Atractylenolide-I Protects Human SH-SY5Y Cells from 1-Methyl-4-Phenylpyridinium-Induced Apoptotic Cell Death
Abstract
:1. Introduction
2. Results
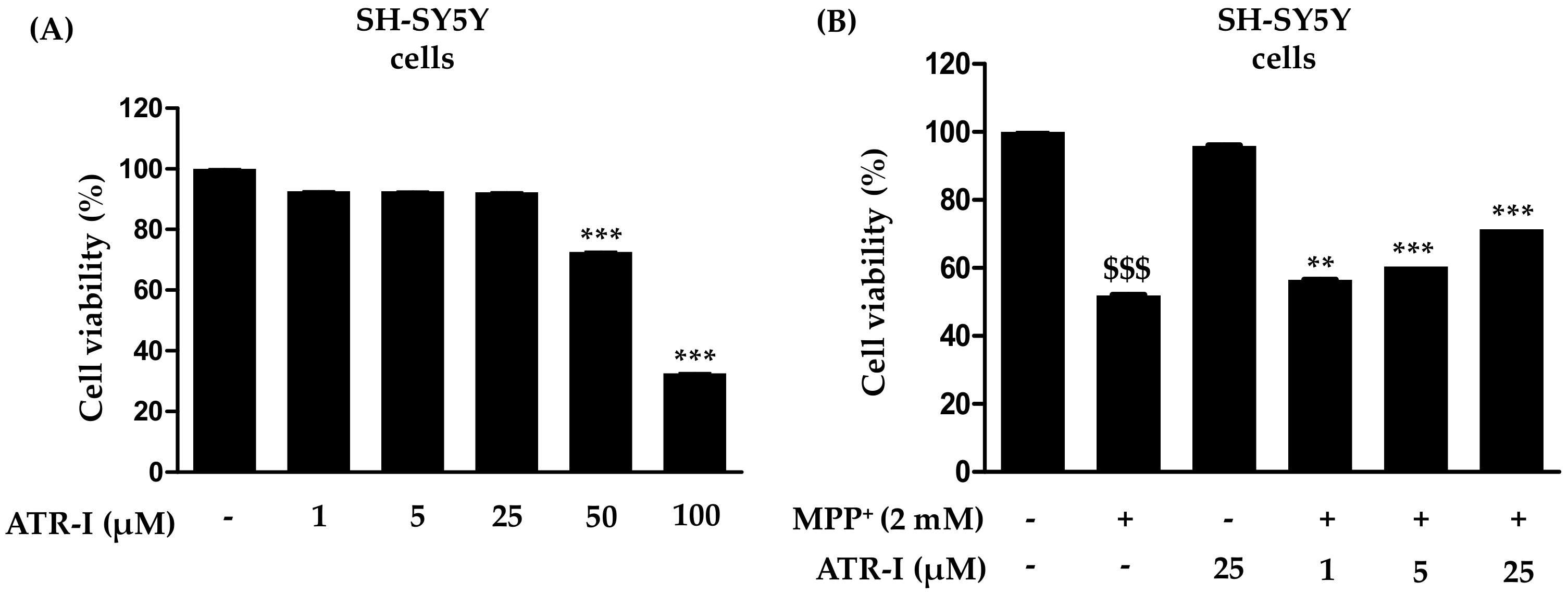
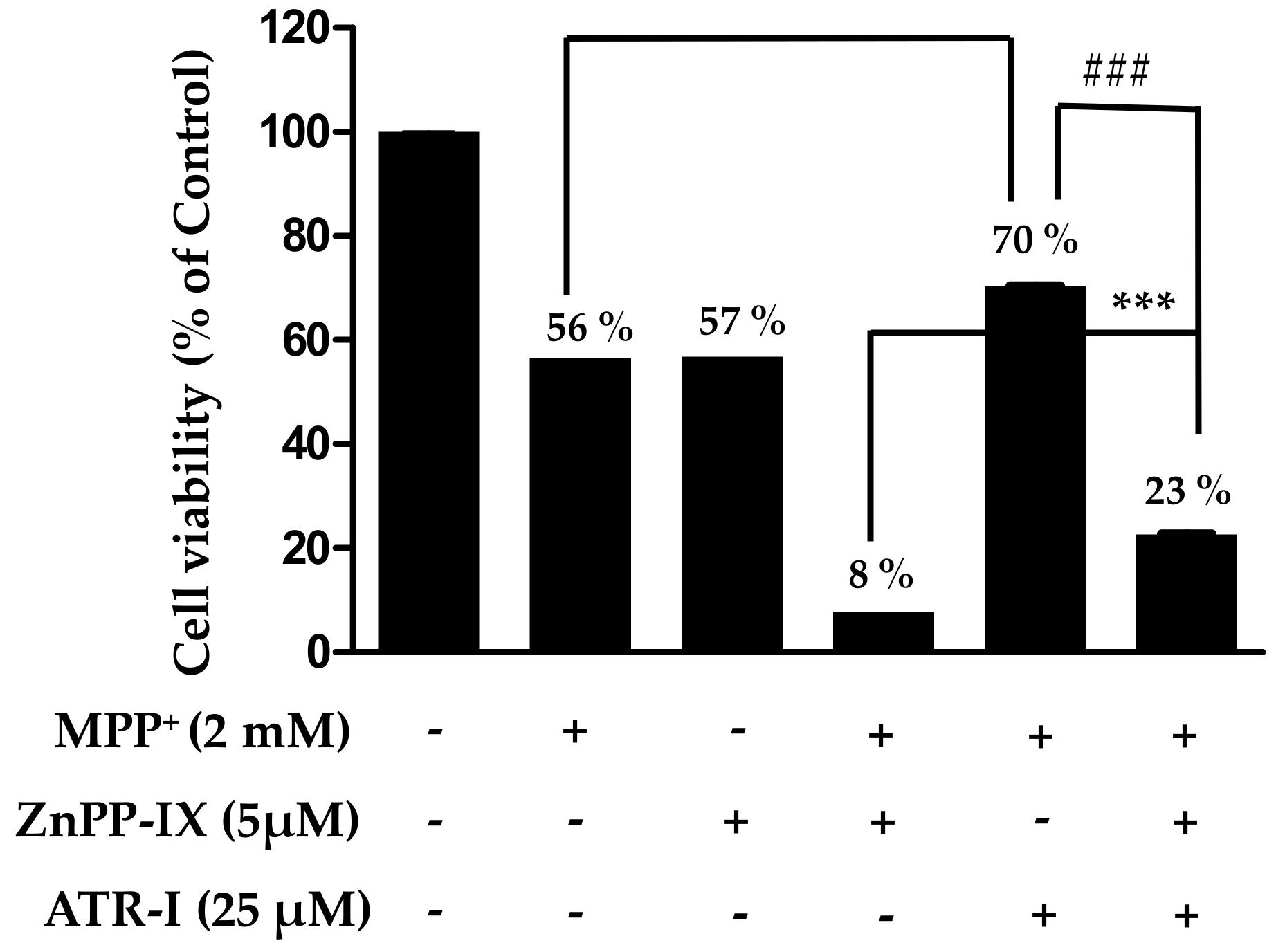
2.1. Effects of Atractylenolide-I (ATR-I) on Cytotoxicity Induced by 1-Methyl-4-Phenylpyridinium (MPP+) in SH-SY5Y Cells
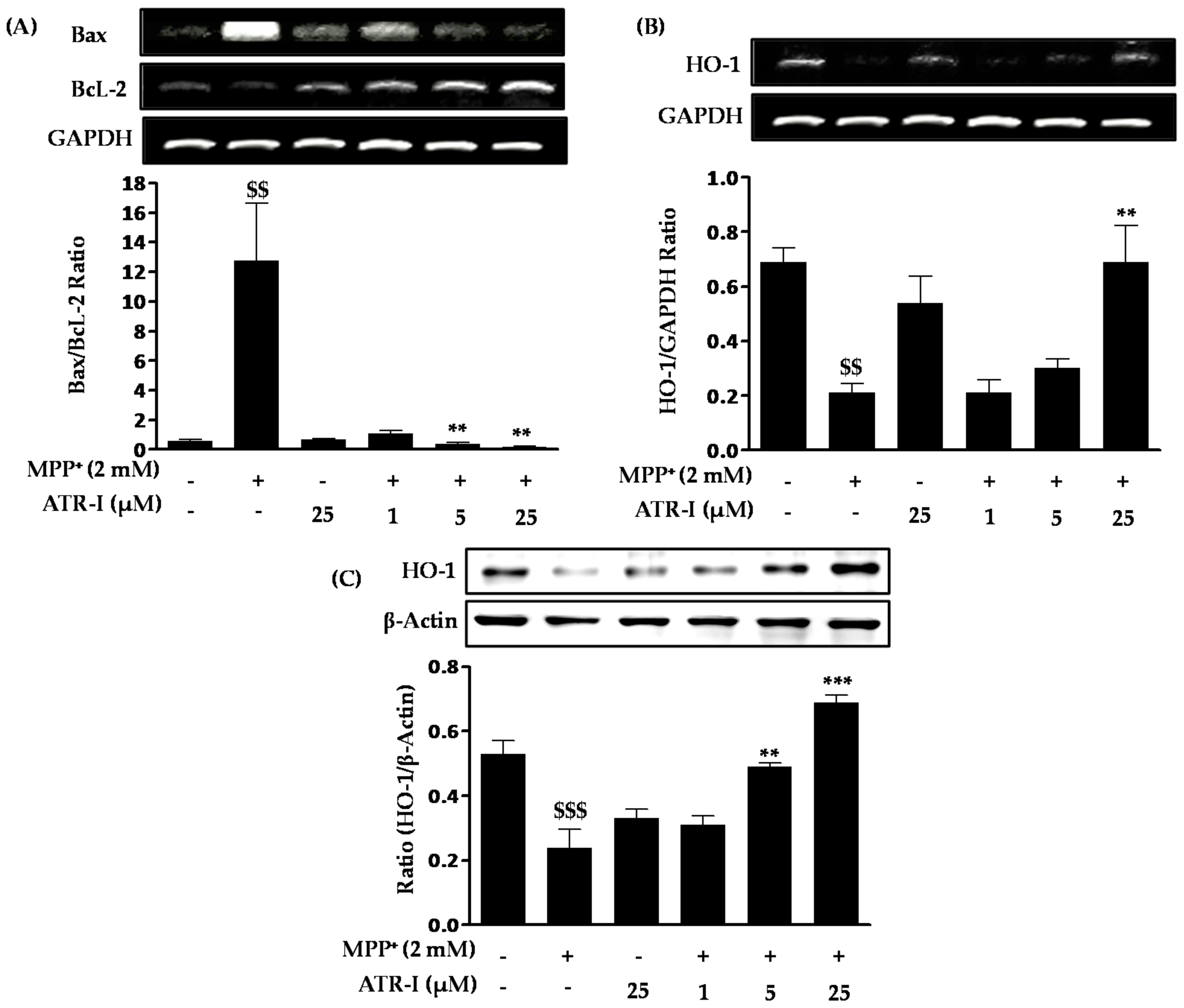
2.2. ATR-I Abates Bax, Bcl-2 Ratios and Upregulates Heme Oxygenase (HO-1) mRNA and Protein Expression in MPP+-Intoxicated SH-SY5Y Cells
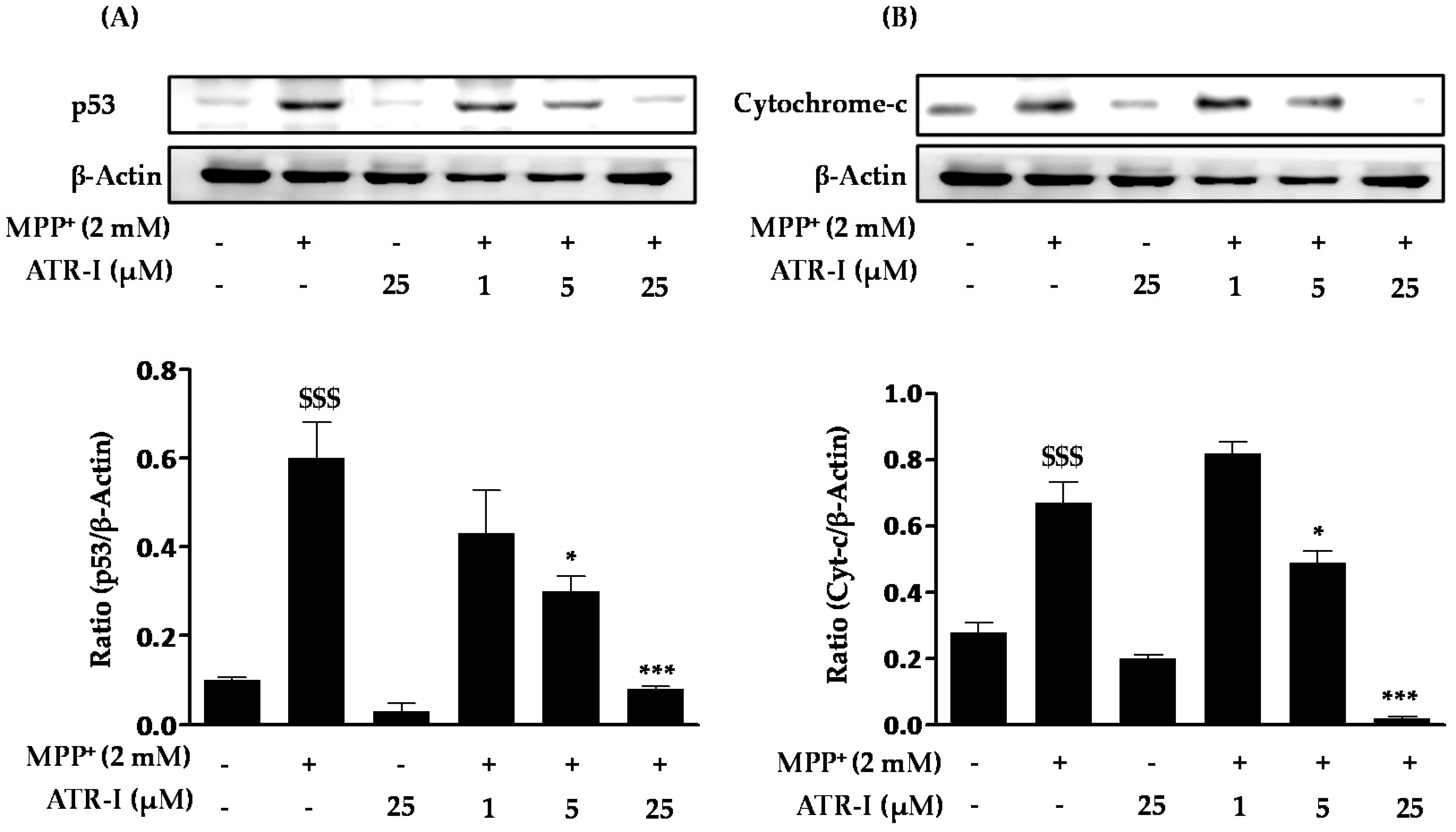
2.3. ATR-I Pre-Treatment Mitigates MPP+-Induced Protein Expression of p53 and Cytochrome-c in SH-SY5Y Cells
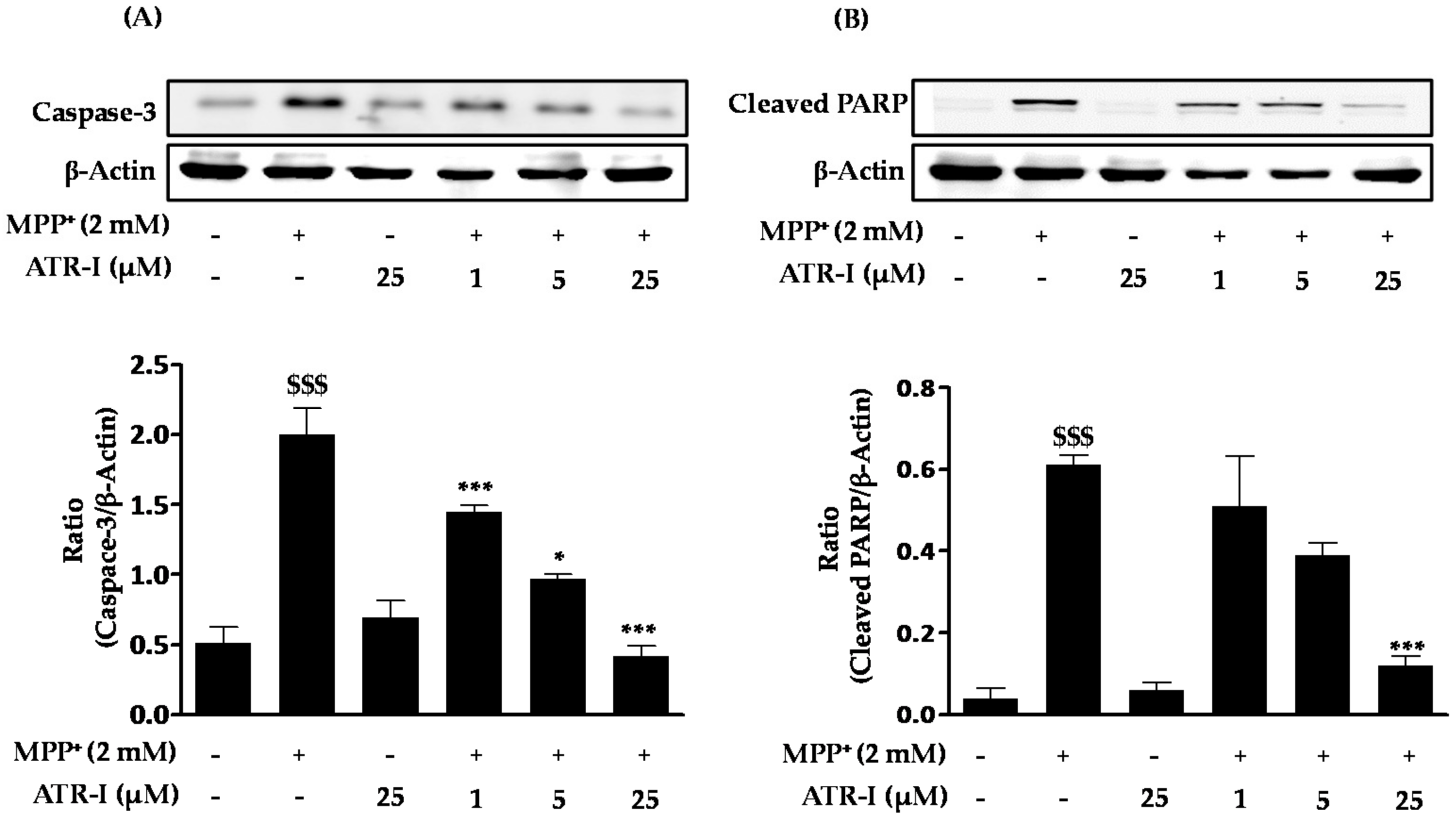
2.4. ATR-I Pre-Treatment Prevents MPP+-Induced Induction of Caspase-3 and Cleaved Poly(ADP-Ribose) Polymerase (PARP) in SH-SY5Y Cells
2.5. Protective Role of HO-1 in MPP+-Intoxicated SH-SY5Y Cells
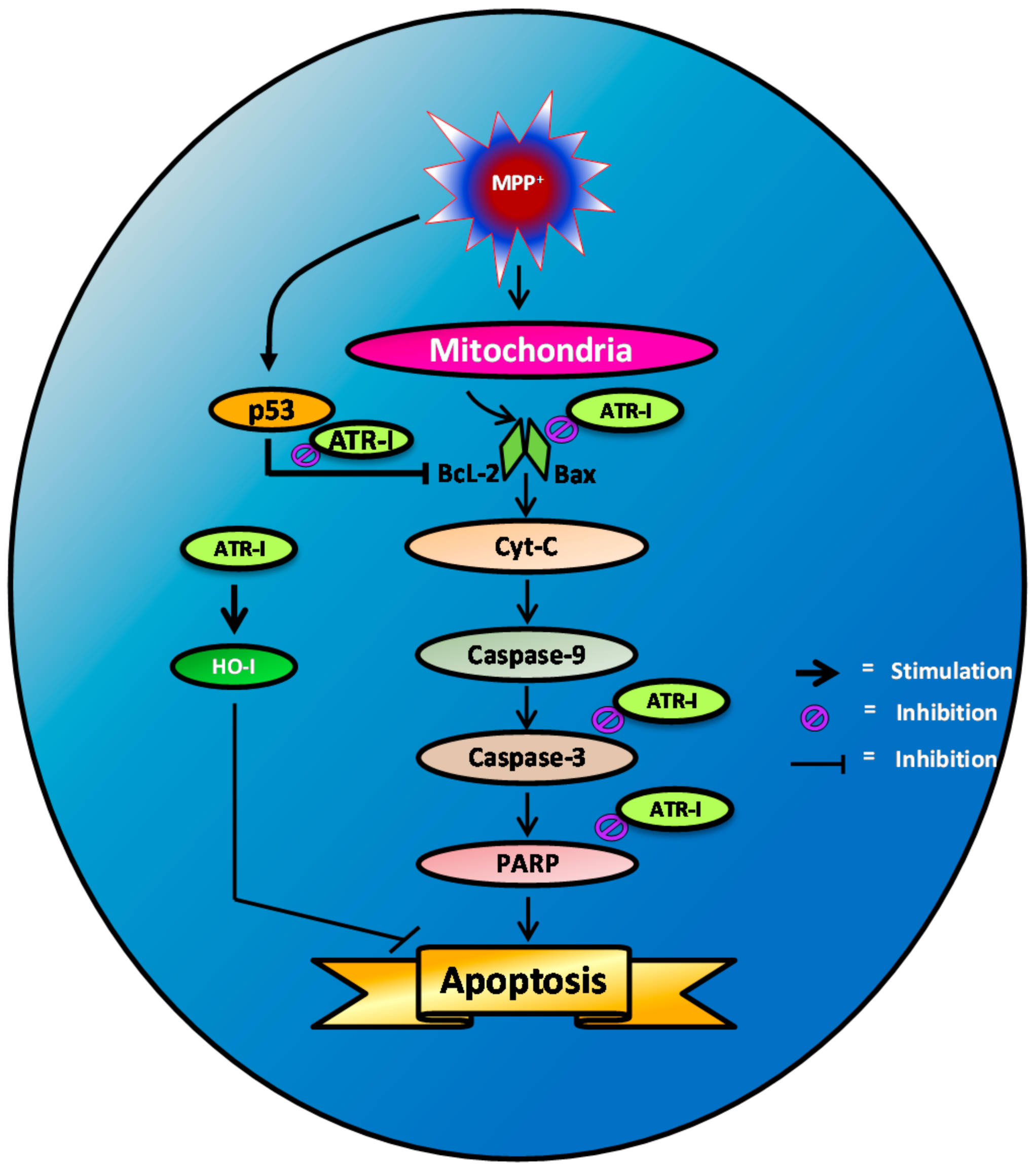
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Treatments
4.3. Measurement of Cell Viability
4.4. Isolation of Total RNA and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot Analysis
4.6. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ATR-I | Atractylenolide-I |
| Apaf | Apoptotic protease activating factor 1 |
| DMSO | Dimethyl sulfoxide |
| HO-1 | Oxygenase-1 |
| MPP | 1-methyl-4-phenylpyridinium |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| PD | Parkinson’s disease |
| PARP | (ADP-ribose) polymerase |
| PCD | Programmed cell death |
| ROS | Reactive oxygen species |
| SN | Substantia nigra |
| ZnPP | Zinc protoporphyrin |
References
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, Y.; Okada, Y.; Akamatsu, W.; Koike, M.; Kuzumaki, N.; Hayakawa, H.; Nihira, T.; Kobayashi, T.; Ohyama, M.; Sato, S.; et al. Mitochondrial dysfunction associated with increased oxidative stress and alpha-synuclein accumulation in PARK2 IPSC-derived neurons and postmortem brain tissue. Mol. Brain 2012, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Licker, V.; Turck, N.; Kovari, E.; Burkhardt, K.; Cote, M.; Surini-Demiri, M.; Lobrinus, J.A.; Sanchez, J.C.; Burkhard, P.R. Proteomic analysis of human substantia nigra identifies novel candidates involved in Parkinson’s disease pathogenesis. Proteomics 2014, 14, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Leist, M.; Jaattela, M. Four deaths and a funeral: From caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2001, 2, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, E.; Fernandez-Moriano, C.; Gomez-Serranillos, M.P. Potential neuroprotective activity of ginseng in Parkinson’s disease: A review. J. Neuroimmune Pharmacol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kost, G.C.; Selvaraj, S.; Lee, Y.B.; Kim, D.J.; Ahn, C.H.; Singh, B.B. Clavulanic acid inhibits MPP+-induced ROS generation and subsequent loss of dopaminergic cells. Brain Res. 2012, 1469, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S.; Jackson-Lewis, V. Mechanisms of mptp toxicity. Mov. Disord. 1998, 13, S35–S38. [Google Scholar]
- Singer, T.P.; Ramsay, R.R. Mechanism of the neurotoxicity of mptp. An update. FEBS Lett. 1990, 274, 1–8. [Google Scholar] [PubMed]
- Dykens, J.A. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated Ca2+ and Na+: Implications for neurodegeneration. J. Neurochem. 1994, 63, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Tieu, K.; Guegan, C.; Caspersen, C.; Jackson-Lewis, V.; Carelli, V.; Martinuzzi, A.; Hirano, M.; Przedborski, S.; Vila, M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc. Natl. Acad. Sci. USA 2005, 102, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Seaton, T.A.; Cooper, J.M.; Schapira, A.H. Free radical scavengers protect dopaminergic cell lines from apoptosis induced by complex I inhibitors. Brain Res. 1997, 777, 110–118. [Google Scholar] [PubMed]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Benabid, A.L.; Sadoul, R.; Verna, J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Zhang, Y.; Dawson, V.L.; Dawson, T.M. Oxidative stress and genetics in the pathogenesis of Parkinson’s disease. Neurobiol. Dis. 2000, 7, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B.; Abbracchio, M.P. To be or not to be (inflamed)—Is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol. Sci. 2005, 26, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- King, T.D.; Bijur, G.N.; Jope, R.S. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3β and attenuated by lithium. Brain Res. 2001, 919, 106–114. [Google Scholar] [CrossRef]
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000, 1, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.-K.; Ou, Y.-C.; Lin, S.-Y.; Pan, H.-C.; Song, P.-J.; Raung, S.-L.; Lai, C.-Y.; Liao, S.-L.; Lu, H.-C.; Chen, C.-J. Luteolin inhibits cytokine expression in endotoxin/cytokine-stimulated microglia. J. Nutr. Biochem. 2011, 22, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.J.; Koh, S.B.; Ban, J.Y.; Seong, Y.H. Protection of NMDA-induced neuronal cell damage by methanol extract of Zizyphi Spinosi Semen in cultured rat cerebellar granule cells. J. Ethnopharmacol. 2004, 95, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Lin, Y. Gastrointestinal inhibitory effects of sesquiterpene lactones from atractylodes macrocephala. Zhong Yao Cai 1999, 22, 636–640. [Google Scholar] [PubMed]
- Wang, Y.; Chang, J.; Li, K.; Wu, C.; Tin, K.; Liu, Y. Studies on the constituents of atractylodes macrocephala koidz. Shanzi Med. J. 1980, 9, 47–51. [Google Scholar]
- Li, C.-Q.; He, L.-C.; Dong, H.-Y.; Jin, J.-Q. Screening for the anti-inflammatory activity of fractions and compounds from Atractylodes macrocephala koidz. J. Ethnopharmacol. 2007, 114, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Chen, C.-C.; Yeh, C.-Y.; Huang, R.-L. Reactive oxygen species mediation of baizhu-induced apoptosis in human leukemia cells. J. Ethnopharmacol. 2005, 97, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, M.A.; Tyrrell, P.J.; King, A.T.; Patel, H.C. Imaging in young adults with intracerebral hemorrhage. Clin. Neurol. Neurosurg. 2012, 114, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, W.C.; Liu, Q.S.; Hu, J.J.; Liu, G.T.; Du, G.H. Pinocembrin prevents glutamate-induced apoptosis in SH-SY5Y neuronal cells via decrease of Bax/Bcl-2 ratio. Eur. J. Pharmacol. 2008, 591, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Kim, M.K.; Kim, H.S.; Chung, H.H.; Song, Y.S. Mitochondrial permeability transition pore as a selective target for anti-cancer therapy. Front. Oncol. 2013, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Robert, G.; Puissant, A.; Dufies, M.; Marchetti, S.; Jacquel, A.; Cluzeau, T.; Colosetti, P.; Belhacene, N.; Kahle, P.; da Costa, C.A.; et al. The caspase 6 derived N-terminal fragment of DJ-1 promotes apoptosis via increased ROS production. Cell Death Differ. 2012, 19, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Park, C.S.; Lee, S.K.; Shin, D.W.; Kang, J.H. Leptin inhibits 1-methyl-4-phenylpyridinium-induced cell death in SH-SY5Y cells. Neurosci. Lett. 2006, 407, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Mena, N.P.; Urrutia, P.J.; Lourido, F.; Carrasco, C.M.; Nunez, M.T. Mitochondrial iron homeostasis and its dysfunctions in neurodegenerative disorders. Mitochondrion 2015, 21, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Fall, C.P.; Bennett, J.P. Characterization and time course of MPP+-induced apoptosis in human SH-SY5Y neuroblastoma cells. J. Neurosci. Res. 1999, 55, 620–628. [Google Scholar] [CrossRef]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.; Soares, M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, H.; Basuroy, S.; Bhattacharya, S.; Tcheranova, D.; Qu, Y.; Regan, R.F.; Leffler, C.W. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: Contributions of HO-1 and HO-2 to cytoprotection. Am. J. Physiol. Cell Physiol. 2006, 290, C1399–C1410. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, A.; Dimova, E.Y.; Horbach, T.; Teplyuk, N.; Immenschuh, S.; Kietzmann, T. Opposite expression of the antioxidant heme oxygenase-1 in primary cells and tumor cells: Regulation by interaction of USF-2 and Fra-1. Antioxid. Redox Signal. 2008, 10, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Dal-Cim, T.; Molz, S.; Egea, J.; Parada, E.; Romero, A.; Budni, J.; Martin de Saavedra, M.D.; del Barrio, L.; Tasca, C.I.; Lopez, M.G. Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase-1 via PI3K/Akt/GSK-3β pathway. Neurochem. Int. 2012, 61, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Cha, Y.N.; Surh, Y.J. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of Nf-E2-related factor 2 in PC12 cells. Free Radic. Biol. Med. 2006, 41, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Chida, A.S.; Rahman, I. Redox modifications of protein-thiols: Emerging roles in cell signaling. Biochem. Pharmacol. 2006, 71, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Alberio, T.; Lopiano, L.; Fasano, M. Cellular models to investigate biochemical pathways in Parkinson’s disease. FEBS J. 2012, 279, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.T.; Dinis-Oliveira, R.J.; de Lourdes Bastos, M.; Tsatsakis, A.M.; Duarte, J.A.; Carvalho, F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases—A mechanistic approach. Toxicol. Lett. 2014, 230, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Culmsee, C.; Mattson, M.P. p53 in neuronal apoptosis. Biochem. Biophys. Res. Commun. 2005, 331, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Trimmer, P.A.; Smith, T.S.; Jung, A.B.; Bennett, J.P., Jr. Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration 1996, 5, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Zhu, X.; Ladenheim, B.; Yu, Q.S.; Guo, Z.; Oyler, J.; Cutler, R.G.; Cadet, J.L.; Greig, N.H.; Mattson, M.P. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann. Neurol. 2002, 52, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Bove, J.; Wu, D.C.; Dehay, B.; Choi, D.K.; Jackson-Lewis, V.; Rathke-Hartlieb, S.; Bouillet, P.; Strasser, A.; Schulz, J.B.; et al. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 8161–8166. [Google Scholar] [CrossRef] [PubMed]
- Ronca, F.; Chan, S.L.; Yu, V.C. 1-(5-isoquinolinesulfonyl)-2-methylpiperazine induces apoptosis in human neuroblastoma cells, SH-SY5Y, through a p53-dependent pathway. J. Biol. Chem. 1997, 272, 4252–4260. [Google Scholar] [CrossRef] [PubMed]
- Sanz, E.; Quintana, A.; Battaglia, V.; Toninello, A.; Hidalgo, J.; Ambrosio, S.; Valoti, M.; Marco, J.L.; Tipton, K.F.; Unzeta, M. Anti-apoptotic effect of Mao-B inhibitor PF9601N [N-(2-propynyl)-2-(5-benzyloxy-indolyl) methylamine] is mediated by p53 pathway inhibition in MPP+-treated SH-SY5Y human dopaminergic cells. J. Neurochem. 2008, 105, 2404–2417. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T.; Reed, J.C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995, 80, 293–299. [Google Scholar] [PubMed]
- Hartmann, A.; Michel, P.P.; Troadec, J.D.; Mouatt-Prigent, A.; Faucheux, B.A.; Ruberg, M.; Agid, Y.; Hirsch, E.C. Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson’s disease? J. Neurochem. 2001, 76, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. Bcl-2 family members and the mitochondria in apoptosis. Gene Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, K.L.; Liu, J.; Lotharius, J.; Holtz, W. Targeted expression of Bcl-2 attenuates MPP+ but not 6-OHDA induced cell death in dopaminergic neurons. Neurobiol. Dis. 2003, 14, 43–51. [Google Scholar] [CrossRef]
- McDonnell, T.J.; Beham, A.; Sarkiss, M.; Andersen, M.M.; Lo, P. Importance of the Bcl-2 family in cell death regulation. Experientia 1996, 52, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.M.; Moulder, K.L.; Knudson, C.M.; Creedon, D.J.; Deshmukh, M.; Korsmeyer, S.J.; Johnson, E.M., Jr. Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J. Cell. Biol. 1997, 139, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J.; Riggins, J.N.; West, J.D. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Investig. 2003, 111, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Doo, A.R.; Kim, S.N.; Kim, S.T.; Park, J.Y.; Chung, S.H.; Choe, B.Y.; Chae, Y.; Lee, H.; Yin, C.S.; Park, H.J. Bee venom protects SH-SY5Y human neuroblastoma cells from 1-methyl-4-phenylpyridinium-induced apoptotic cell death. Brain Res. 2012, 1429, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kanthasamy, A.G.; Anantharam, V.; Zhang, D.; Latchoumycandane, C.; Jin, H.; Kaul, S.; Kanthasamy, A. A novel peptide inhibitor targeted to caspase-3 cleavage site of a proapoptotic kinase protein kinase c δ (PKCδ) protects against dopaminergic neuronal degeneration in Parkinson’s disease models. Free Radical Biol. Med. 2006, 41, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Le, D.A.; Wu, Y.; Huang, Z.; Matsushita, K.; Plesnila, N.; Augustinack, J.C.; Hyman, B.T.; Yuan, J.; Kuida, K.; Flavell, R.A.; et al. Caspase activation and neuroprotection in caspase-3-deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 15188–15193. [Google Scholar] [CrossRef] [PubMed]
- Datki, Z.; Juhasz, A.; Galfi, M.; Soos, K.; Papp, R.; Zadori, D.; Penke, B. Method for measuring neurotoxicity of aggregating polypeptides with the mtt assay on differentiated neuroblastoma cells. Brain Res. Bull. 2003, 62, 223–229. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Park, J.Y.; Kim, B.W.; Kumar, H.; Lim, H.W.; Kang, S.M.; Koppula, S.; Yoon, S.H.; Choi, D.K. Anti-neuroinflammatory activity of a novel cannabinoid derivative by inhibiting the NF-κ signaling pathway in lipopolysaccharide-induced BV-2 microglial cells. J. Pharmacol. Sci. 2013, 121, 119–130. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
More, S.V.; Choi, D.-K. Atractylenolide-I Protects Human SH-SY5Y Cells from 1-Methyl-4-Phenylpyridinium-Induced Apoptotic Cell Death. Int. J. Mol. Sci. 2017, 18, 1012. https://doi.org/10.3390/ijms18051012
More SV, Choi D-K. Atractylenolide-I Protects Human SH-SY5Y Cells from 1-Methyl-4-Phenylpyridinium-Induced Apoptotic Cell Death. International Journal of Molecular Sciences. 2017; 18(5):1012. https://doi.org/10.3390/ijms18051012
Chicago/Turabian StyleMore, Sandeep Vasant, and Dong-Kug Choi. 2017. "Atractylenolide-I Protects Human SH-SY5Y Cells from 1-Methyl-4-Phenylpyridinium-Induced Apoptotic Cell Death" International Journal of Molecular Sciences 18, no. 5: 1012. https://doi.org/10.3390/ijms18051012





