Impaired Platelet Aggregation and Rebalanced Hemostasis in Patients with Chronic Hepatitis C Virus Infection
Abstract
:1. Introduction
2. Results
2.1. Altered Standard Coagulation Tests in CHC-Infected Patients
2.2. Normal Functional Whole Blood Hemostasis in CHC-Infected Patients
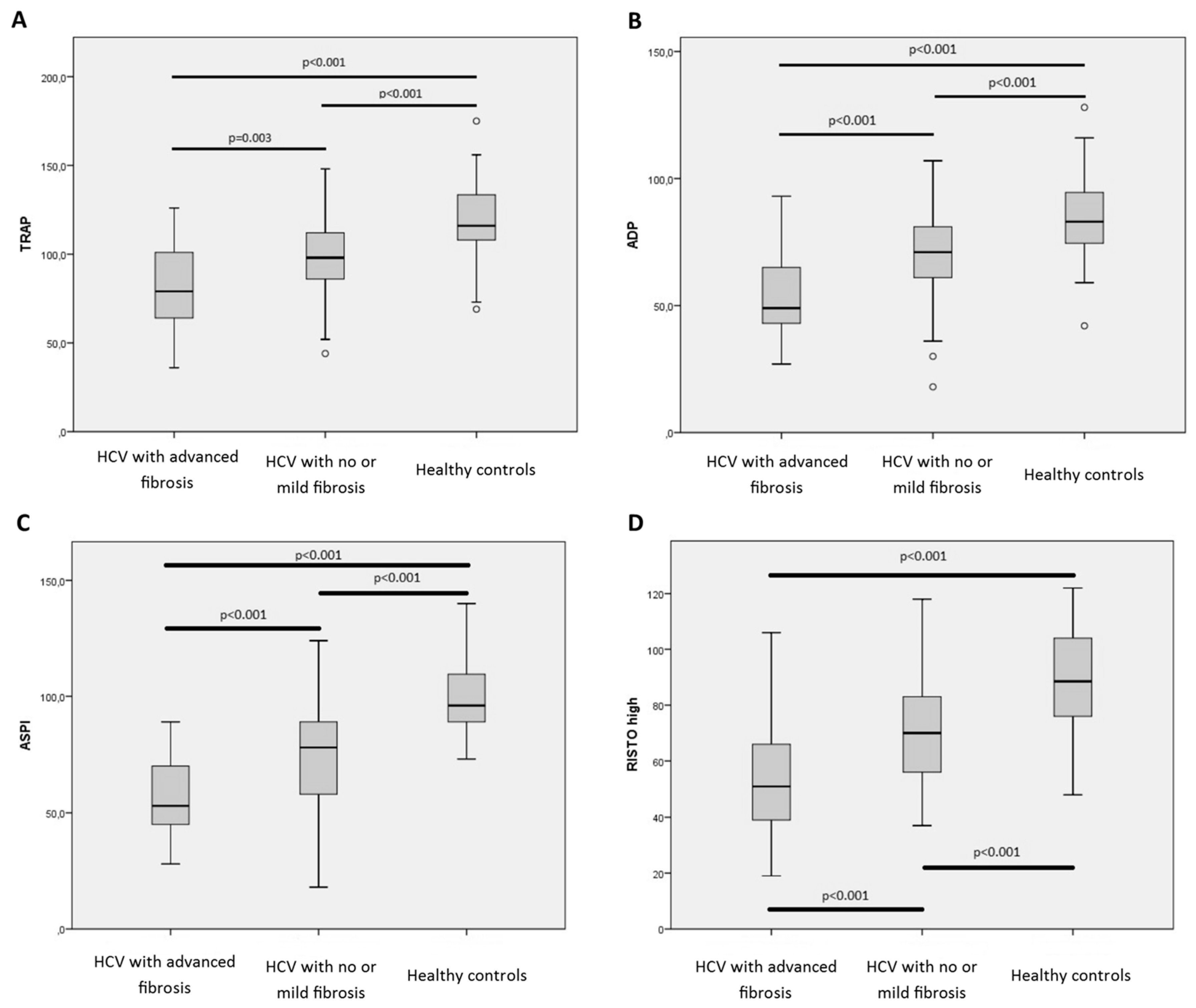
2.3. Impaired Whole Blood Platelet Aggregation in Patients with CHC Infection
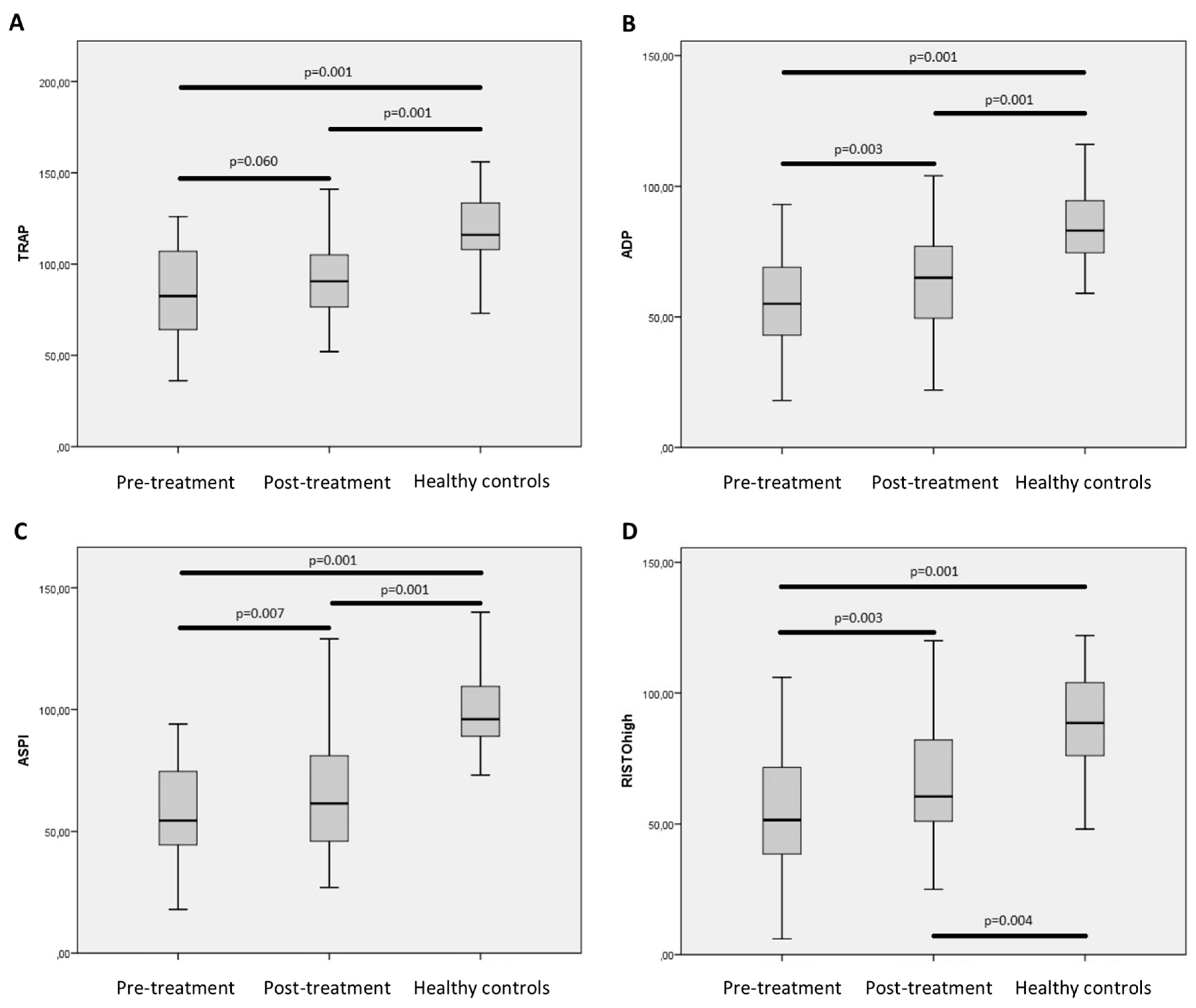
2.4. Minor Changes in Functional Hemostasis after Treatment
3. Discussion
4. Methods
4.1. Patients
4.2. Blood Sampling
4.3. Conventional Plasma Based and Functional Haemostatic Whole-Blood Tests
4.4. Tromboelastography (TEG)
4.5. Impedance Aggregometry (Multiplate)
4.6. Statistics and Data
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular disease |
| CHC | Chronic Hepatitis C |
| HCV | Hepatitis C virus |
| SVR | Sustained Virological Response |
| TEG | Tromboelastography |
| HCC | Hepatocellular carcinoma |
| vWF | Von Willebrand factor |
| APTT | Activated partial thromboplastin time |
| INR | International normalized ratio |
| HIV | Human immunodeficiency virus |
| DAA | Direct-acting antivirals |
| DVT | Deep vein thrombosis |
| EASL | European Association for the Study of the Liver |
References
- World Health Organization (WHO). Hepatitis C; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Chen, S.L.; Morgan, T.R. The natural history of hepatitis c virus (HCV) infection. Int. J. Med. 2006, 3, 47–52. [Google Scholar] [CrossRef]
- Zampino, R.; Marrone, A.; Restivo, L.; Guerrera, B.; Sellitto, A.; Rinaldi, L.; Romano, C.; Adinolfi, L.E. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J. Hepatol. 2013, 5, 528–540. [Google Scholar] [PubMed]
- Vassalle, C.; Masini, S.; Bianchi, F.; Zucchelli, G.C. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart 2004, 90, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Wang, X.; Budoff, M.; Leaf, D.; Kuller, L.H.; Justice, A.C. Hepatitis c virus infection and the risk of coronary disease. Clin. Infect. Diseases 2011, 49, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, J. Assessing cardiovascular risk in hepatitis c: An unmet need. World J. Hepatol. 2015, 7, 2214. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.M. Chronic hepatitis c-associated thrombocytopenia: Aetiology and management. Trop. Gastroenterol. 2013, 34, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sherman, K.E. Advanced liver disease: What every hepatitis c virus treater should know. Top. Antivir. Med. 2011, 19, 121–125. [Google Scholar] [PubMed]
- Lisman, T.; Leebeek, F.W.G. Hemostatic alterations in liver disease: A review on pathophysiology, clinical consequences, and treatment. Dig. Surg. 2007, 24, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T. Hemostatic dysfunction in liver diseases. Semin. Thromb. Hemost. 2015, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Northup, P.G.; Caldwell, S.H. Coagulation in liver disease: A guide for the clinician. Clin. Gastroenterol. Hepatol. 2013, 11, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Mannucci, P.M. The coagulopathy of chronic liver disease. N. Engl. J. Med. 2011, 365, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T.; Porte, R.J. Rebalanced hemostasis in patients with liver disease: Evidence and clinical consequences. Blood 2010, 116, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T.; Bongers, T.N.; Adelmeijer, J.; Janssen, H.L.A.; de Maat, M.P.M.; de Groot, P.G.; Leebeek, F.W.G. Elevated levels of von willebrand factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 2006, 44, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hollestelle, M.J.; Geertzen, H.G.M.; Straatsburg, I.H.; Gulik, T.M.V.; Mourik, J.A.V. Factor VIII expression in liver disease. Thromb. Haemost. 2004, 91, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Mallett, S.V.; Chowdary, P.; Burroughs, A.K. Clinical utility of viscoelastic tests of coagulation in patients with liver disease. Liver Int. 2013, 33, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Görlinger, K.; Jambor, C.; Hanke, A.A.; Dirkmann, D.; Adamzik, M.; Hartmann, M.; Rahe-Meyer, N. Perioperative coagulation management and control of platelet transfusion by point-of-care platelet function analysis. Transf. Med. Hemother. 2007, 34, 396–411. [Google Scholar] [CrossRef]
- Mannucci, P.M. Abnormal hemostasis tests and bleeding in chronic liver disease: Are they related? No. J. Thromb. Haemost. 2006, 4, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Cotton, B.A.; Minei, K.M.; Radwan, Z.A.; Matijevic, N.; Pivalizza, E.; Podbielski, J.; Wade, C.E.; Kozar, R.A.; Holcomb, J.B. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J. Trauma Acute Care Surg. 2012, 72, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Kashuk, J.L.; Moore, E.E.; Sabel, A.; Barnett, C.; Haenel, J.; Le, T.; Pezold, M.; Lawrence, J.; Biffl, W.L.; Cothren, C.C.; et al. Rapid thrombelastography (r-teg) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery 2009, 146, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Lee, A.; Critchley, L.A.H.; White, P.F. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth. Analg. 2009, 108, 734–742. [Google Scholar] [CrossRef] [PubMed]
- McCrath, D.J.; Cerboni, E.; Frumento, R.J.; Hirsh, A.L.; Bennett-Guerrero, E. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth. Analg. 2005, 100, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.C.; Meijers, J.C.; Vroom, M.B.; Juffermans, N.P. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: A systematic review. Crit. Care 2014, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, K.J.; Troxler, M.; Homer-Vanniasinkam, S. The surgical application of point-of-care haemostasis and platelet function testing. Br. J. Surg. 2008, 95, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Johansson, P.I.; Ostrowski, S.R.; Stissing, T.; Steinbrüchel, D.A. Hypercoagulability in patients undergoing coronary artery bypass grafting: Prevalence, patient characteristics and postoperative outcome. Eur. J. Card. Thorac. Surg. 2012, 41, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Primignani, M.; Chantarangkul, V.; Viscardi, Y.; Dell’Era, A.; Fabris, F.M.; Mannucci, P.M. The coagulopathy of cirrhosis assessed by thromboelastometry and its correlation with conventional coagulation parameters. Thromb. Res. 2009, 124, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Stravitz, R.T. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol. Hepatol. 2012, 8, 513–520. [Google Scholar]
- Gurbel, P.A.; Bliden, K.P.; Guyer, K.; Cho, P.W.; Zaman, K.A.; Kreutz, R.P.; Bassi, A.K.; Tantry, U.S. Platelet reactivity in patients and recurrent events post-stenting. J. Am. Coll. Cardiol. 2005, 46, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, R.; Priora, R.; Liotta, A.A.; Abbate, R. Platelet function tests: A comparative review. Vasc. Health Risk Manag. 2015, 11, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Braun, S.; Morath, T.; Mehilli, J.; Vogt, W.; Schömig, A.; Kastrati, A.; von Beckerath, N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 2009, 53, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Morath, T.; Braun, S.; Stegherr, J.; Mehilli, J.; Vogt, W.; Schomig, A.; Kastrati, A.; von Beckerath, N. Clopidogrel response status assessed with multiplate point-of-care analysis and the incidence and timing of stent thrombosis over six months following coronary stenting. Thromb. Haemost. 2010, 103, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Paarup Dridi, N.; Johansson, P.I.; Lønborg, J.T.; Clemmensen, P.; Radu, M.D.; Qayyum, A.; Pedersen, F.; Kollslid, R.; Helqvist, S.; Saunamäki, K.; et al. Tailored antiplatelet therapy to improve prognosis in patients exhibiting clopidogrel low-response prior to percutaneous coronary intervention for stable angina or non-st elevation acute coronary syndrome. Platelets 2015, 26, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Oliveri, R.; Wade, C.E.; Ostrowski, S.R.; Holcomb, J.B. How I treat patients with massive hemorrhage. Blood 2014, 124, 3052–3058. [Google Scholar] [CrossRef] [PubMed]
- European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH clinical practice guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar]
- Giannini, E.G. Review article: Thrombocytopenia in chronic liver disease and pharmacologic treatment options. Aliment. Pharmacol. Ther. 2006, 23, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Primignani, M.; Chantarangkul, V.; Clerici, M.; Dell’Era, A.; Fabris, F.; Salerno, F.; Mannucci, P.M. Thrombin generation in patients with cirrhosis: The role of platelets. Hepatology 2006, 44, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Escolar, G.; Cases, A.; Vinas, M.; Pino, M.; Calls, J.; Cirera, I.; Ordinas, A. Evaluation of acquired platelet dysfunctions in uremic and cirrhotic patients using the platelet function analyzer (pfa-100): Influence of hematocrit elevation. Haematologica 1999, 84, 614–619. [Google Scholar] [PubMed]
- Laffi, G.; Cominelli, F.; Ruggiero, M.; Fedi, S.; Chiarugi, V.P.; La Villa, G.; Pinzani, M.; Gentilini, P. Altered platelet function in cirrhosis of the liver: Impairment of inositol lipid and arachidonic acid metabolism in response to agonists. Hepatology 1988, 8, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Alkozai, E.M.; Porte, R.J.; Adelmeijer, J.; Zanetto, A.; Simioni, P.; Senzolo, M.; Lisman, T. No evidence for increased platelet activation in patients with hepatitis b- or c-related cirrhosis and hepatocellular carcinoma. Thromb. Res. 2014, 135, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.; Heller, T.; Haynes-Williams, V.; Hara, K.; Zhao, X.; Feld, J.J.; Kleiner, D.E.; Rotman, Y.; Ghany, M.G.; Liang, T.J.; et al. Long term outcome of chronic hepatitis c after sustained virological response to interferon-based therapy. Aliment. Pharmacol. Ther. 2013, 37, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Serfaty, L. Follow-up of patients with chronic hepatitis c and a sustained viral response. Liver Int. 2016, 36, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Tachi, Y.; Hirai, T.; Miyata, A.; Ohara, K.; Iida, T.; Ishizu, Y.; Honda, T.; Kuzuya, T.; Hayashi, K.; Ishigami, M.; et al. Progressive fibrosis significantly correlates with hepatocellular carcinoma in patients with a sustained virological response. Hepatol. Res. 2015, 45, 238–246. [Google Scholar] [CrossRef] [PubMed]
- George, S.L.; Bacon, B.R.; Brunt, E.M.; Mihindukulasuriya, K.L.; Hoffmann, J.; Di Bisceglie, A.M. Clinical, virologic, histologic, and biochemical outcomes after successful hcv therapy: A 5 year follow-up of 150 patients. Hepatology 2009, 49, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.M.; Foucher, J.; Combis, J.-M.; Métivier, S.; Brunetto, M.; Capron, D.; Bourlière, M.; Bronowicki, J.-P.; Dao, T.; Maynard-Muet, M.; et al. Longitudinal liver stiffness assessment in patients with chronic hepatitis c undergoing antiviral therapy. PLoS ONE 2012, 7, e47715. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Moussalli, J.; Munteanu, M.; Thabut, D.; Lebray, P.; Rudler, M.; Ngo, Y.; Thibault, V.; Mkada, H.; Charlotte, F.; et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. J. Hepatol. 2013, 59, 675–683. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | HCV Infected with No or Mild Fibrosis (n = 43) * | HCV Infected with Advanced Fibrosis (n = 39) | Healthy Controls (n = 39) | p-Value | HCV Infected + Treatment (n = 33) |
|---|---|---|---|---|---|
| Gender, n (Male) (m%) | 27 (62.8) | 23 (59) | 20 (51.3) | 0.495 | 21 (61.8) |
| Age, year, median (IQR) | 49 (37–56) | 57 (50–61) L | 51 (42–56) | 0.001 | 55 (45–60) |
| Current smoker n (%) | 18 (42) L | 15 (38) | 10 (25) | 0.358 | 18 (53) |
| HCV-RNA at inclusion, IU/mL, median, (IQR) | 1.2 × 106 (0.28 × 106–3.00 × 106) | 1.6 × 106 (0.68 × 106–4.05 × 106) | N/A | 0.166 | 1.55 × 106 (0.8 × 106–3.8 × 106) |
| Years since diagnosis of HCV infection, median (IQR) | 14 (6–23) | 10 (3–27) | N/A | 0.266 | 11 (5–25) |
| Genotype 1/2/3/4 n | 22/8/9/3 | 18/2/16/3 | N/A | N/A | 20/1/12/1 |
| Fibroscan kPa, median (IQR) | 5.7 (4.7–6.2) | 14.1 (11.1–21.3) | N/A | <0.001 | 13.5 (6.6–20.2) |
| Bilirubin level, µmol/L, median (IQR) | 7 (6–10) | 11 (6–16) | N/A | 0.009 | 11 (6–16) |
| Albumin level, g/L, median (IQR) | 39 (37–40) | 37 (34–38) | N/A | 0.002 | 36 (34–38) |
| Creatinine level, µmol/L, medicn (IQR) | 75 (68–86) | 68 (61–80) | N/A | 0.068 | 70 (62–82) |
| INR, median (IQR) | 1.1 (1.1–1.2) | 1.1 (1.1–1.2) L | 1.0 (1.0–1.1) | 0.002 | 1.1 (1.0–1.2) |
| Patients with presence of portal hypertension/esophagal varices at enrollment (n) | 5/2 | 4/2 | |||
| Treatment used, n A(r+)/B(r+)/C(r+)/D(r+)/E(r+) | 5(2)/17(10)/5(0)/3(0)/3(1) | ||||
| Treatment duration, n 8/12/16/24 weeks | 8/19/3/3 |
| Coagulation Tests | Normal Range | HCV Infected with Advanced Fibrosis | HCV Infected with no or Mild Fibrosis | p-Value | Healthy Controls |
|---|---|---|---|---|---|
| Standard coagulation tests | |||||
| Platelet count, median (IQR) | 145–390 × 109 cells/L | 139 (113–187) L | 232 (184–267) L | <0.001 | 254 (230–293) |
| Coagulation factors II-VII-X, median (IQR) | >0.60 arb.units/L | 0.76 (0.6–0.86) L | 0.88 (0.76–1.03) | 0.002 | 0.93 (0.81–1.06) |
| D-dimer, median (IQR) | >0.5 mg FEU/L | 0.3 (0.3–0.4) L | 0.3 (0.3–0.3) | 0.002 | 0.3 (0.3–0.3) |
| Above threshold, n (%) | 8 (21) L | 1 (2) | 0.009 | 0 (0) | |
| Antithrombin, median (IQR) | 0.83–1.15 × 103 IU/L | 0.86 (0.7–0.92) L | 1.01 (0.95–1.11) L | 0.001 | 1.10 (1.05–1.16) |
| APTT, median (IQR) | 25–37 s | 28 (26–30) | 28 (27–30) | 0.738 | 29 (28–31) |
| Fibrinogen, median (IQR) | 5.3–10.3 µmol/L | 8.5 (7.6–10) | 8.1 (7.1–9.5) L | 0.333 | 8.7 (8.1–10.2) |
| Whole blood functional hemostasis tests | |||||
| R, median (IQR) | 4–9 min | 6,7 (5.7–7.9) | 6.4 (5.5–7.5) | 0.239 | 6.8 (5.8–7.6) |
| Angle, median (IQR) | 55–78 degrees | 65 (61–68) | 67 (64–69) | 0.122 | 67 (62–69) |
| MA, median (IQR) | 51–69 mm | 58 (54–61) | 61 (57–63) | 0.044 | 52 (56–66) |
| Ly30, %, median (IQR) | 0–4% | 0.9 (0.0–2.6) | 1.4 (0.2–3.2) | 0.393 | 1.6 (0.3–4.0) |
| Coagulation in CHC-Infected Patients before and after Treatment against HCV | Pre Treatment | Post Treatment | p-Value | Healthy Controls |
|---|---|---|---|---|
| Standard coagulation tests | ||||
| Platelet count, median (IQR) | 166 (113–209) L | 170 (118–230) L | 0.033 | 254 (230–293) |
| Coagulation factors II-VII-X, median (IQR) | 0.79 (0.63–0.86) L | 0.73 (0.62–0.90) L | 0.837 | 0.93 (0.81–1.06) |
| D-dimer, median (IQR) | 0.3 (0.3–0.4) L | 0.3 (0.3–0.4) L | 0.495 | 0.3 (0.3–0.3) |
| Above threshold (%) | 5 (14) L | 4 (12) L | 0 (0) | |
| Antithrombin, median (IQR) | 0.88 (0.73–1.05) L | 0.93 (0.80–0.98) L | 0.865 | 1.10 (1.05–1.16) |
| APTT, median (IQR) | 29 (27–30) | 28 (26–31) | 0.116 | 29 (28–31) |
| Fibrinogen, median (IQR) | 8.2 (7.2–10.1) | 9.6 (8.4–10.7) | 0.001 | 8.7 (8.1–10.2) |
| Whole blood functional hemostasis tests | ||||
| R, median (IQR) | 6.6 (5.8–7.6) | 6.4 (5.5–7.2) | 0.543 | 6.8 (5.8–7.6) |
| Angle, median (IQR) | 65 (60–67) | 66 (60–69) | 0.294 | 67 (62–69) |
| MA, median (IQR) | 58 (54–61) | 59 (54–64) | 0.058 | 52 (56–66) |
| Ly30, %, median (IQR) | 0.8 (0.7–2.4) | 1.2 (1.2–3.4) | 0.808 | 1.6 (0.3–4.0) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, N.S.; Jespersen, S.; Gaardbo, J.C.; Arnbjerg, C.J.; Clausen, M.R.; Kjær, M.; Gerstoft, J.; Ballegaard, V.; Ostrowski, S.R.; Nielsen, S.D. Impaired Platelet Aggregation and Rebalanced Hemostasis in Patients with Chronic Hepatitis C Virus Infection. Int. J. Mol. Sci. 2017, 18, 1016. https://doi.org/10.3390/ijms18051016
Nielsen NS, Jespersen S, Gaardbo JC, Arnbjerg CJ, Clausen MR, Kjær M, Gerstoft J, Ballegaard V, Ostrowski SR, Nielsen SD. Impaired Platelet Aggregation and Rebalanced Hemostasis in Patients with Chronic Hepatitis C Virus Infection. International Journal of Molecular Sciences. 2017; 18(5):1016. https://doi.org/10.3390/ijms18051016
Chicago/Turabian StyleNielsen, Nick S., Sofie Jespersen, Julie C. Gaardbo, Caroline J. Arnbjerg, Mette R. Clausen, Mette Kjær, Jan Gerstoft, Vibe Ballegaard, Sisse R. Ostrowski, and Susanne D. Nielsen. 2017. "Impaired Platelet Aggregation and Rebalanced Hemostasis in Patients with Chronic Hepatitis C Virus Infection" International Journal of Molecular Sciences 18, no. 5: 1016. https://doi.org/10.3390/ijms18051016






