.; Ozel, Y.; Engstler, A.J.; Puchinger, S.; Wagnerberger, S.; Hulpke-Wette, M.; Bischoff, S.C.; Bergheim, I. Differences in the prevalence of metabolic disorders between prepubertal boys and girls from 5 to 8 years of age. Acta Paediatr. 2014, 103, e154–e160. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Xu, M.; Schultze, F.C.; Wilting, J.; Mihm, S.; Raddatz, D.; Ramadori, G. Combination of alcohol and fructose exacerbates metabolic imbalance in terms of hepatic damage, dyslipidemia, and insulin resistance in rats. PLoS ONE 2014, 9, e104220. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.; Ramadori, G. How does bariatric surgery improve type II diabetes. The neglected importance of the liver in clearing glucose and insulin from the portal blood. J. Obes. Weight Loss Ther. 2015, 5, 5–8. [Google Scholar] [CrossRef]
- Dib, N.; Kiciak, A.; Pietrzak, P.; Ferenc, K.; Jaworski, P.; Kapica, M.; Tarnowski, W.; Zabielski, R. Early-effect of bariatric surgery (scopinaro method) on intestinal hormones and adipokines in insulin resistant wistar rat. J. Physiol. Pharmacol. 2013, 64, 571–577. [Google Scholar] [PubMed]
- Spruss, A.; Kanuri, G.; Stahl, C.; Bischoff, S.C.; Bergheim, I. Metformin protects against the development of fructose-induced steatosis in mice: Role of the intestinal barrier function. Lab. Investig. 2012, 92, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Federico, A.; Dallio, M.; Scazzina, F. Mediterranean diet and nonalcoholic fatty liver disease: Molecular mechanisms of protection. Int. J. Food Sci. Nutr. 2017, 68, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Akbulut, U.E.; Okten, A. Association between adherence to the mediterranean diet and presence of nonalcoholic fatty liver disease in children. Child. Obes. 2016, 12, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, T.; Li, J.; Wang, S.; Qiu, F.; Yu, H.; Zhang, Y.; Wang, T. Effects of natural products on fructose-induced nonalcoholic fatty liver disease (NAFLD). Nutrients 2017, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Alwahsh, S.M.; Gebhardt, R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease (NAFLD). Arch. Toxicol. 2016, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Carey, A.; Watt, M.; Febbraio, M. Cytokine gene expression in human skeletal muscle during concentric contraction: Evidence that IL-8, like IL-6, is influenced by glycogen availability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 322–327. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herat, L.; Rudnicka, C.; Okada, Y.; Mochizuki, S.; Schlaich, M.; Matthews, V. The Metalloproteinase ADAM28 Promotes Metabolic Dysfunction in Mice. Int. J. Mol. Sci. 2017, 18, 884. https://doi.org/10.3390/ijms18040884
Herat L, Rudnicka C, Okada Y, Mochizuki S, Schlaich M, Matthews V. The Metalloproteinase ADAM28 Promotes Metabolic Dysfunction in Mice. International Journal of Molecular Sciences. 2017; 18(4):884. https://doi.org/10.3390/ijms18040884
Chicago/Turabian StyleHerat, Lakshini, Caroline Rudnicka, Yasunori Okada, Satsuki Mochizuki, Markus Schlaich, and Vance Matthews. 2017. "The Metalloproteinase ADAM28 Promotes Metabolic Dysfunction in Mice" International Journal of Molecular Sciences 18, no. 4: 884. https://doi.org/10.3390/ijms18040884





