Inhibiting HDAC1 Enhances the Anti-Cancer Effects of Statins through Downregulation of GGTase-Iβ Expression
Abstract
:1. Introduction
2. Results
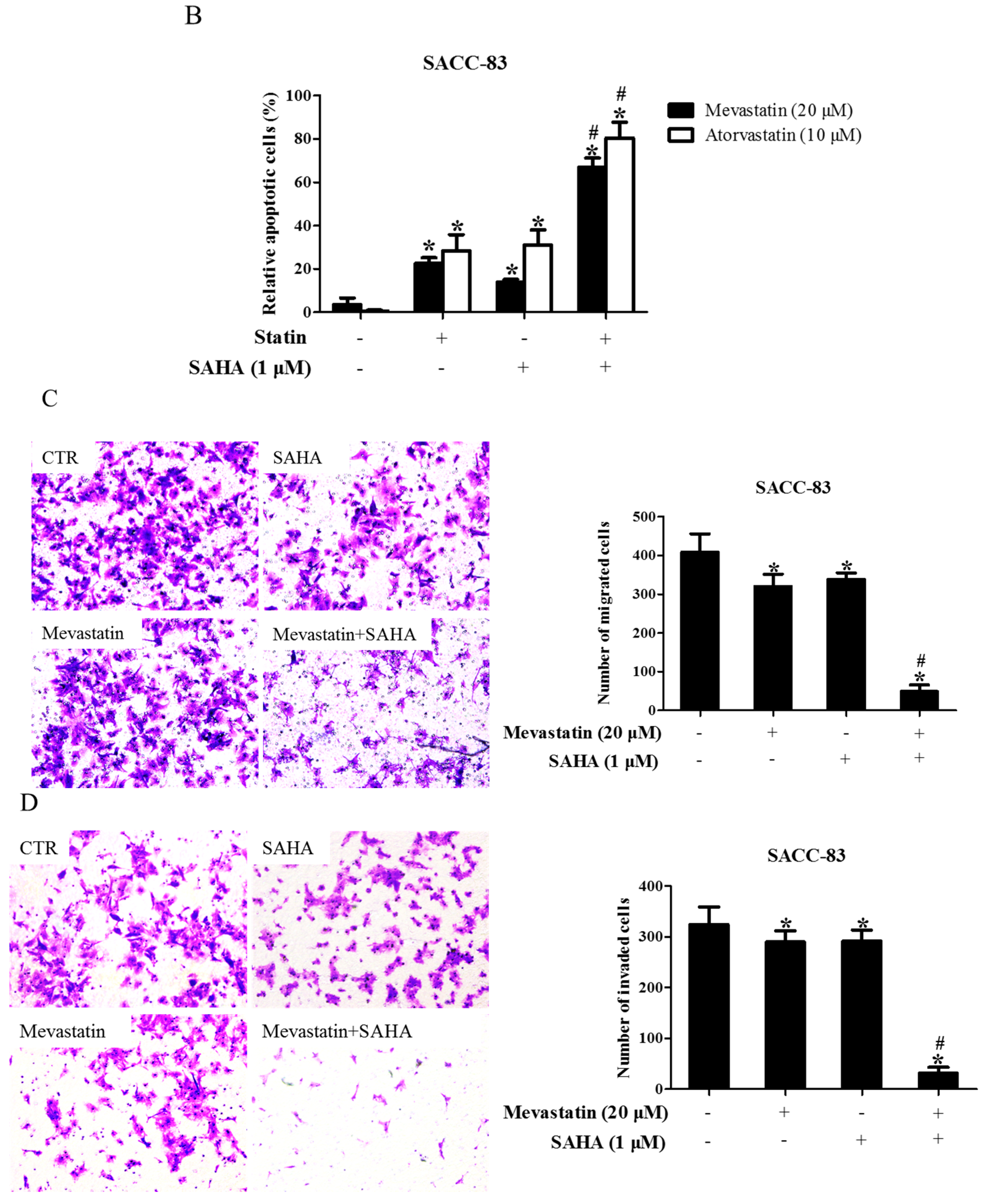
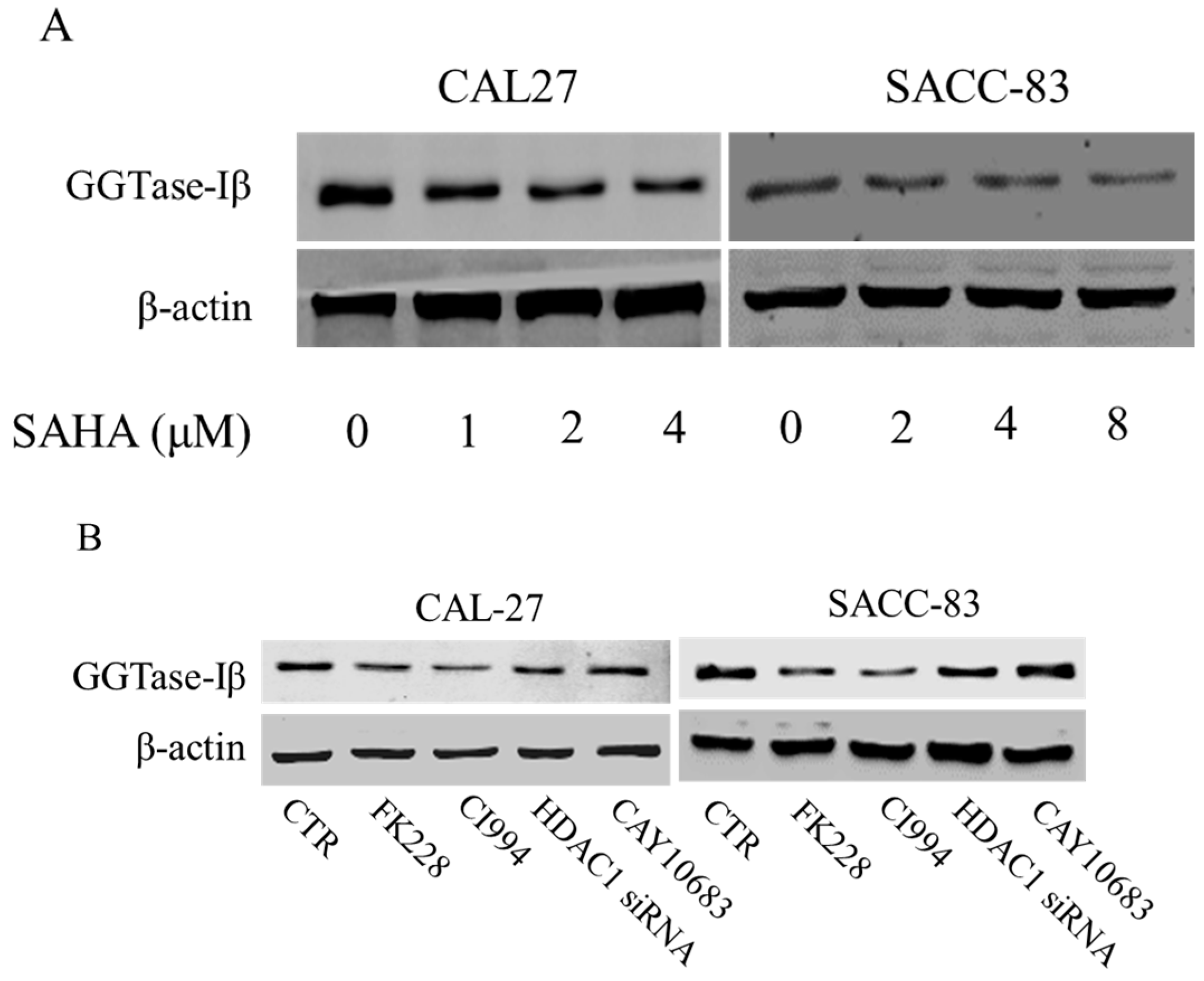
2.1. Suberoylanilide Hydroxamic Acid (SAHA) Enhanced Statin-Induced Anti-Cancer Effects
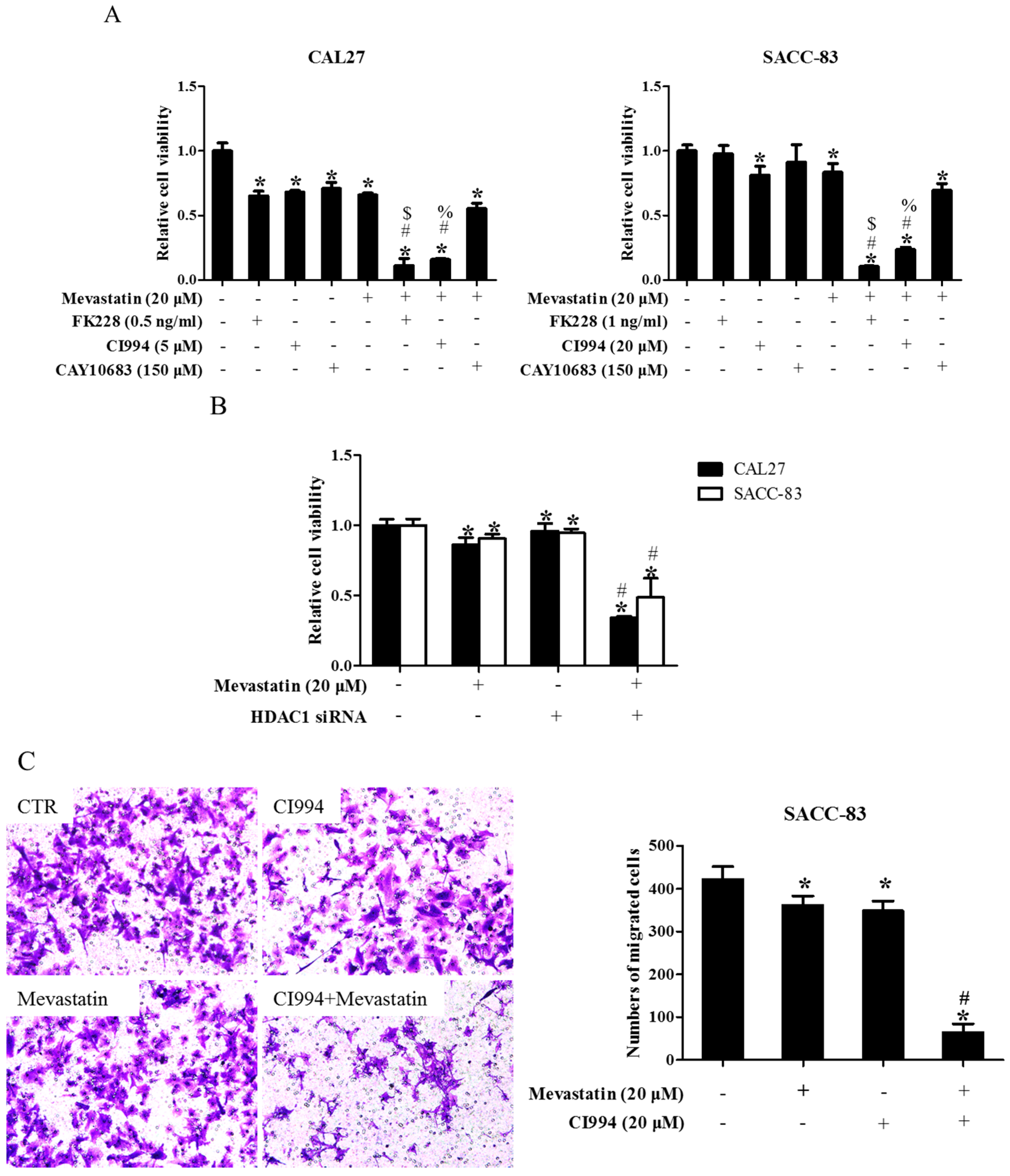
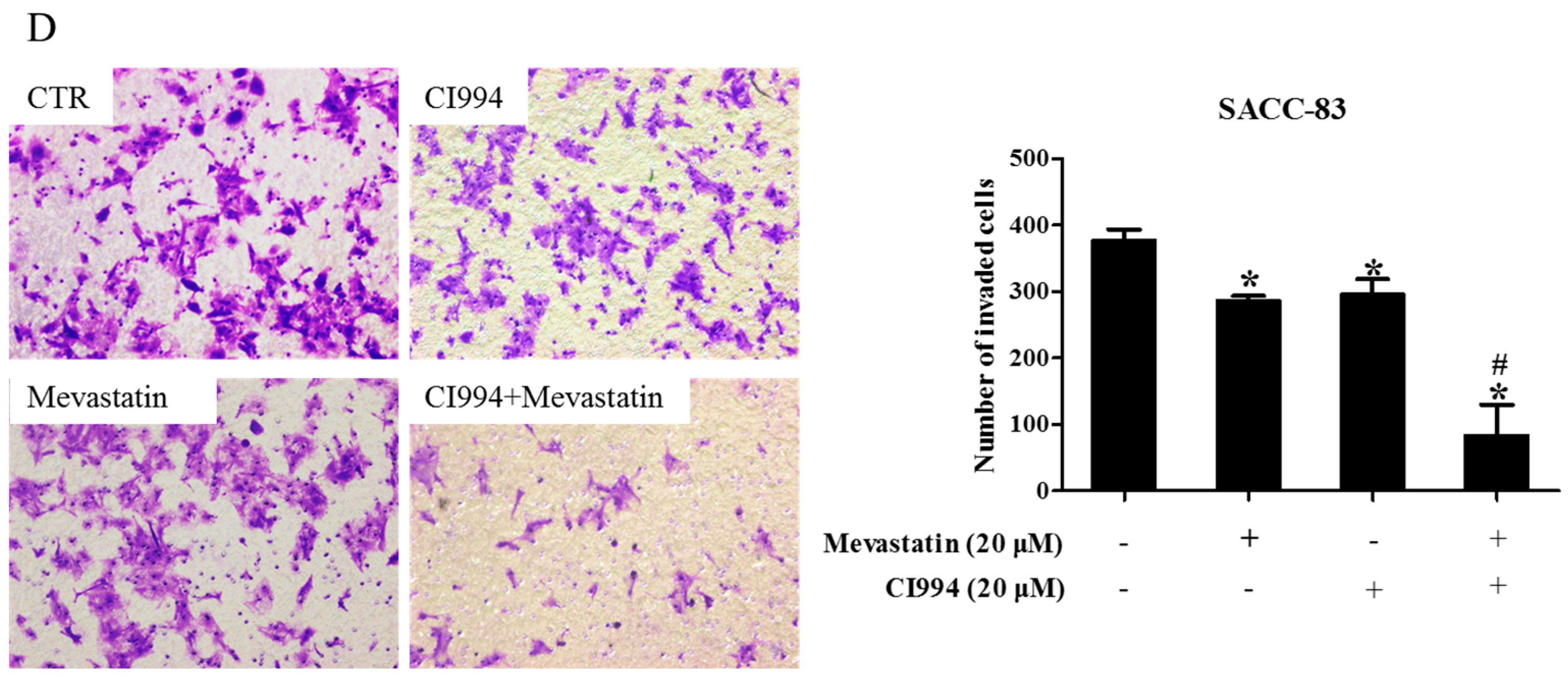
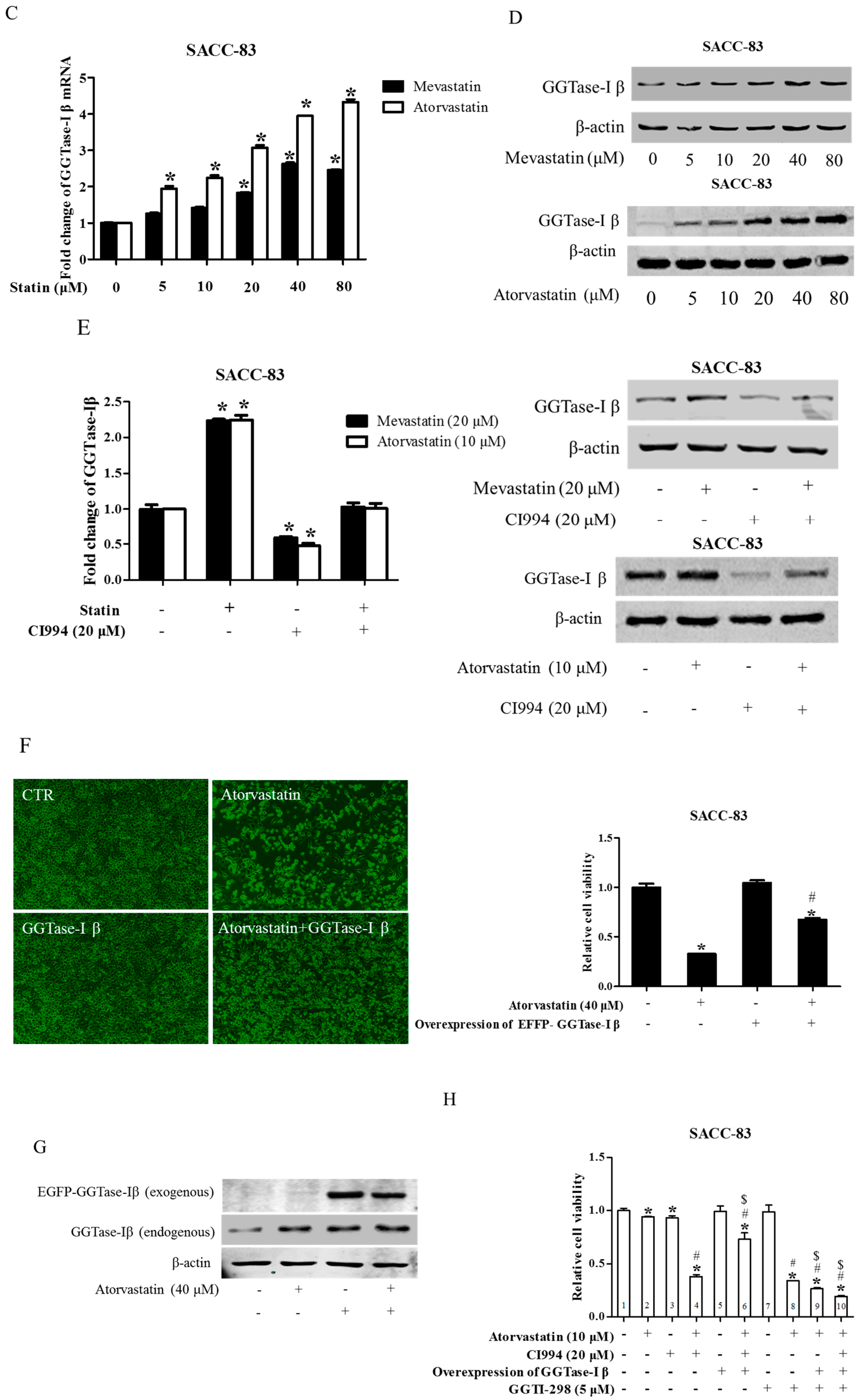
2.2. Inhibition of HDAC1 Was Responsible for Pan-HDAC Inhibitor to Enhance Anti-Cancer Effects of Statins
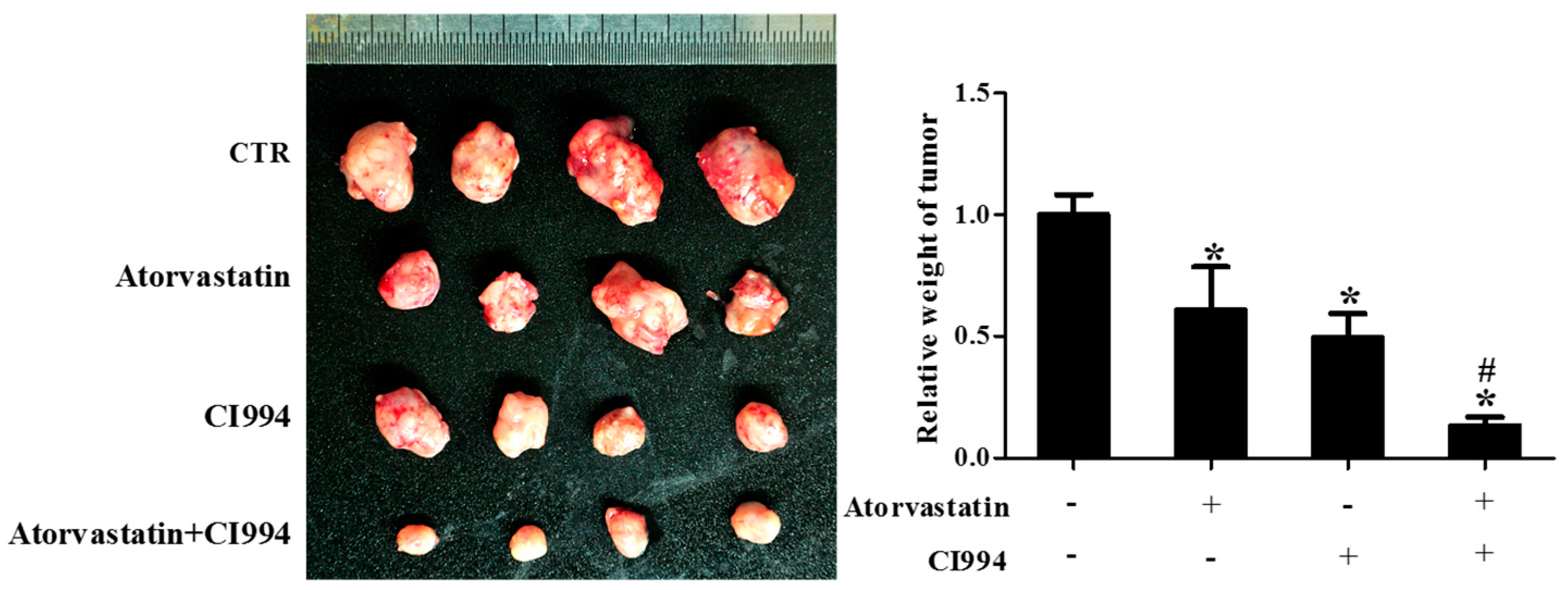
2.3. HDAC1 Inhibitor and Atorvastatin Synergistically Inhibited CAL27 Xenograft Growth in Nude Mice
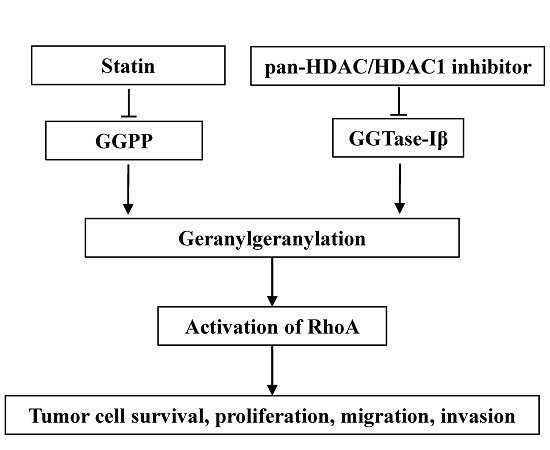
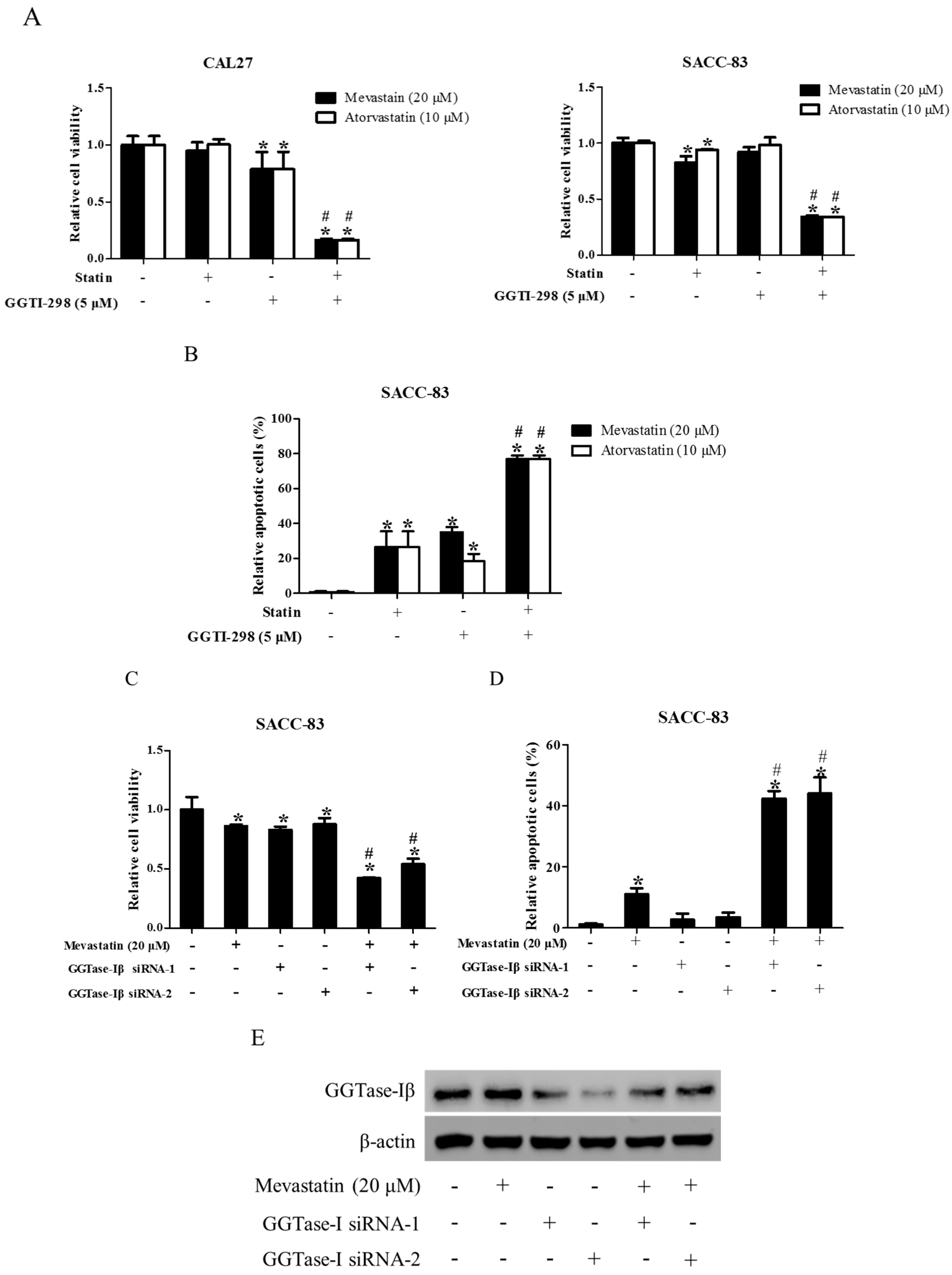
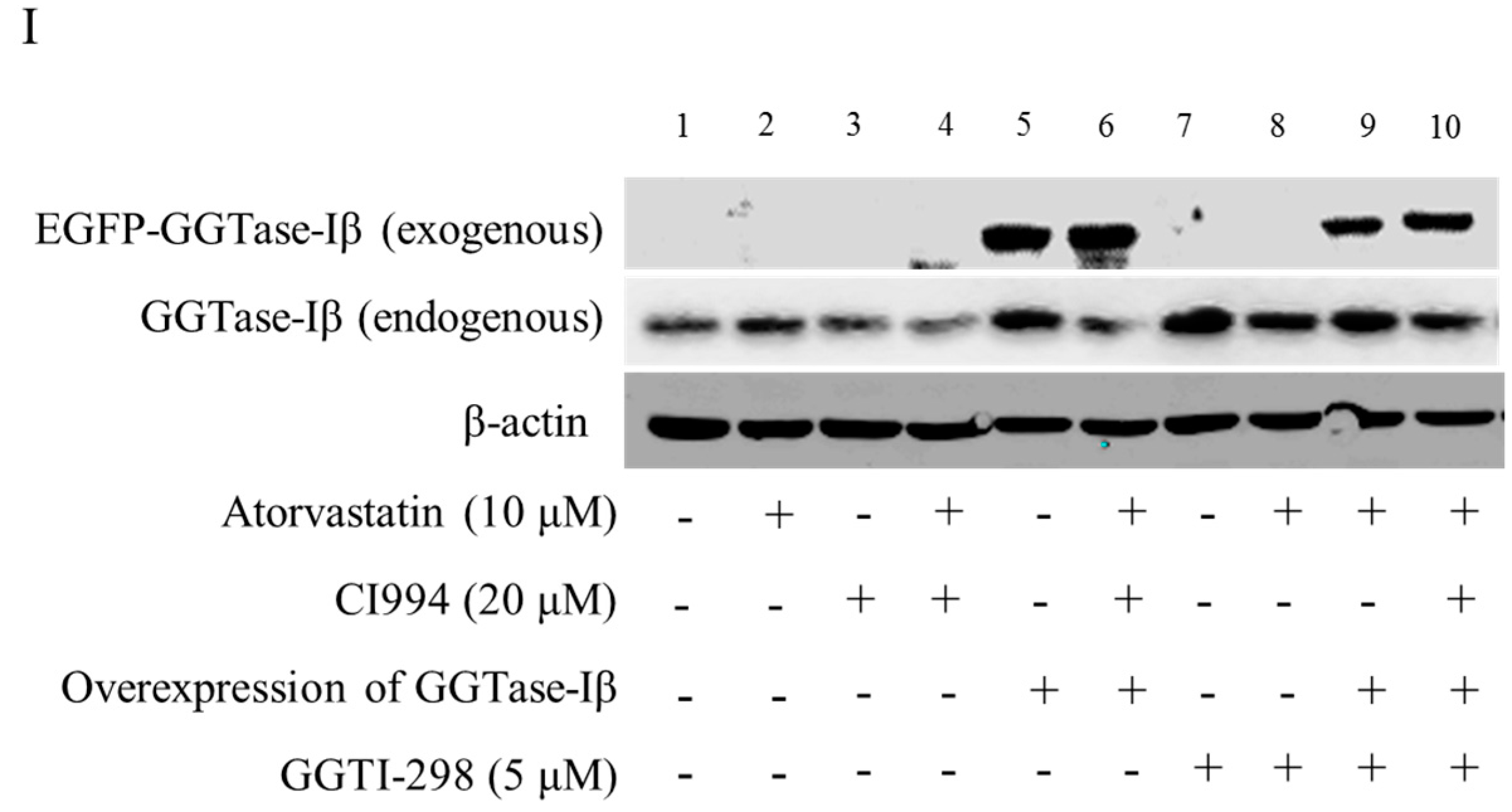
2.4. Inhibition of GGTase-Iβ also Enhanced the Anti-Cancer Effects of Statin
2.5. GGTase-Iβ Mediated the Synergistic Anti-Cancer Effects of HDAC1 Inhibitor and Statin
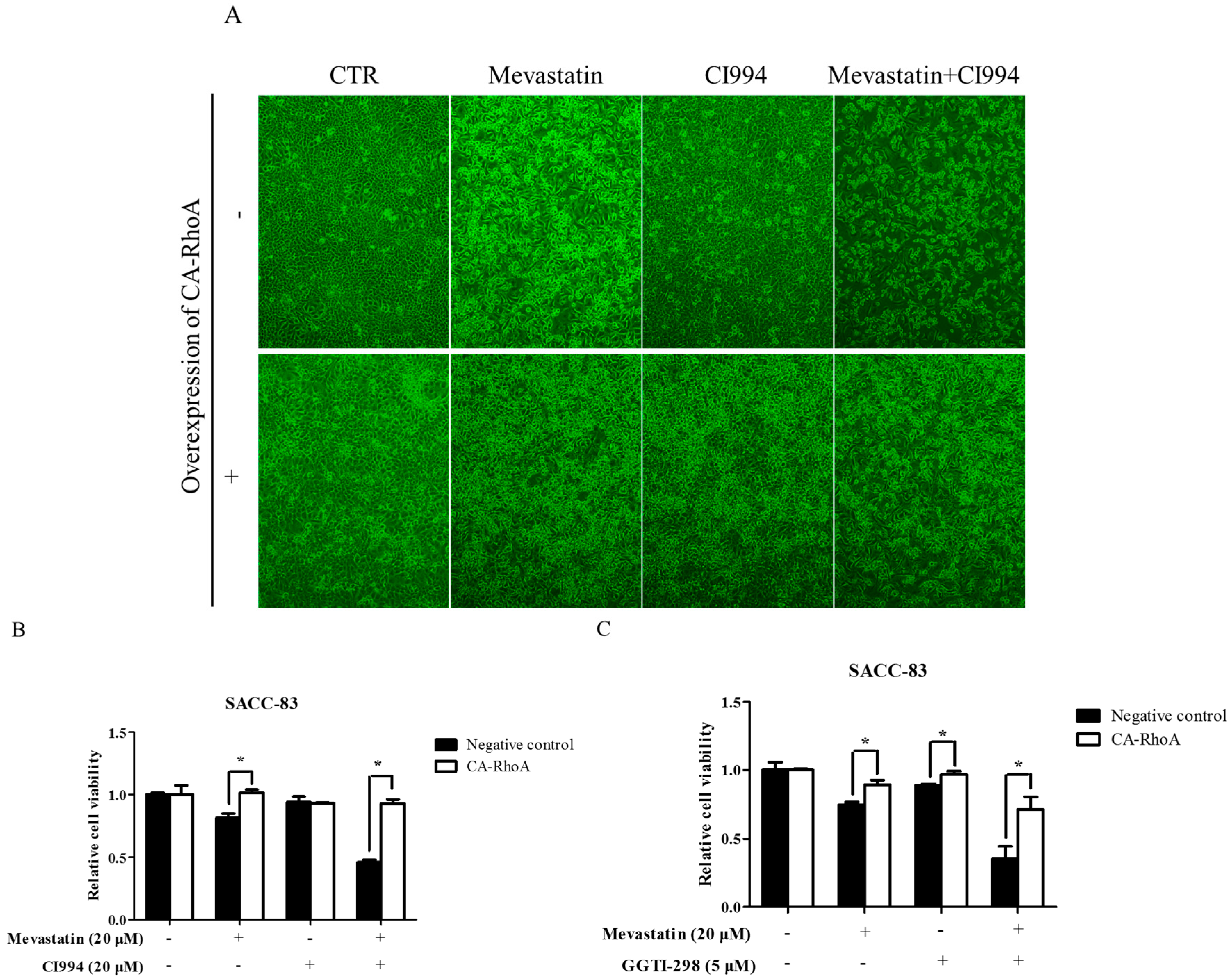
2.6. Overexpression of Constitutively Active RhoA Abolished Enhancement by HDAC1 or GGTase-I Inhibitor of Statin-Induced Anti-Cancer Effects
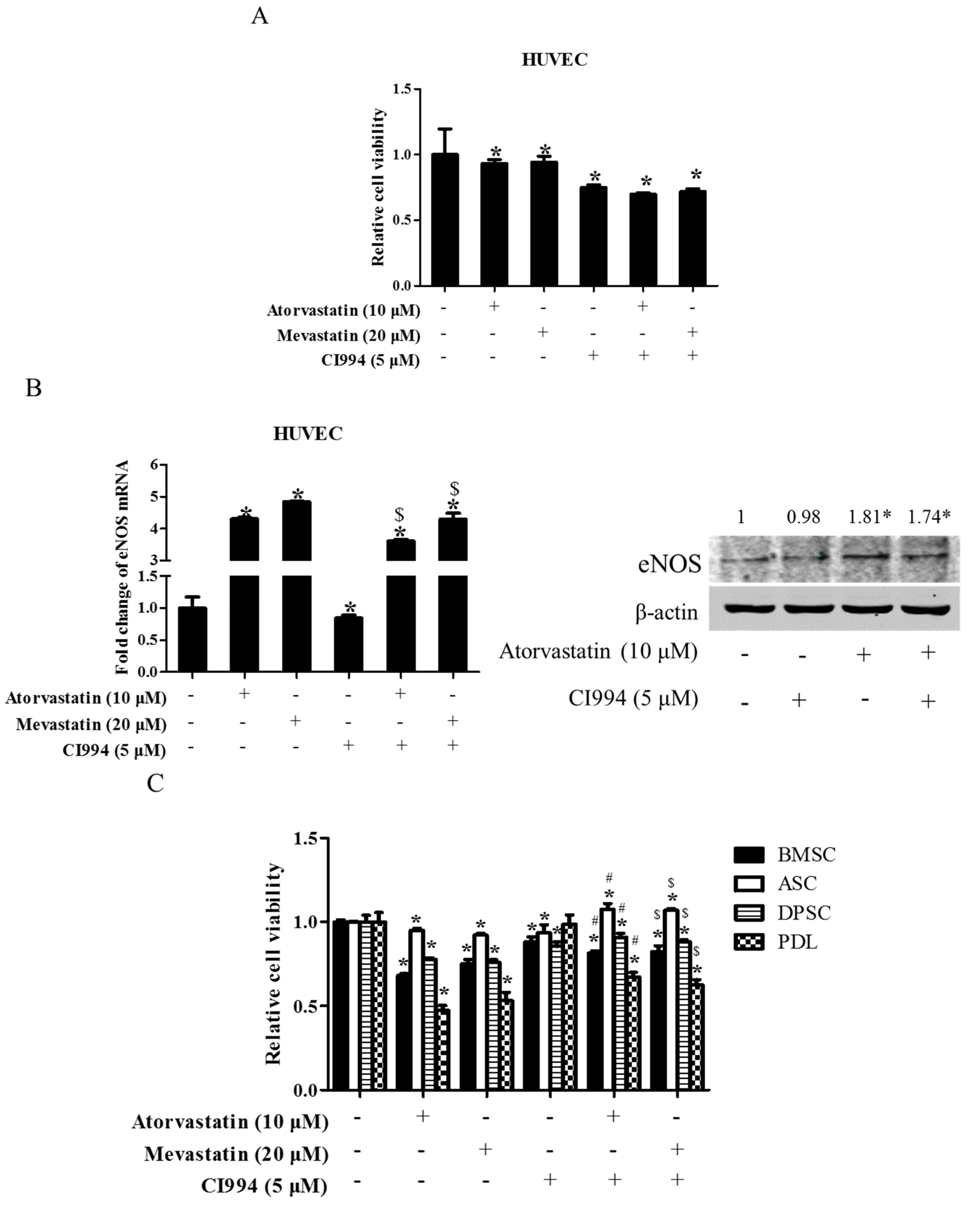
2.7. HDAC1 Inhibitor Did Not Enhance Statin-Induced Inhibition of Cell Proliferation in Non-Tumor Primary Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Reagents and Antibodies
4.3. Plasmids and siRNAs
4.4. Stable Transfection with Lentivirus
4.5. Real-Time Quantitative PCR
4.6. Protein Extraction and Western Blot Analysis
4.7. Cell Proliferation Assay
4.8. Assessment of Cell Apoptosis
4.9. Transwell Migration and Invasion Assay
4.10. Xenograft Tumor Inoculation
4.11. Luciferase Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Escobar, C.; Echarri, R.; Barrios, V. Relative safety profiles of high dose statin regimens. Vasc. Health Risk Manag. 2008, 4, 525–533. [Google Scholar] [PubMed]
- Athyros, V.G.; Tziomalos, K.; Karagiannis, A.; Mikhailidis, D.P. Atorvastatin: Safety and tolerability. Expert Opin. Drug Saf. 2010, 9, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G. HMG-CoA reductase inhibitors (statins) as anticancer drugs (review). Int. J. Oncol. 2005, 27, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Gauthaman, K.; Fong, C.Y.; Bongso, A. Statins, stem cells, and cancer. J. Cell Biochem. 2009, 106, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Osmak, M. Statins and cancer: Current and future prospects. Cancel Lett. 2012, 324, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Al-Haidari, A.A.; Syk, I.; Thorlacius, H. HMG-CoA reductase regulates CCL17-induced colon cancer cell migration via geranylgeranylation and RhoA activation. Biochem. Biophys. Res. Commun. 2014, 446, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Gelb, M.H.; Brunsveld, L.; Hrycyna, C.A.; Michaelis, S.; Tamanoi, F.; Van Voorhis, W.C.; Waldmann, H. Therapeutic intervention based on protein prenylation and associated modifications. Nat. Chem. Biol. 2006, 2, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Fromigue, O.; Hamidouche, Z.; Marie, P.J. Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces osteosarcoma cell invasion. J. Biol. Chem. 2008, 283, 30549–30556. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Li, C.H.; Lin, C.L.; Liang, J.A. Long-term statin use in patients with lung cancer and dyslipidemia reduces the risk of death. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Hsieh, M.C.; Chow, J.M.; Liu, S.H.; Chang, C.L.; Wu, S.Y. Statins improve outcomes of nonsurgical curative treatments in hepatocellular carcinoma patients. Medicine 2016, 95, e4639. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.H.; Miller, C.M.; Tenney, M.E.; Lee, N.K.; Yamada, S.D.; Hasan, Y. Statin Use Significantly Improves Overall Survival in High-Grade Endometrial Cancer. Int. J. Gynecol. Cancer 2016, 137, 36. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Ezer, N.; Sigel, K.; Mhango, G.; Wisnivesky, J.P. The effect of statins on survival in patients with stage IV lung cancer. Lung Cancer 2016, 99, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Aragaki, A.K.; Tang, J.Y.; Kurian, A.W.; Manson, J.E.; Chlebowski, R.T.; Simon, M.; Desai, P.; Wassertheil-Smoller, S.; Liu, S.; et al. Statin use and all-cancer survival: Prospective results from the Women’s Health Initiative. Br. J. Cancer 2016, 115, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, K.H.; Park, Y.S.; Ahn, J.B.; Shin, S.J.; Im, S.A.; Oh, D.Y.; Shin, D.B.; Kim, T.W.; Lee, N.; et al. Simvastatin plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as first-line chemotherapy in metastatic colorectal patients: A multicenter phase II study. Cancer Chemother. Pharmacol. 2009, 64, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Aguilar, E.; Sepulveda-Vildosola, A.C.; Betanzos-Cabrera, Y.; Rocha-Moreno, Y.G.; Gascon-Lastiri, G.; Rivera-Marquez, H.; Wanzke-del-Angel, V.; Cerecedo-Diaz, F.; de la Cruz-Yanez, H. Phase II study of metronomic chemotherapy with thalidomide, carboplatin-vincristine-fluvastatin in the treatment of brain stem tumors in children. Arch. Med. Res. 2008, 39, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Kawata, S.; Yamasaki, E.; Nagase, T.; Inui, Y.; Ito, N.; Matsuda, Y.; Inada, M.; Tamura, S.; Noda, S.; Imai, Y.; et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br. J. Cancer 2001, 84, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kim, M.M.; Choi, H.J.; Yoon, S.S.; Lee, M.H.; Park, K.; Park, C.H.; Kang, W.K. Phase II study of high-dose lovastatin in patients with advanced gastric adenocarcinoma. Invest. New Drugs 2001, 19, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Thibault, A.; Samid, D.; Tompkins, A.C.; Figg, W.D.; Cooper, M.R.; Hohl, R.J.; Trepel, J.; Liang, B.; Patronas, N.; Venzon, D.J.; et al. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin. Cancer Res. 1996, 2, 483–491. [Google Scholar] [PubMed]
- Zhang, F.L.; Casey, P.J. Protein prenylation: Molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996, 65, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ohkanda, J.; Coppola, D.; Yin, H.; Kothare, M.; Busciglio, B.; Hamilton, A.D.; Sebti, S.M. Geranylgeranyltransferase I inhibitor GGTI-2154 induces breast carcinoma apoptosis and tumor regression in H-Ras transgenic mice. Cancer Res. 2003, 63, 8922–8929. [Google Scholar] [PubMed]
- Vogt, A.; Sun, J.; Qian, Y.; Hamilton, A.D.; Sebti, S.M. The geranylgeranyltransferase-I inhibitor GGTI-298 arrests human tumor cells in G0/G1 and induces p21(WAF1/CIP1/SDI1) in a p53-independent manner. J. Biol. Chem. 1997, 272, 27224–27229. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, A.K.; Andersson, K.M.; Liu, M.; Cutts, B.A.; Karlsson, C.; Wahlstrom, A.M.; Dalin, M.; Weinbaum, C.; Casey, P.J.; Tarkowski, A.; Swolin, B.; Young, S.G.; Bergo, M.O. GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer. J. Clin. Investig. 2007, 117, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sjogren, A.K.; Karlsson, C.; Ibrahim, M.X.; Andersson, K.M.; Olofsson, F.J.; Wahlstrom, A.M.; Dalin, M.; Yu, H.; Chen, Z.; Yang, S.H.; Young, S.G.; Bergo, M.O. Targeting the protein prenyltransferases efficiently reduces tumor development in mice with K-RAS-induced lung cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 6471–6476. [Google Scholar] [CrossRef] [PubMed]
- Philips, M.R.; Cox, A.D. Geranylgeranyltransferase I as a target for anti-cancer drugs. J. Clin. Investig. 2007, 117, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Witt, O.; Deubzer, H.E.; Milde, T.; Oehme, I. HDAC family: What are the cancer relevant targets? Cancer Lett. 2009, 277, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Dovey, O.M.; Foster, C.T.; Cowley, S.M. Histone deacetylase 1 (HDAC1), but not HDAC2, controls embryonic stem cell differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 8242–8247. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.M.; Jana, L.; Kasajima, A.; Lehmann, A.; Prinzler, J.; Budczies, J.; Winzer, K.J.; Dietel, M.; Weichert, W.; Denkert, C. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer—Overexpression of HDAC2 and HDAC3 is associated with clinicopathological ndicators of disease progression. BMC Cancer 2013, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Noh, J.H.; Lee, J.H.; Eun, J.W.; Ahn, Y.M.; Kim, S.Y.; Lee, S.H.; Park, W.S.; Yoo, N.J.; Lee, J.Y.; Nam, S.W. Increased expression of histone deacetylase 2 is found in human gastric cancer. APMIS 2005, 113, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Weichert, W.; Roske, A.; Gekeler, V.; Beckers, T.; Stephan, C.; Jung, K.; Fritzsche, F.R.; Niesporek, S.; Denkert, C.; Dietel, M.; Kristiansen, G. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br. J. Cancer 2008, 98, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Agus, D.B.; Scher, H.I.; Higgins, B.; Rose, A.; Cordon-Cardo, C.; Thaler, H.T.; Rifkind, R.A.; Marks, P.A.; Richon, V.M. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000, 60, 5165–5170. [Google Scholar] [PubMed]
- Herold, C.; Ganslmayer, M.; Ocker, M.; Hermann, M.; Geerts, A.; Hahn, E.G.; Schuppan, D. The histone-deacetylase inhibitor Trichostatin A blocks proliferation and triggers apoptotic programs in hepatoma cells. J. Hepatol. 2002, 36, 233–240. [Google Scholar] [CrossRef]
- Duvic, M.; Vu, J. Vorinostat: A new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin. Investig. Drugs 2007, 16, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Frew, A.J.; Johnstone, R.W.; Bolden, J.E. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009, 280, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Rosato, R.R.; Almenara, J.A.; Grant, S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003, 63, 3637–3645. [Google Scholar] [PubMed]
- Insinga, A.; Monestiroli, S.; Ronzoni, S.; Gelmetti, V.; Marchesi, F.; Viale, A.; Altucci, L.; Nervi, C.; Minucci, S.; Pelicci, P.G. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat. Med. 2005, 11, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.H.; Zhang, S. PTEN/AKT pathway involved in histone deacetylases inhibitor induced cell growth inhibition and apoptosis of oral squamous cell carcinoma cells. Oral. Oncol. 2009, 45, e150–e154. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Jia, L.F.; Gan, Y.H. PTEN activation through K163 acetylation by inhibiting HDAC6 contributes to tumour inhibition. Oncogene 2016, 35, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Wang, J.; Coselli, J.; Wang, X.L. Synergistic induction of apoptosis by HMG-CoA reductase inhibitor and histone deacetylases inhibitor in HeLa cells. Biochem. Biophys. Res. Commun. 2008, 365, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Arrighetti, N.; Corno, C.; Gatti, L. Drug Combinations with HDAC Inhibitors in Antitumor Therapy. Crit. Rev. Oncog. 2015, 20, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, L.Y.; Zuraw, B.L.; Ye, R.D.; Pan, Z.K. Chemoattractant-stimulated NF-κβ activation is dependent on the low molecular weight GTPase RhoA. J. Biol. Chem. 2001, 276, 40977–40981. [Google Scholar] [CrossRef] [PubMed]
- Minamiya, Y.; Ono, T.; Saito, H.; Takahashi, N.; Ito, M.; Mitsui, M.; Motoyama, S.; Ogawa, J. Expression of histone deacetylase 1 correlates with a poor prognosis in patients with adenocarcinoma of the lung. Lung Cancer 2011, 74, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, J.; Kurita, N.; Miyatani, T.; Yoshikawa, K.; Morimoto, S.; Nishioka, M.; Iwata, T.; Shimada, M. Expression of histone deacetylase 1 and metastasis-associated protein 1 as prognostic factors in colon cancer. Oncol. Rep. 2011, 26, 343–348. [Google Scholar] [PubMed]
- Witt, A.E.; Lee, C.W.; Lee, T.I.; Azzam, D.J.; Wang, B.; Caslini, C.; Petrocca, F.; Grosso, J.; Jones, M.; Cohick, E.B.; et al. Identification of a cancer stem cell-specific function for the histone deacetylases, HDAC1 and HDAC7, in breast and ovarian cancer. Oncogene 2016, 36, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Horinouchi, S.; Beppu, T. Trichostatin A and trapoxin: Novel chemical probes for the role of histone acetylation in chromatin structure and function. Bioessays 1995, 17, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, W.K.; Johnstone, R.W.; Prince, H.M. Histone deacetylase inhibitors in cancer therapy. Expert Opin. Investig. Drugs 2007, 16, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, J.H.; Chou, C.W.; Chang, Y.F.; Yeh, S.H.; Chen, C.C. Statins increase p21 through inhibition of histone deacetylase activity and release of promoter-associated HDAC1/2. Cancer Res. 2008, 68, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Nezasa, K.; Higaki, K.; Matsumura, T.; Inazawa, K.; Hasegawa, H.; Nakano, M.; Koike, M. Liver-specific distribution of rosuvastatin in rats: Comparison with pravastatin and simvastatin. Drug Metab. Dispos. 2002, 30, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.S.; Tayek, J.A. Lipophilicity and cardiovascular outcome in patients with CHF. Am. Heart J. 2008, 156, e7–e9. [Google Scholar] [CrossRef] [PubMed]
- Gioanni, J.; Fischel, J.L.; Lambert, J.C.; Demard, F.; Mazeau, C.; Zanghellini, E.; Ettore, F.; Formento, P.; Chauvel, P.; Lalanne, C.M.; et al. Two new human tumor cell lines derived from squamous cell carcinomas of the tongue: Establishment, characterization and response to cytotoxic treatment. Eur. J. Cancer Clin. Oncol. 1988, 24, 1445–1455. [Google Scholar] [CrossRef]
- Li, S.L. Establishment of a human cancer cell line from adenoid cystic carcinoma of the minor salivary gland. Zhonghua Kou Qiang Yi Xue Za Zhi 1990, 25, 29–31. [Google Scholar] [PubMed]
- Cao, H.; Kou, X.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Feng, L.; He, D.; Gan, Y.; Zhou, Y. Force-induced Adrb2 in periodontal ligament cells promotes tooth movement. J. Dent. Res. 2014, 93, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Xiao, E.; Yang, H.Q.; Gan, Y.H.; Duan, D.H.; He, L.H.; Guo, Y.; Wang, S.Q.; Zhang, Y. Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells 2015, 33, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.X.; Hao, T.; Meng, Z.; Zhou, Y.H.; Gan, Y.H. Acetylated Sp1 inhibits PTEN expression through binding to PTEN core promoter and recruitment of HDAC1 and promotes cancer cell migration and invasion. Carcinogenesis 2013, 34, 58–67. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Gan, Y.-H. Inhibiting HDAC1 Enhances the Anti-Cancer Effects of Statins through Downregulation of GGTase-Iβ Expression. Int. J. Mol. Sci. 2017, 18, 1010. https://doi.org/10.3390/ijms18051010
Li R, Gan Y-H. Inhibiting HDAC1 Enhances the Anti-Cancer Effects of Statins through Downregulation of GGTase-Iβ Expression. International Journal of Molecular Sciences. 2017; 18(5):1010. https://doi.org/10.3390/ijms18051010
Chicago/Turabian StyleLi, Ran, and Ye-Hua Gan. 2017. "Inhibiting HDAC1 Enhances the Anti-Cancer Effects of Statins through Downregulation of GGTase-Iβ Expression" International Journal of Molecular Sciences 18, no. 5: 1010. https://doi.org/10.3390/ijms18051010