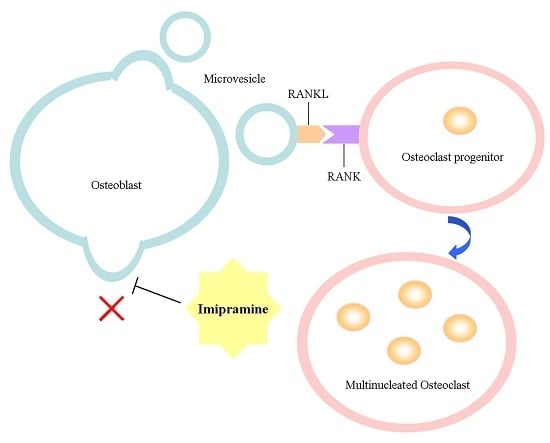
Imipramine Protects against Bone Loss by Inhibition of Osteoblast-Derived Microvesicles
Abstract
:1. Introduction
2. Results
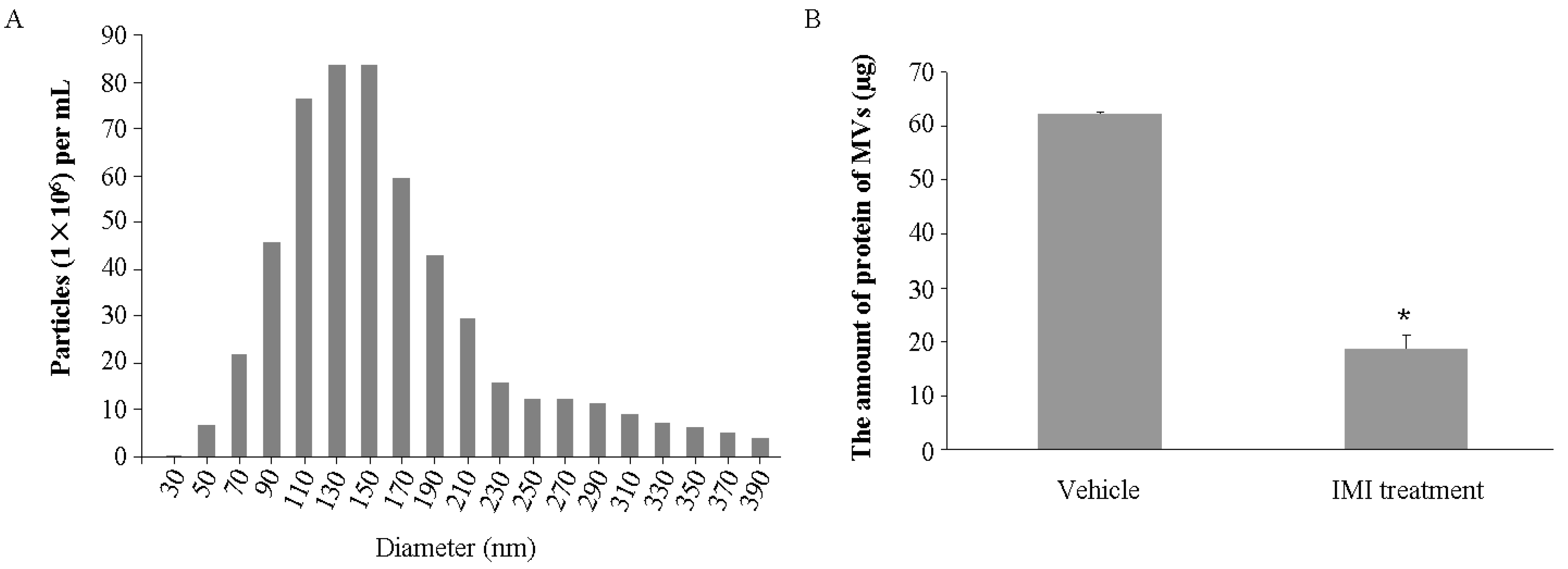
2.1. Imipramine Blocks MV Generation from Osteoblasts
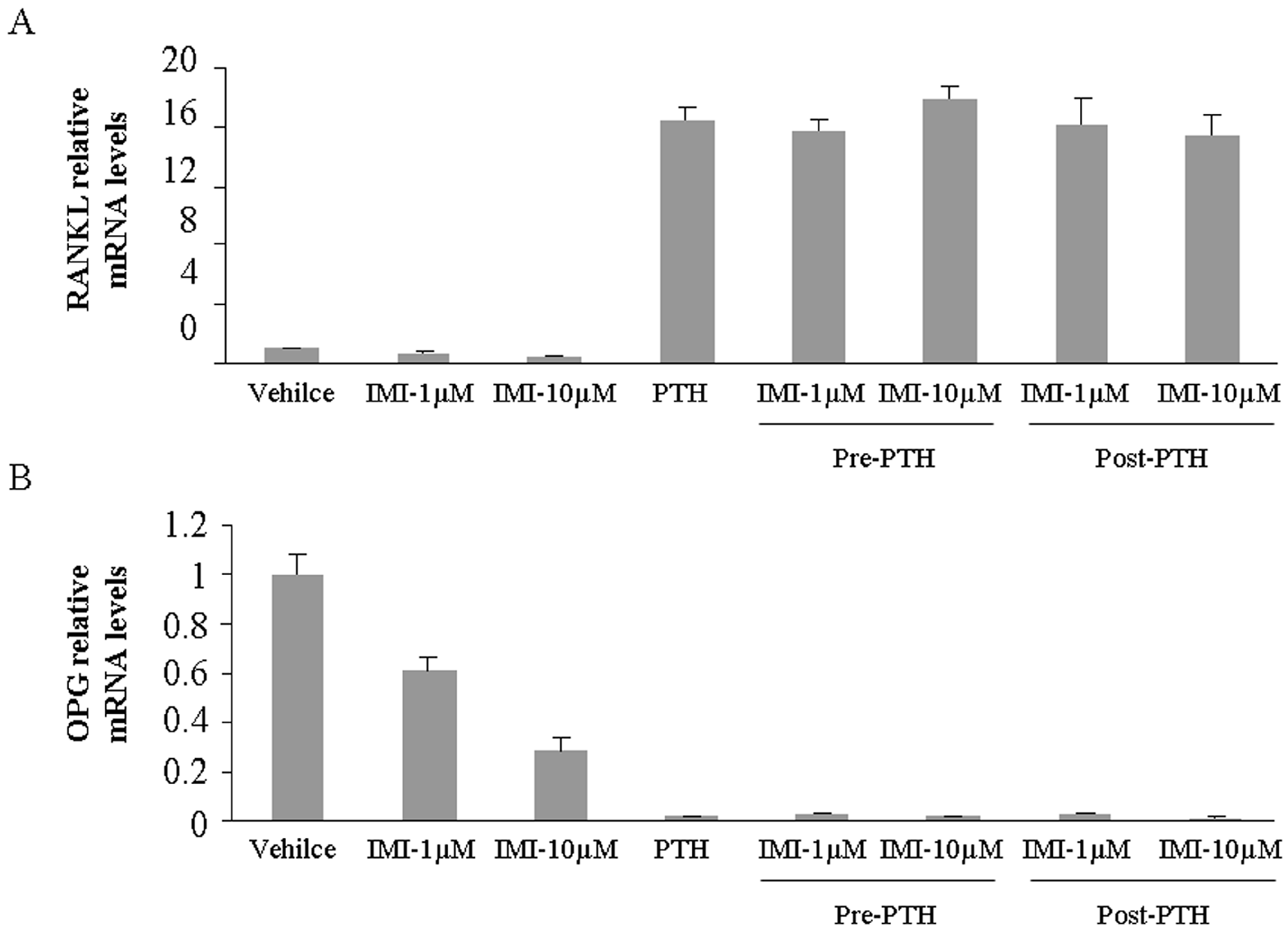
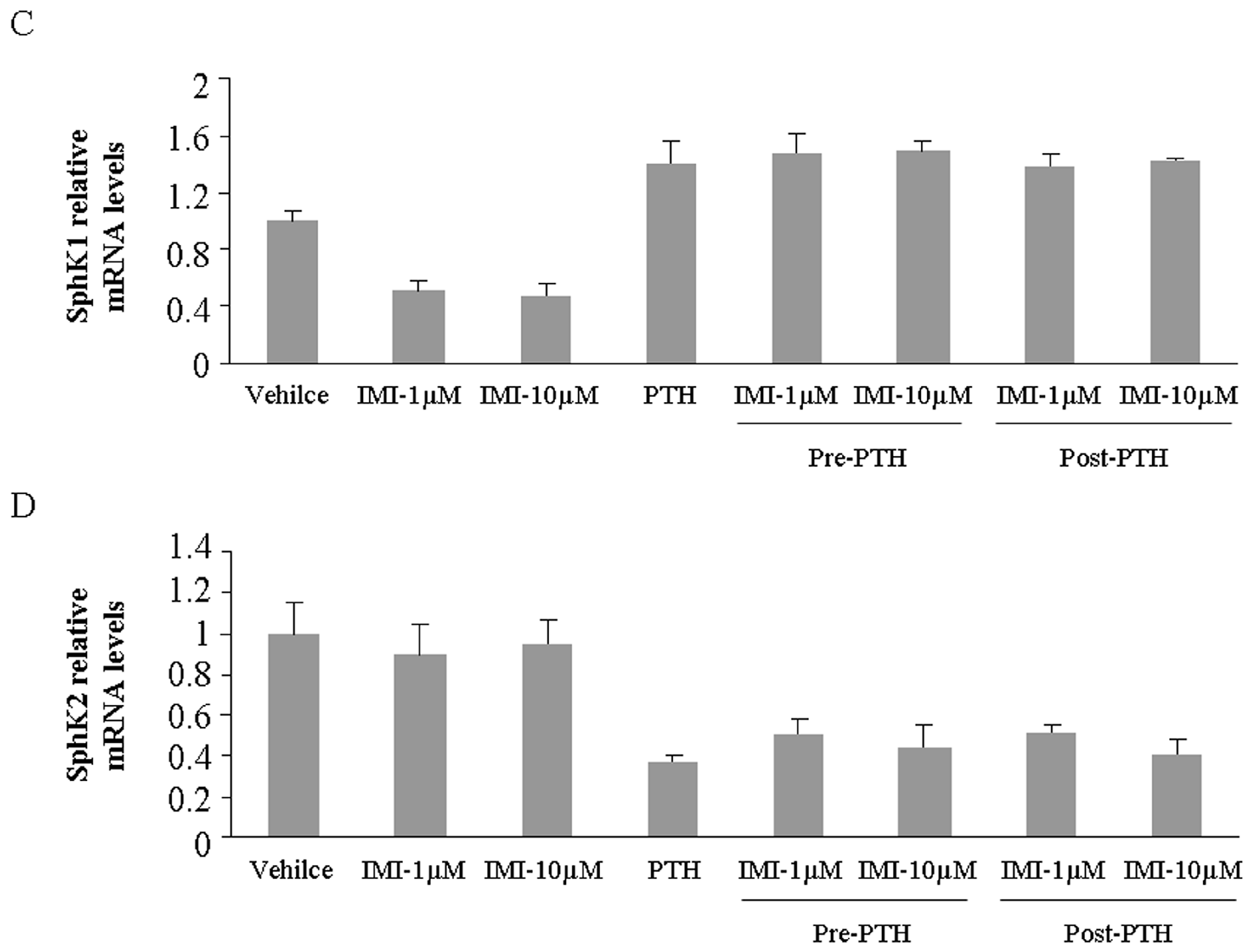
2.2. Imipramine Slightly Reduces Osteoblast Differentiation Gene Expression, but Does Not Affect Parathyroid Hormone Regulation
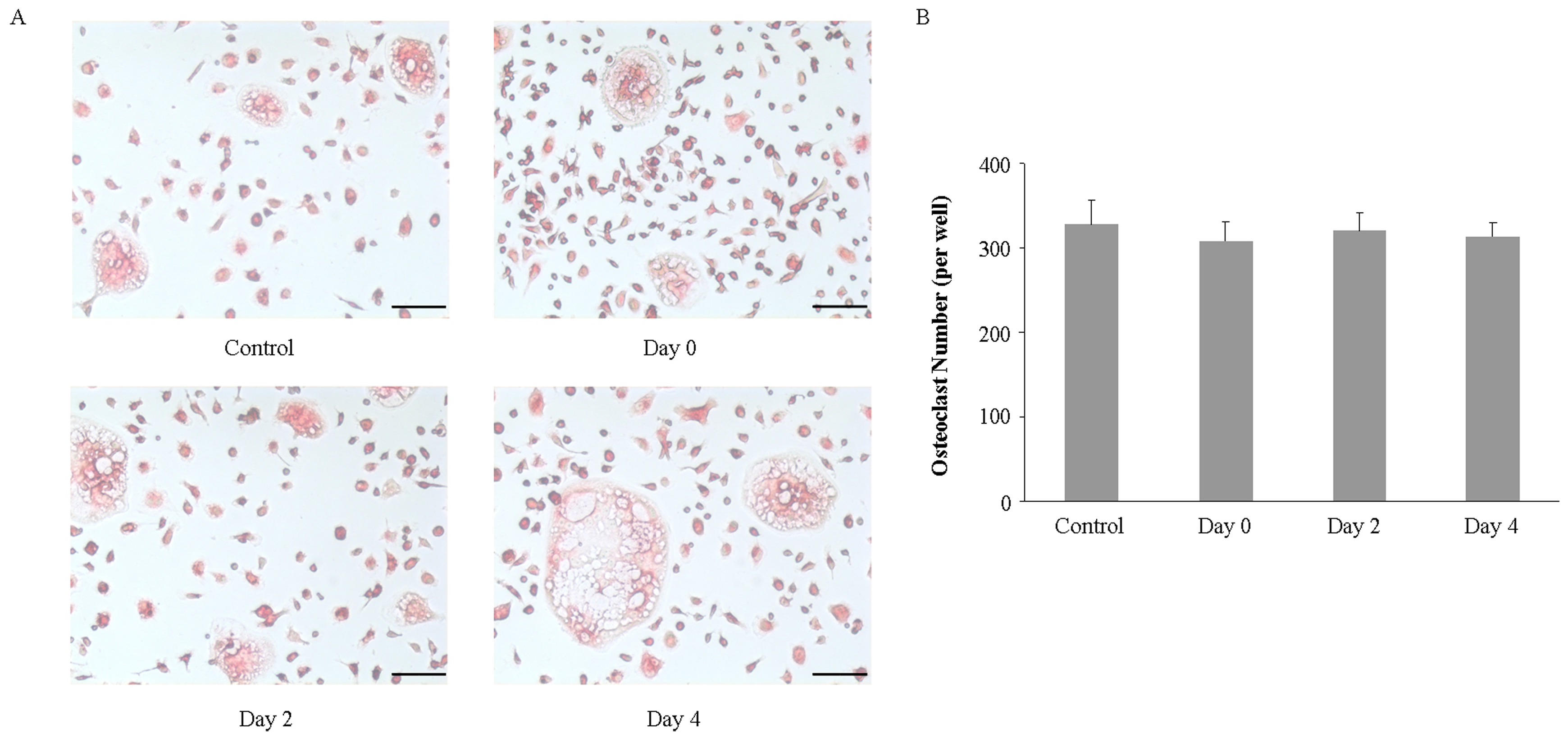
2.3. Imipramine Does Not Affect RANKL-Mediated Osteoclast Formation
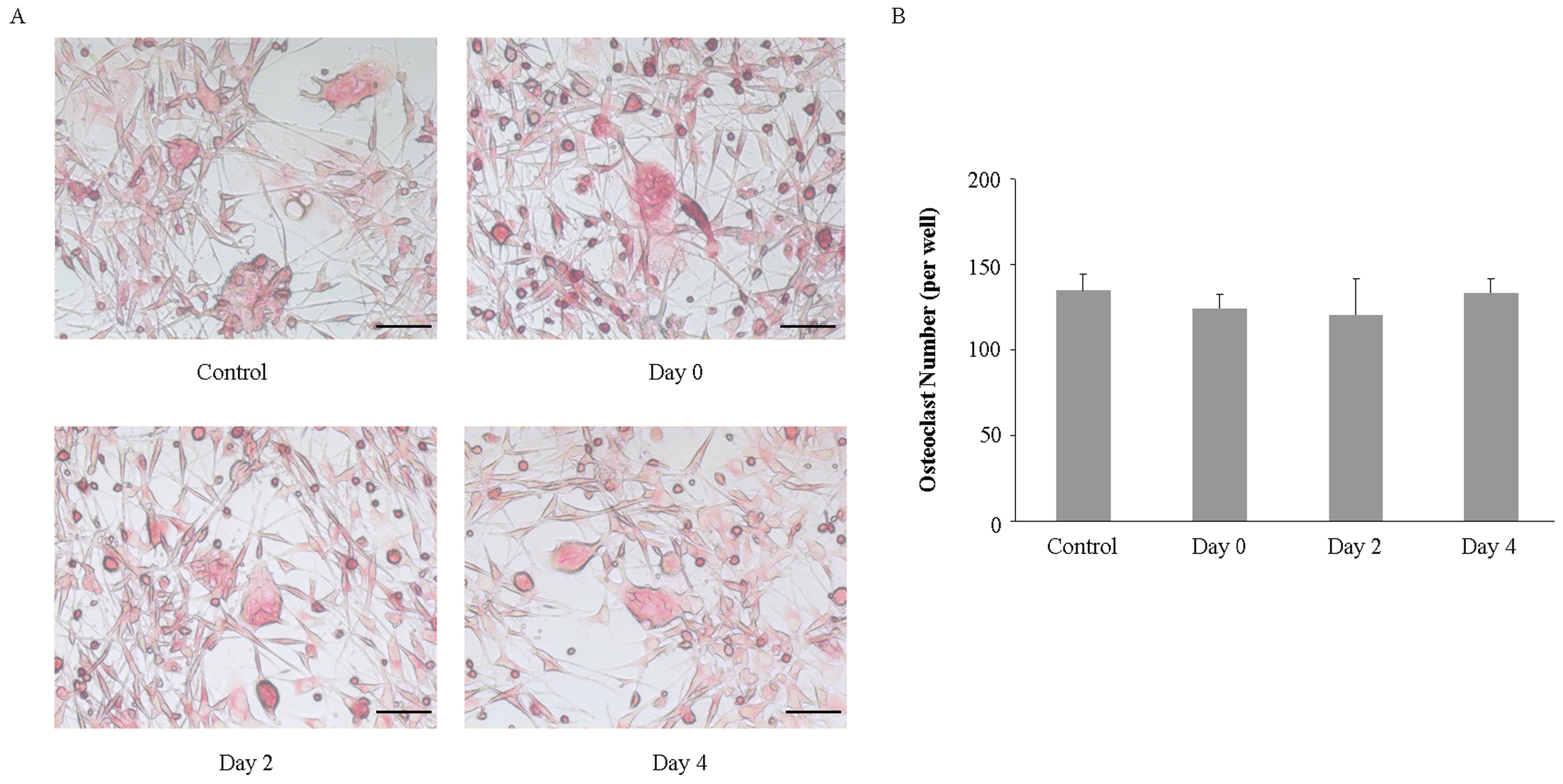
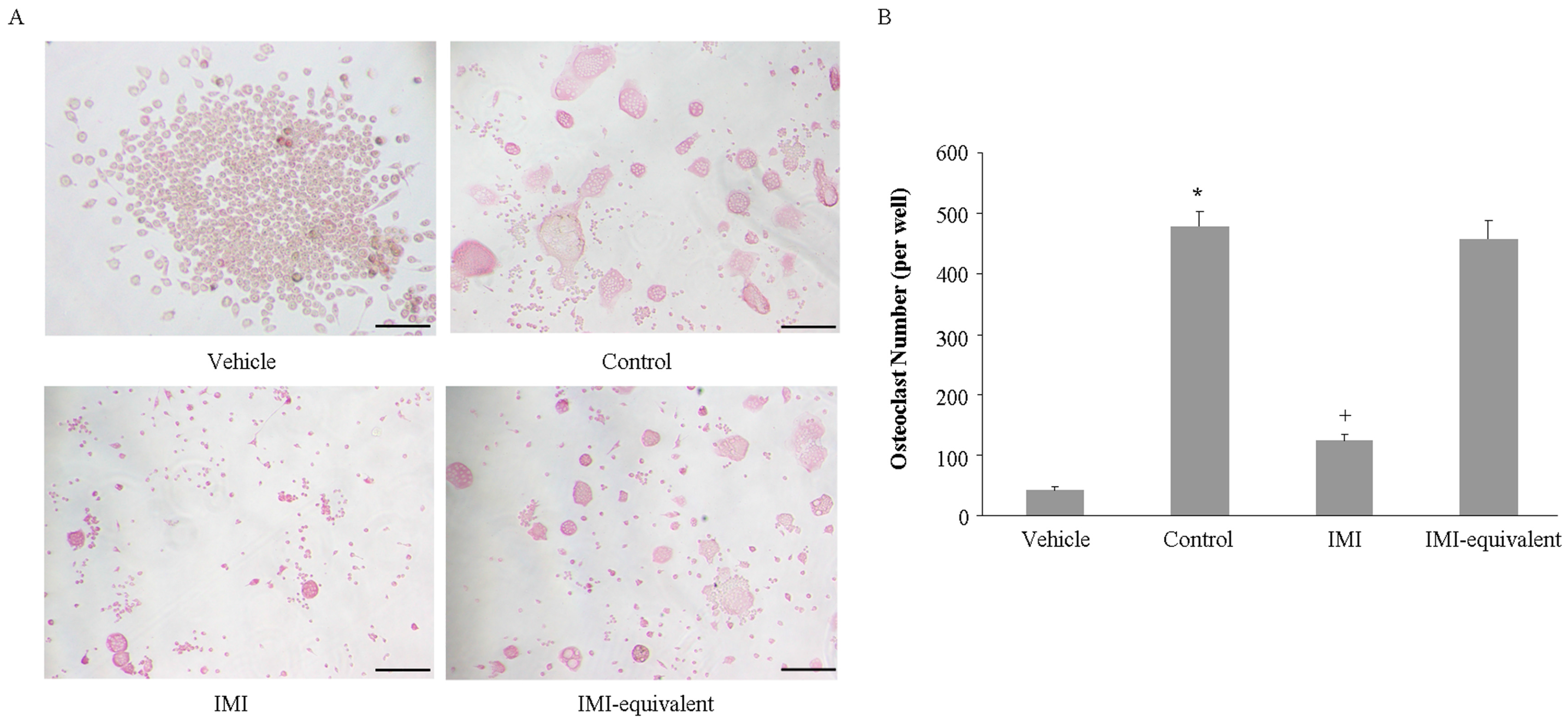
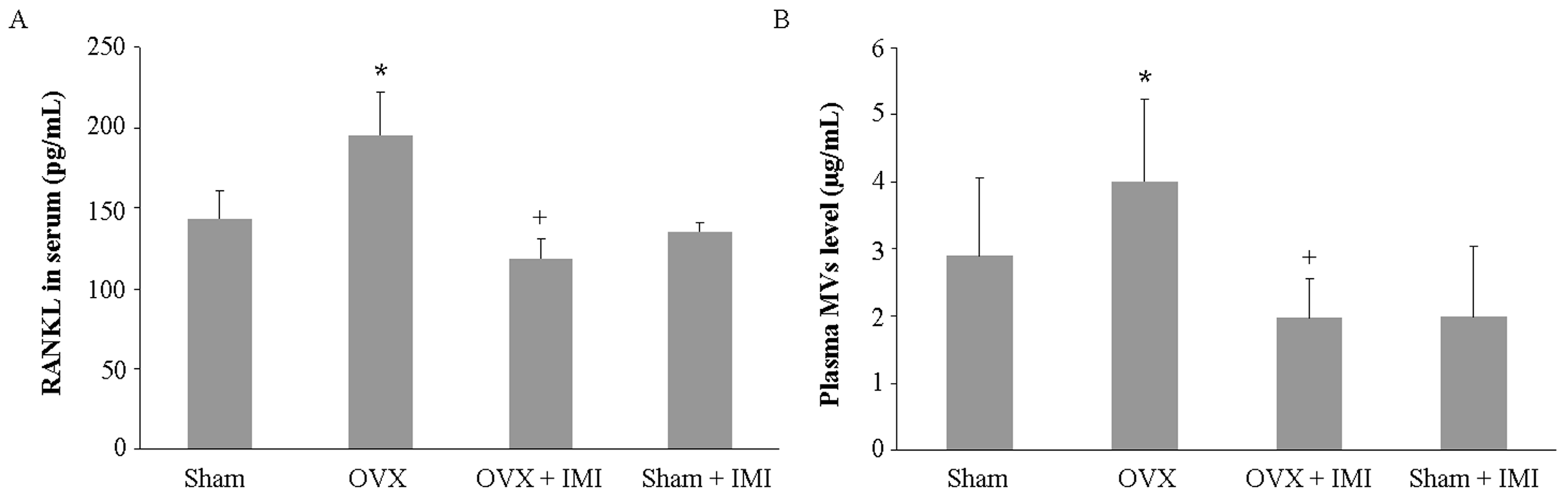
2.4. Imipramine Affects MV-Induced Osteoclast Formation
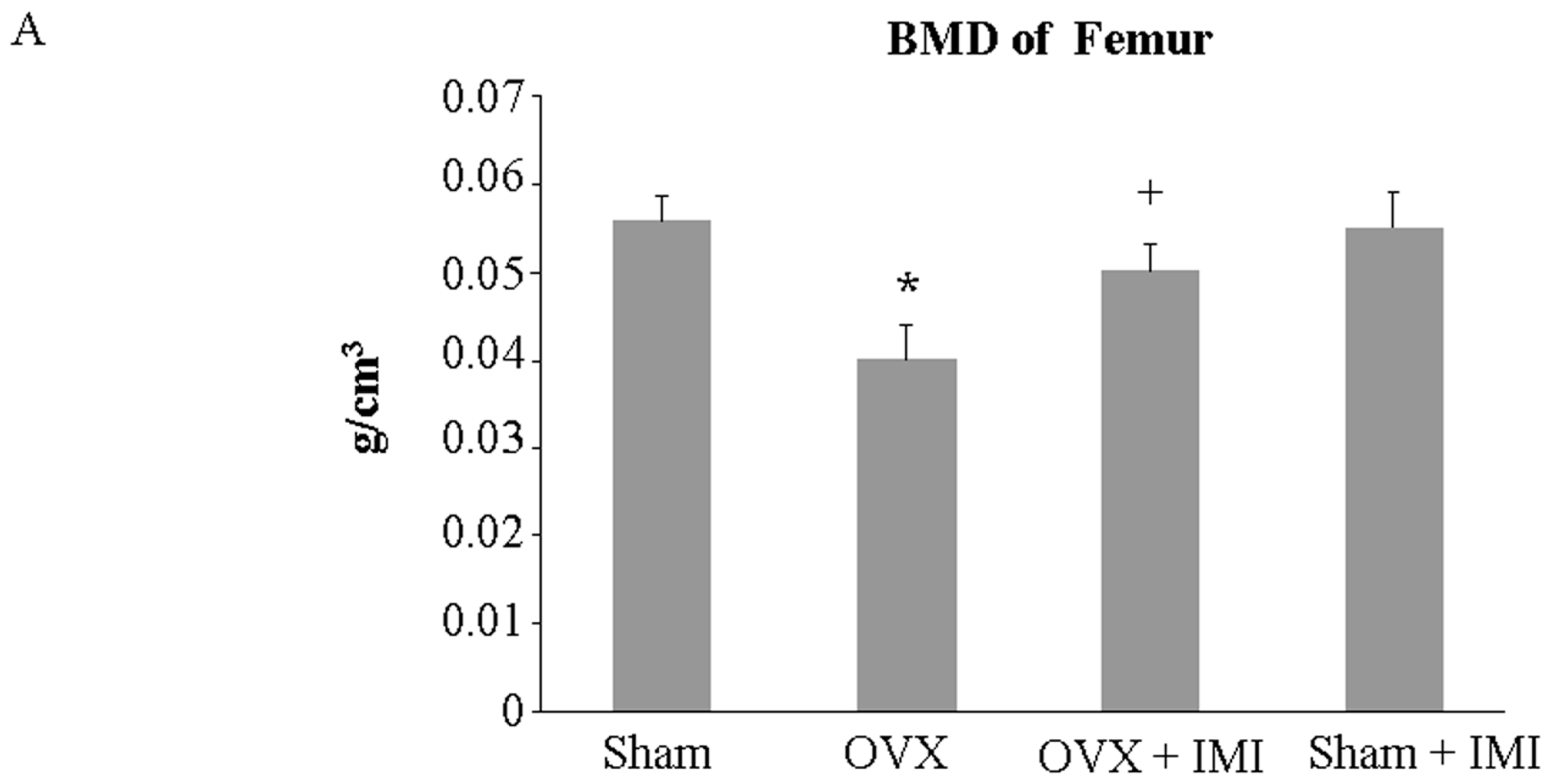
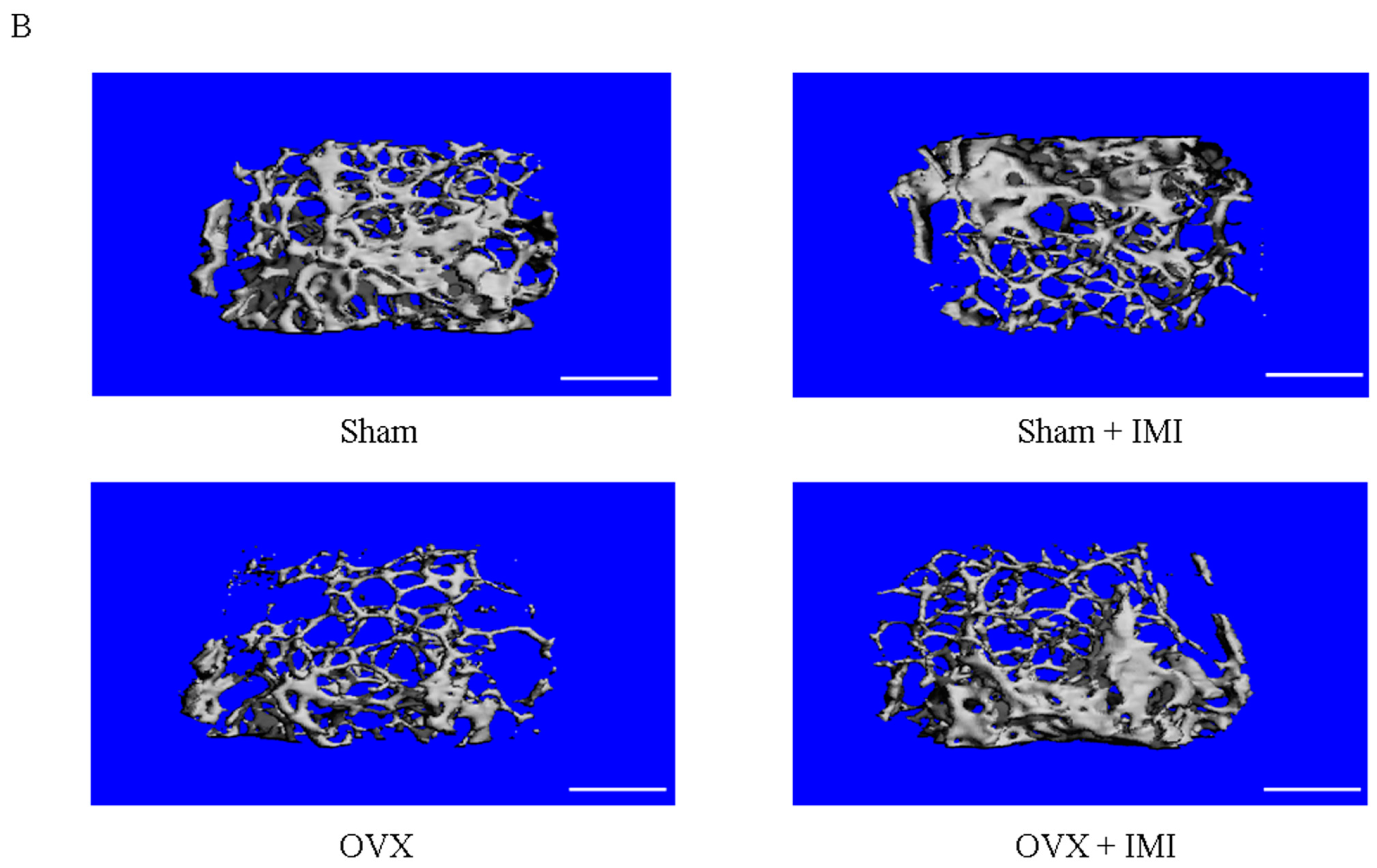
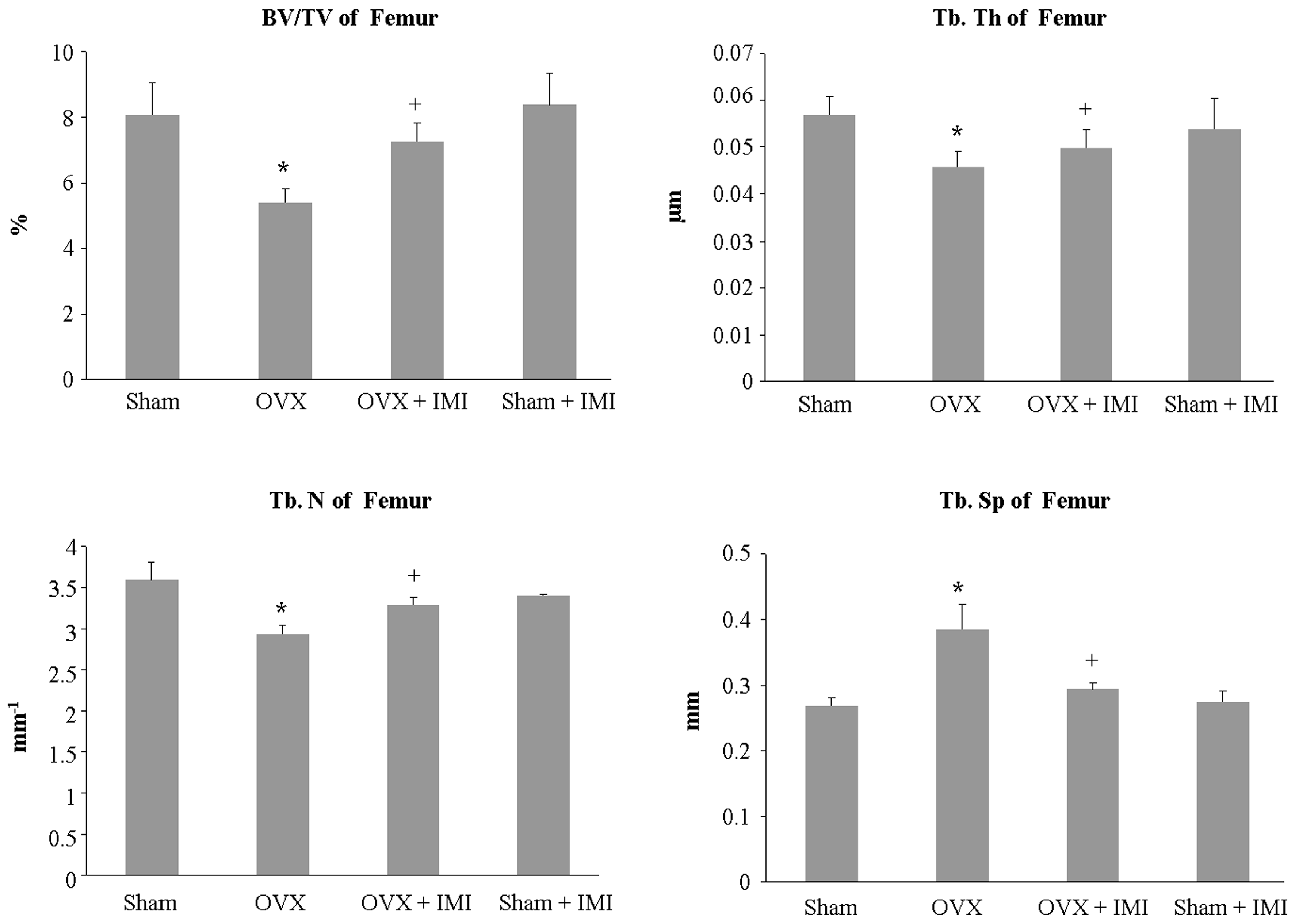
2.5. Imipramine Protects against Ovariectomy-Induced Bone Loss
3. Discussion
4. Materials and Methods
4.1. MV Isolation from Osteoblastic Cell Culture and Plasma
4.2. MV Characterization and Quantification
4.3. Osteoclast Formation Assay
4.4. Animals
4.5. Bone Histomorphometric and Micro-CT Analysis
4.6. Serum Biochemical Marker of Bone Metabolism
4.7. Gene Expression Analysis
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Muller, G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab. Syndr. Obes. 2012, 5, 247–282. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; D'Asti, E.; Magnus, N.; Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles as mediators of intercellular communication in cancer—The emerging science of cellular ”debris”. Semin. Immunopathol. 2011, 33, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, A.; Wilson, H.L.; Kiss-Toth, E.; Dower, S.K.; North, R.A.; Surprenant, A. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity 2001, 15, 825–835. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Slotte, J.P.; Hedstrom, G.; Rannstrom, S.; Ekman, S. Effects of sphingomyelin degradation on cell cholesterol oxidizability and steady-state distribution between the cell surface and the cell interior. Biochim. Biophys. Acta 1989, 985, 90–96. [Google Scholar] [CrossRef]
- Neufeld, E.B.; Cooney, A.M.; Pitha, J.; Dawidowicz, E.A.; Dwyer, N.K.; Pentchev, P.G.; Blanchette-Mackie, E.J. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J. Biol. Chem. 1996, 271, 21604–21613. [Google Scholar] [PubMed]
- Chang, C.P.; Zhao, J.; Wiedmer, T.; Sims, P.J. Contribution of platelet microparticle formation and granule secretion to the transmembrane migration of phosphatidylserine. J. Biol. Chem. 1993, 268, 7171–7178. [Google Scholar] [PubMed]
- Tepper, A.D.; Ruurs, P.; Wiedmer, T.; Sims, P.J.; Borst, J.; van Blitterswijk, W.J. Sphingomyelin hydrolysis to ceramide during the execution phase of apoptosis results from phospholipid scrambling and alters cell-surface morphology. J. Cell Biol. 2000, 150, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Albouz, S.; Hauw, J.J.; Berwald-Netter, Y.; Boutry, J.M.; Bourdon, R.; Baumann, N. Tricyclic antidepressants induce sphingomyelinase deficiency in fibroblast and neuroblastoma cell cultures. Biomedicine 1981, 35, 218–220. [Google Scholar] [PubMed]
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009, 28, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, Y.; Peng, Y.; Wu, Y.; Ding, Y.; Jiang, Y.; Shen, Z.; Fu, Q. Osteoblast-derived microvesicles: A novel mechanism for communication between osteoblasts and osteoclasts. Bone 2015, 79, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Jilka, R.L.; Manolagas, S.C.; O’Brien, C.A. Parathyroid hormone stimulates receptor activator of NF-κBligand and inhibits osteoprotegerin expression via protein kinase a activation of camp-response element-binding protein. J. Biol. Chem. 2002, 277, 48868–48875. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kim, H.J.; Chang, E.J.; Huang, H.; Banno, Y.; Kim, H.H. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006, 25, 5840–5851. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Ogawa, T.; Se, S.; Akiyama, M.; Bahtiar, A.; Takeya, T.; Ishida-Kitagawa, N. Acid sphingomyelinase regulates osteoclastogenesis by modulating sphingosine kinases downstream of rankl signaling. Biochem. Biophys. Res. Commun. 2011, 405, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Hugel, B.; Martinez, M.C.; Kunzelmann, C.; Freyssinet, J.M. Membrane microparticles: Two sides of the coin. Physiology 2005, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.G.; Chammas, R.; Monteiro, R.Q.; Moreira, M.E.; Barcinski, M.A. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 2009, 283, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.M.; Angelova, M.I.; Kinnunen, P.K. Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophys. J. 2000, 78, 830–838. [Google Scholar] [CrossRef]
- Brown, W.A.; Rosdolsky, M. The clinical discovery of imipramine. Am. J. Psychiatry 2015, 172, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.; Sharma, D.; Gulbins, E.; Becker, K.A.; Edelmann, B. Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Front. Physiol. 2014, 5, 331. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, F.; Martinez, P.E.; Torvik, S.; Phillips, T.M.; Sternberg, E.M.; Mistry, S.; Ronsaville, D.; Wesley, R.; Toomey, C.; Sebring, N.G.; et al. Low bone mass in premenopausal women with depression. Arch. Intern Med. 2007, 167, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Gagne, J.J.; Patrick, A.R.; Mogun, H.; Solomon, D.H. Antidepressants and fracture risk in older adults: A comparative safety analysis. Clin. Pharmacol. Ther. 2011, 89, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Rauma, P.H.; Honkanen, R.J.; Williams, L.J.; Tuppurainen, M.T.; Kroger, H.P.; Koivumaa-Honkanen, H. Effects of antidepressants on postmenopausal bone loss—A 5-year longitudinal study from the ostpre cohort. Bone 2016, 89, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.J.; Blackwell, T.L.; Stone, K.L.; Yaffe, K.; Haney, E.M.; Bliziotes, M.M.; Ensrud, K.E. Use of antidepressants and rates of hip bone loss in older women: The study of osteoporotic fractures. Arch. Intern. Med. 2007, 167, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W.; Pavlatou, M.G.; Michelson, D.; Mouro, C.M.; Kling, M.A.; Wong, M.L.; Licinio, J.; Goldstein, S.A. Chronic administration of anticonvulsants but not antidepressants impairs bone strength: Clinical implications. Transl. Psychiatry 2015, 5, e576. [Google Scholar] [CrossRef] [PubMed]
- Atteritano, M.; Lasco, A.; Mazzaferro, S.; Macri, I.; Catalano, A.; Santangelo, A.; Bagnato, G.; Frisina, N. Bone mineral density, quantitative ultrasound parameters and bone metabolism in postmenopausal women with depression. Intern. Emerg. Med. 2013, 8, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Cutolo, M.; Pacifici, R. Evolutionary medicine and bone loss in chronic inflammatory diseases—A theory of inflammation-related osteopenia. Semin. Arthritis Rheum. 2015, 45, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Trzonkowski, P.; Mysliwska, J.; Godlewska, B.; Szmit, E.; Lukaszuk, K.; Wieckiewicz, J.; Brydak, L.; Machala, M.; Landowski, J.; Mysliwski, A. Immune consequences of the spontaneous pro-inflammatory status in depressed elderly patients. Brain Behav. Immun. 2004, 18, 135–148. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G. Purinoceptors: Are there families of P2X and P2Ypurinoceptors? Pharmacol. Ther. 1994, 64, 445–475. [Google Scholar] [CrossRef]
- Orriss, I.R.; Key, M.L.; Brandao-Burch, A.; Patel, J.J.; Burnstock, G.; Arnett, T.R. The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: The role of P2X receptors. Bone 2012, 51, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Buckley, K.A.; Hipskind, R.A.; Gartland, A.; Bowler, W.B.; Gallagher, J.A. Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-kappa b ligand. Bone 2002, 31, 582–590. [Google Scholar] [CrossRef]
- Grol, M.W.; Panupinthu, N.; Korcok, J.; Sims, S.M.; Dixon, S.J. Expression, signaling, and function of P2X7 receptors in bone. Purinergic Signal. 2009, 5, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Gartland, A.; Hipskind, R.A.; Gallagher, J.A.; Bowler, W.B. Expression of a P2X7 receptor by a subpopulation of human osteoblasts. J. Bone Miner. Res. 2001, 16, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Gulinelli, S.; Salaro, E.; Vuerich, M.; Bozzato, D.; Pizzirani, C.; Bolognesi, G.; Idzko, M.; di Virgilio, F.; Ferrari, D. IL-18 associates to microvesicles shed from human macrophages by a lps/tlr-4 independent mechanism in response to P2X receptor stimulation. Eur. J. Immunol. 2012, 42, 3334–3345. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Sheridan, J.F. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav. Immun. 2016, 57, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Hale, J.S.; Li, M.; Lathia, J.D. The malignant social network: Cell–cell adhesion and communication in cancer stem cells. Cell Adhes. Migr. 2012, 6, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Manolagas, S.C.; O'Brien, C.A. Parathyroid hormone controls receptor activator of NF-κBligand gene expression via a distant transcriptional enhancer. Mol. Cell. Biol. 2006, 26, 6453–6468. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, C.A.; Gubrij, I.; Lin, S.C.; Saylors, R.L.; Manolagas, S.C. STAT3 activation in stromal/osteoblasticcells is required for induction of the receptor activator of NF-κBligand and stimulation of osteoclastogenesis by GP130-utilizing cytokines or interleukin-1 but not 1,25-dihydroxyvitamin D3 or parathyroid hormone. J. Biol. Chem. 1999, 274, 19301–19308. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCt) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Peng, Y.; Jiang, Y.; Wu, Y.; Ding, Y.; Wang, Y.; Xu, D.; Fu, Q. Imipramine Protects against Bone Loss by Inhibition of Osteoblast-Derived Microvesicles. Int. J. Mol. Sci. 2017, 18, 1013. https://doi.org/10.3390/ijms18051013
Deng L, Peng Y, Jiang Y, Wu Y, Ding Y, Wang Y, Xu D, Fu Q. Imipramine Protects against Bone Loss by Inhibition of Osteoblast-Derived Microvesicles. International Journal of Molecular Sciences. 2017; 18(5):1013. https://doi.org/10.3390/ijms18051013
Chicago/Turabian StyleDeng, Lili, Ying Peng, Yuhai Jiang, Yu Wu, Yuedi Ding, Yaping Wang, Dong Xu, and Qiang Fu. 2017. "Imipramine Protects against Bone Loss by Inhibition of Osteoblast-Derived Microvesicles" International Journal of Molecular Sciences 18, no. 5: 1013. https://doi.org/10.3390/ijms18051013