The Selective Centrifugation Ensures a Better In Vitro Isolation of ASCs and Restores a Soft Tissue Regeneration In Vivo
Abstract
:1. Introduction
2. Results
2.1. In Vitro Experiments
2.2. In Vivo Experiment
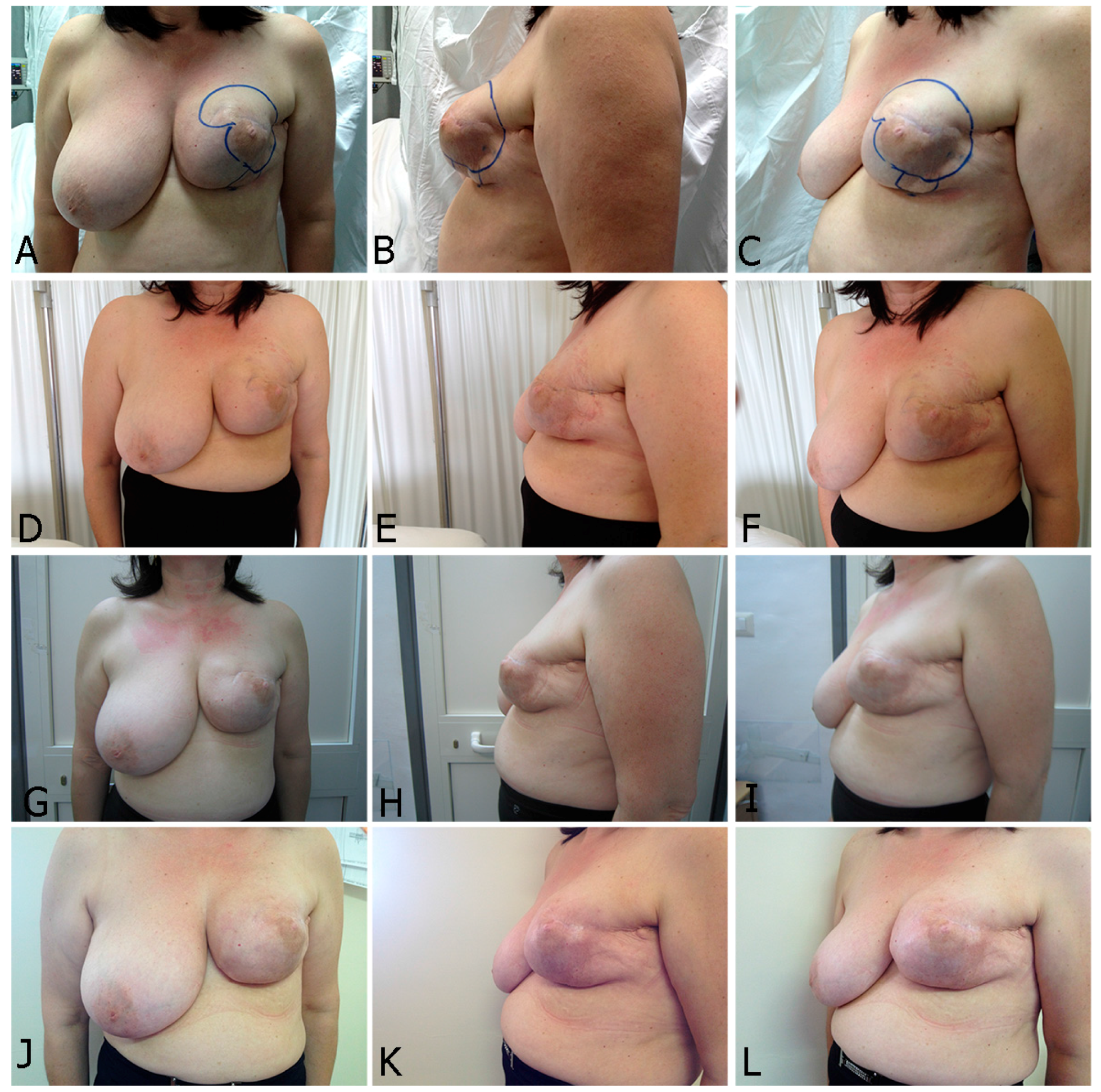
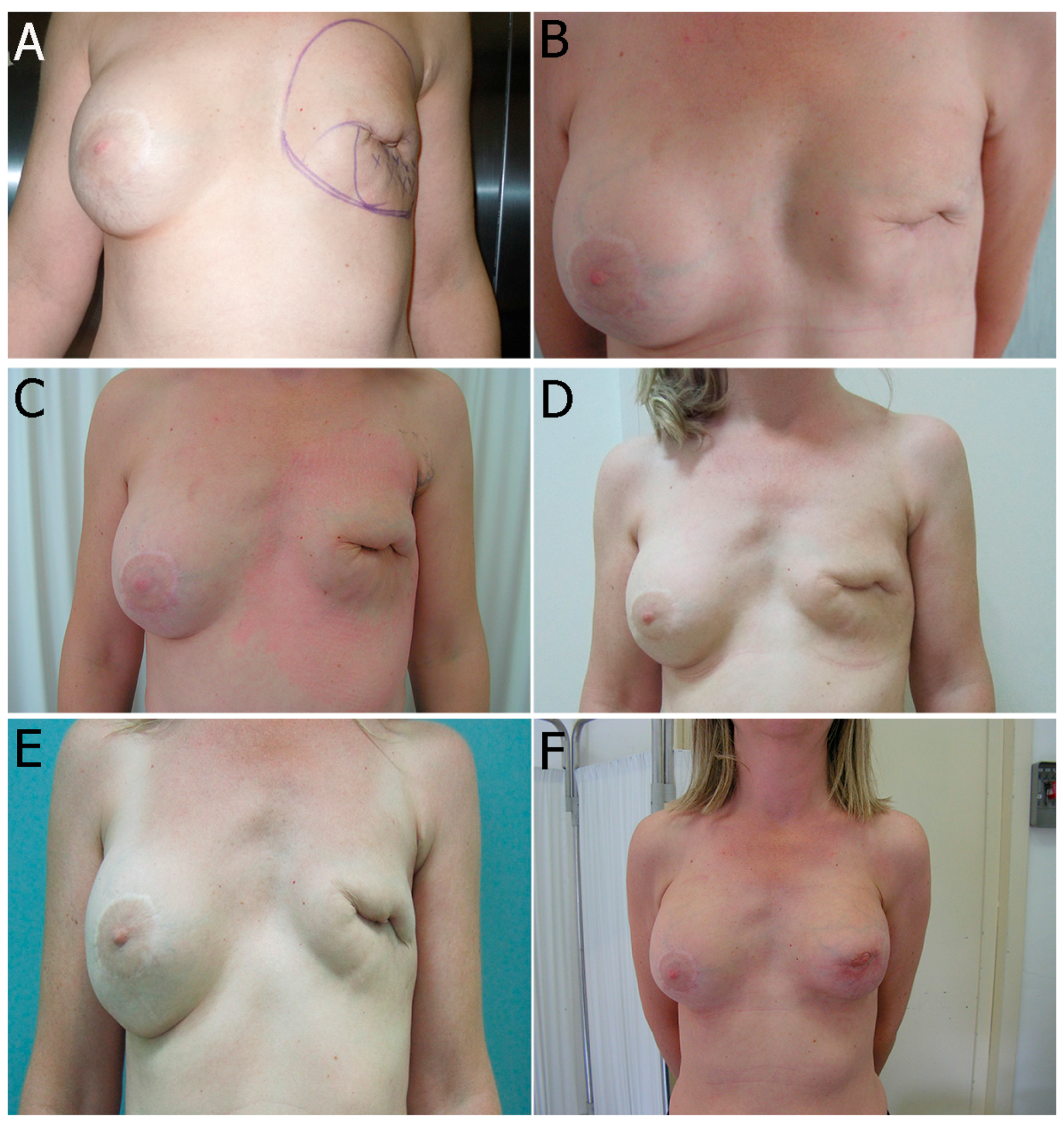
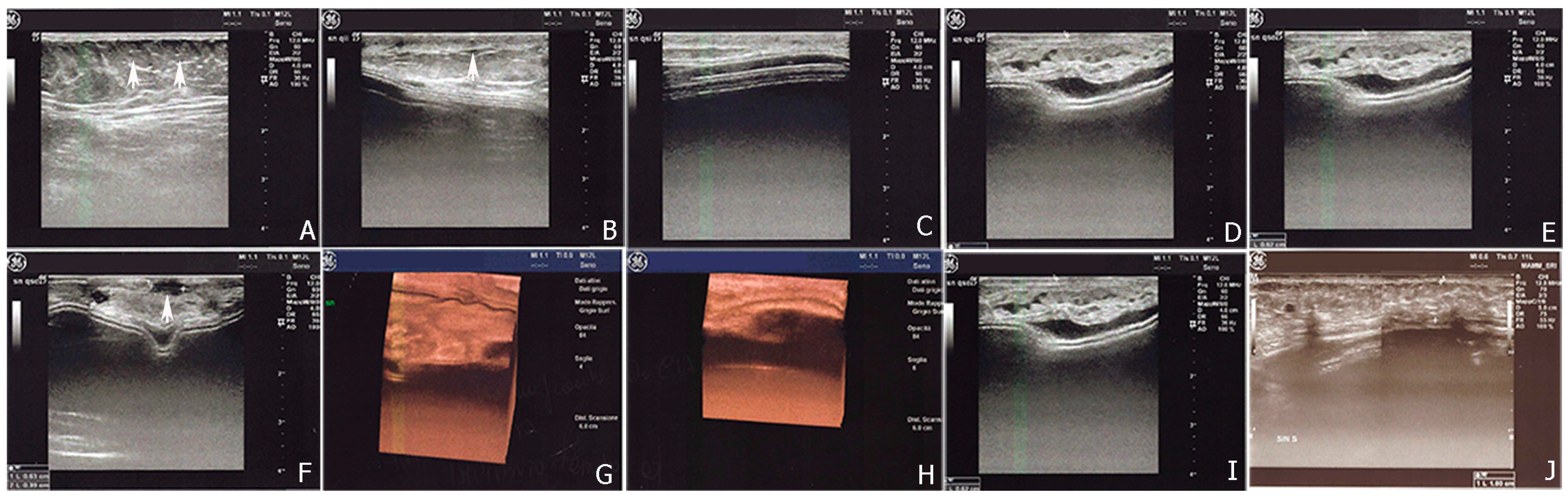
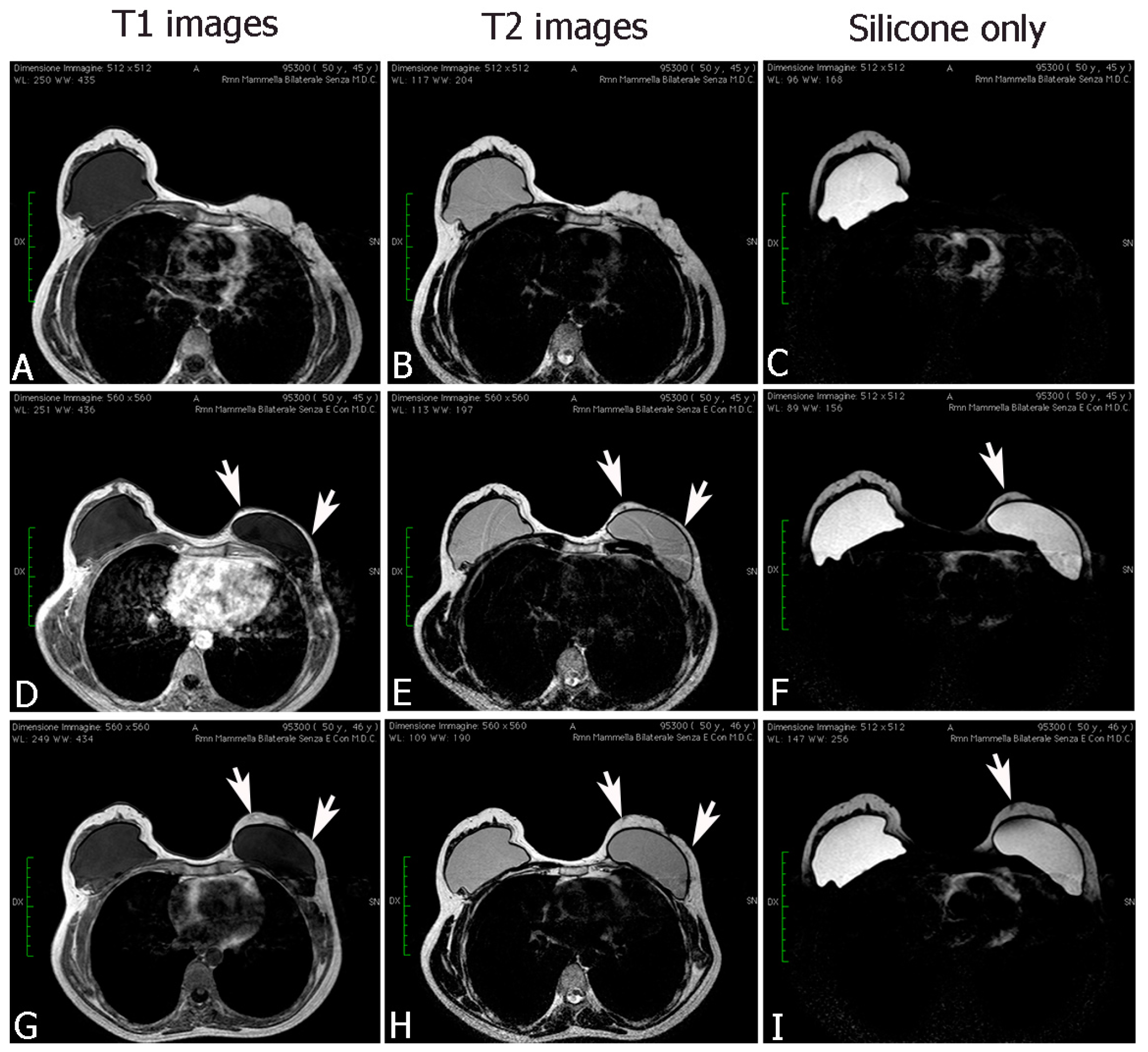
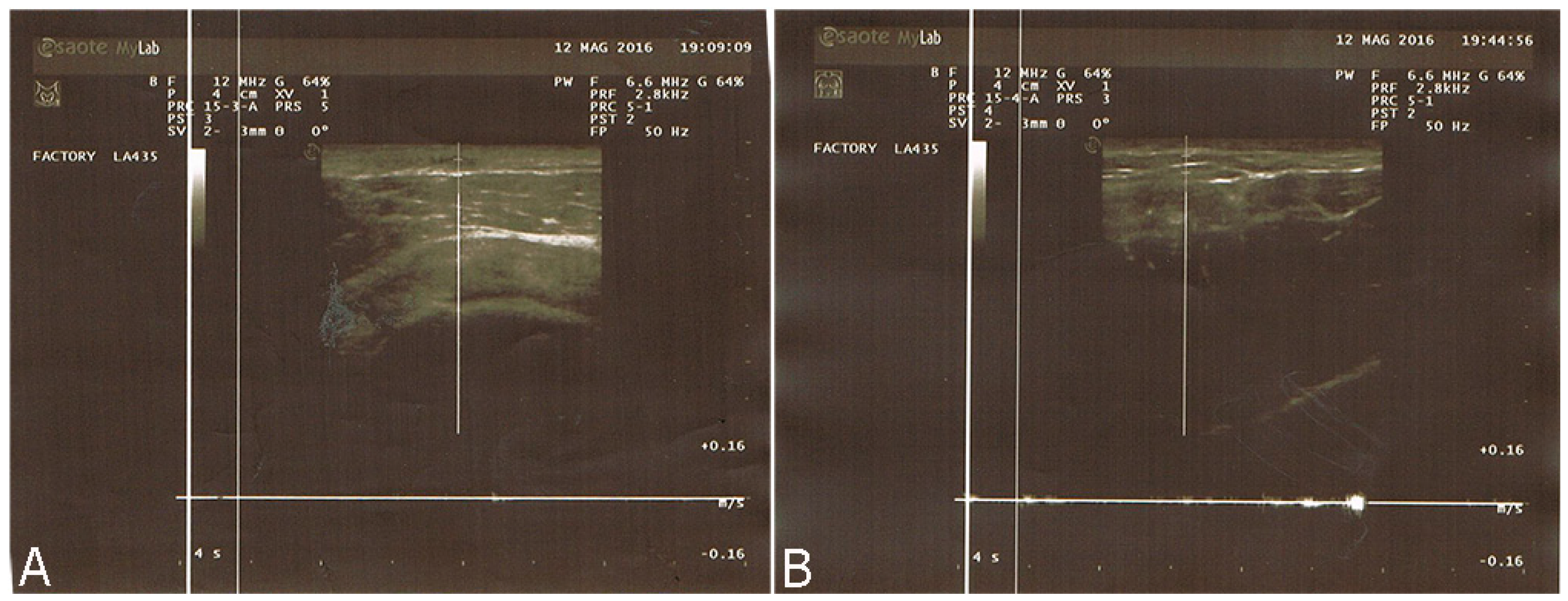
2.2.1. Breast Fat Grafting
2.2.2. Pathological Scar Fat Grafting
3. Discussion
4. Materials and Methods
4.1. In Vitro Experiments
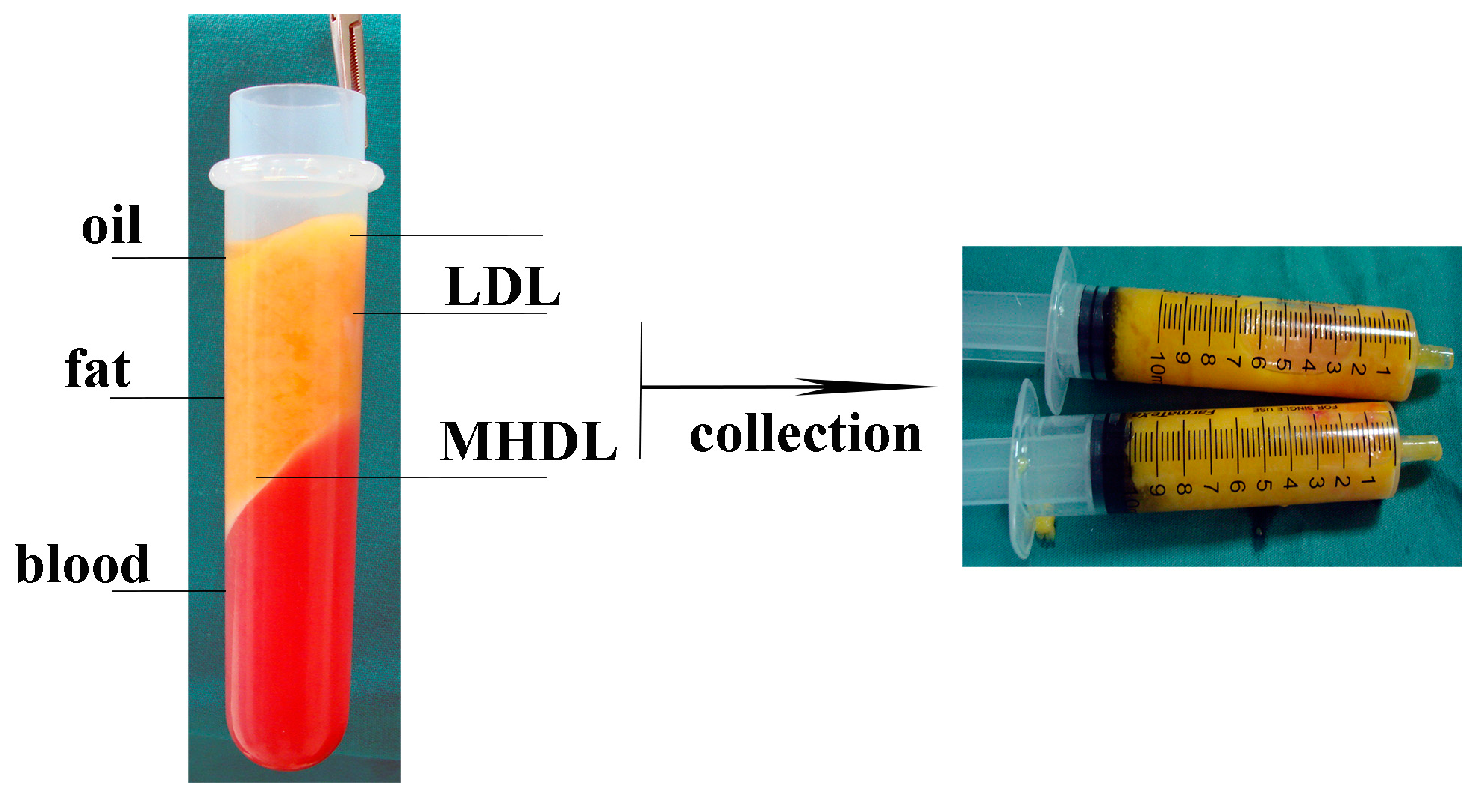
Adipose Tissue Extraction and Digestion
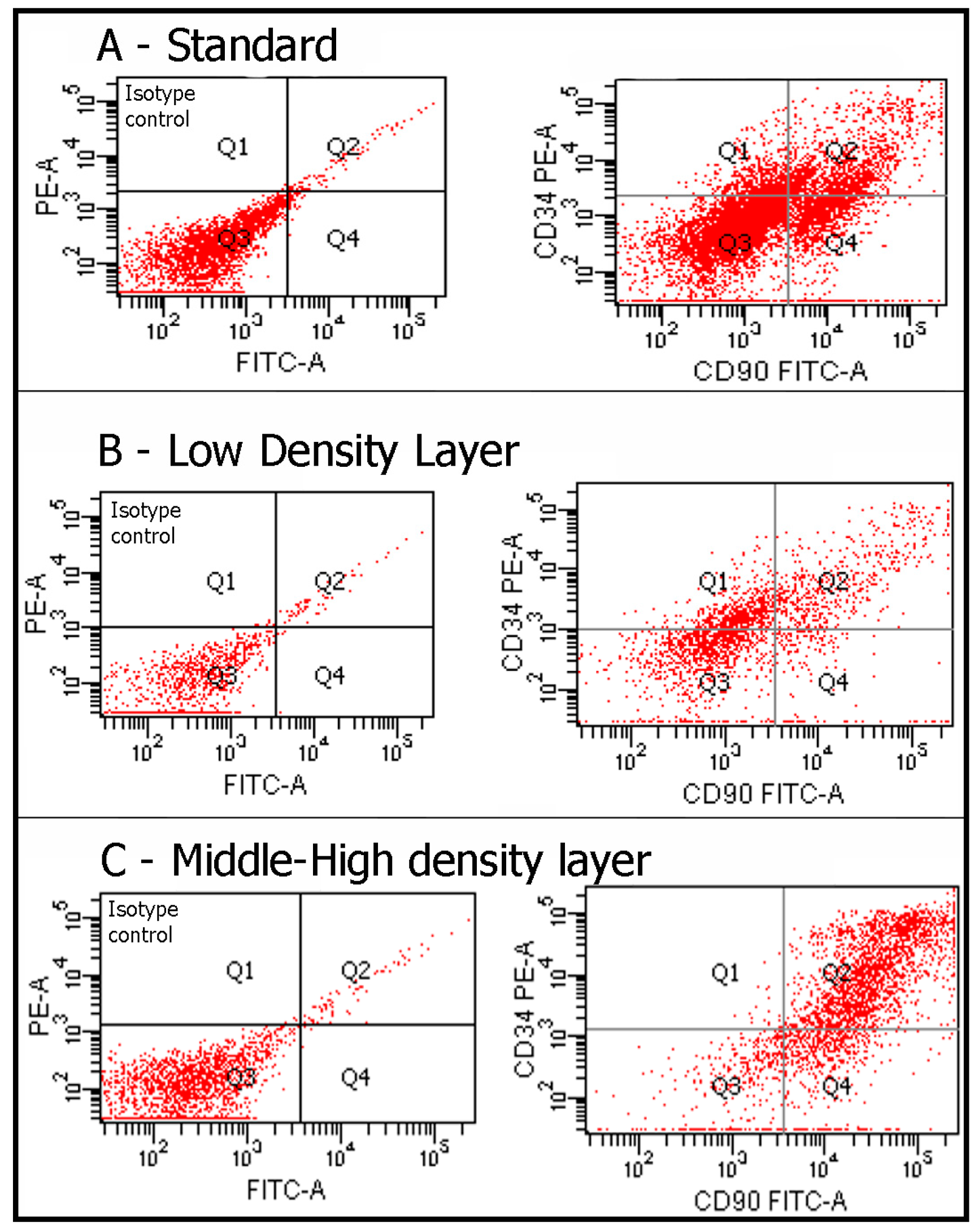
4.2. Flow Cytometry
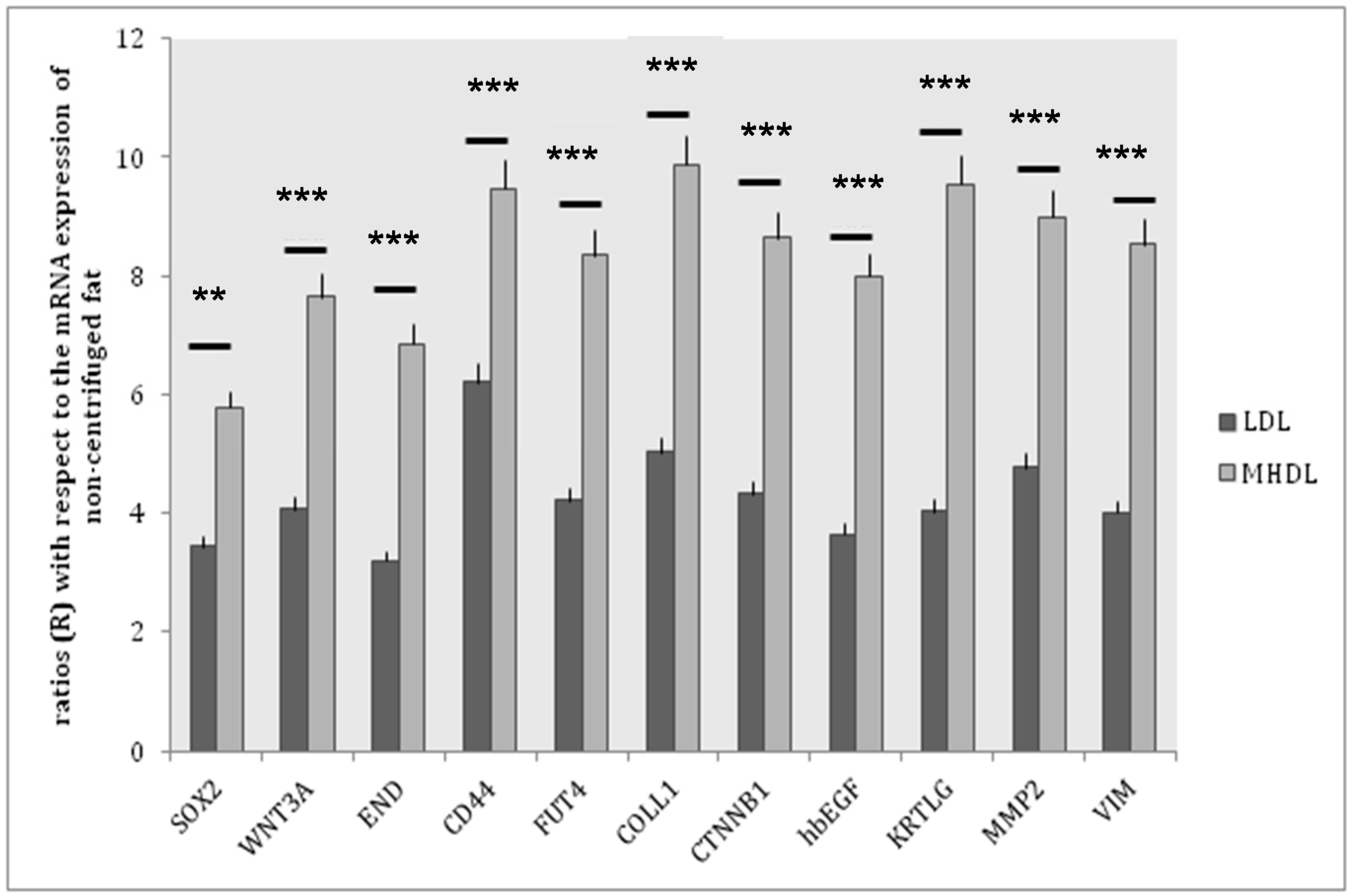
4.3. Real-Time PCR
4.4. In Vivo Experiments
Patients
4.5. Surgical Technique
4.6. Statistical Analysis
4.7. Ethics Statement
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zuk, P.A.; Zhu, M.; Ashjian, P.; de Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Hoy, E. State of the art: Reconstructing partial mastectomy defected with autologous fat grafting. Del. Med. J. 2016, 88, 20–23. [Google Scholar] [PubMed]
- Losken, A.; Pinell-White, X.; Hodges, M.; Egro, F.M. Evaluating outcomes after correction of the breast conservation therapy deformity. Ann. Plast. Surg. 2015, 74, S209–S213. [Google Scholar] [CrossRef] [PubMed]
- Agha, R.A.; Fowler, A.J.; Herlin, C.; Goodacre, T.E.; Orgill, D.P. Use of autologous fat grafting for breast reconstruction: A systematic review with meta-analysis of oncological outcomes. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Mestak, O.; Mestak, J.; Bohac, M.; Edriss, A.; Sukop, A. Breast reconstruction after bilateral mastectomy using the BRAVA expansion system and Fat grafting. Plast. Reconstr. Surg. Glob. Open 2013, 1, e71. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R.; Katzel, E.B. Fat grafting for facial filling and regeneration. Clin. Plast. Surg. 2015, 42, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Dollfus, C.; Blanche, S.; Trocme, N.; Funck-Brentano, I.; Bonnet, F.; Levan, P. Correction of facial lipoatrophy using autologous fat transplants in HIV-infected adolescents. HIV Med. 2009, 10, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Ducic, Y. Fat grafting in trauma and reconstructive surgery. Facial Plast. Surg. Clin. N. Am. 2008, 16, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Franchi, G.; Ansari, E.; Billotet, B.; Diner, P.A.; Cassier, S.; Vazquez, M.P.; Picard, A.; Kadlub, N. Fat graft transfer in children’s facial malformations: A prospective three-dimensional evaluation. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 799–804. [Google Scholar]
- Hunstad, J.P.; Shifrin, D.A.; Kortesis, B.G. Successful treatment of Parry-Romberg syndrome with autologous fat grafting: 14-year follow-up and review. Ann. Plast. Surg. 2011, 67, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Peault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult human adipose tissue. Cytometric A 2010, 77, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Donnenberg, V.S.; Rubin, J.P.; Donnenberg, A.D. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A 2013, 83, 134–140. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbay, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Shukla, L.; Morrison, W.A.; Shayan, R. Adipose-derived stem cells in radiotherapy injury: A new frontiers. Front. Surg. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, G.; Marchi, A.; Galiè, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Asano, Y.; Aoi, N.; Kurita, M.; Oshima, Y.; Sato, K.; Inoue, K.; Suga, H.; Eto, H.; Kato, H.; et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010, 16, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Kobler, J.B.; Herrera, V.L.; Zeitels, S.M. Perspectives on adipose-derived stem/stromal cells as potential treatment for scarred vocal folds: Opportunity and challenges. Curr. Stem. Cell Res. Ther. 2010, 5, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M.; Caviggioli, F.; Klinger, F.M.; Giannasi, S.; Bandi, V.; Banzatti, B.; Forcellini, D.; Maione, L.; Catania, B.; Vinci, V. Autologous fat graft in scar treatment. J. Craniofac. Surg. 2013, 24, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Shingyochi, Y.; Orbay, H.; Mizuno, H. Adipose-derived stem cells for wound repair and regeneration. Expert. Opin. Biol. Ther. 2015, 15, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Romano, M.; Zarantonello, L.; Ruffolo, C.; Neri, D.; Bassi, N.; Giordano, A.; Zanus, G.; Ferraro, G.A.; Cillo, U. The role of adipose stem cells in inflammatory bowel disease: From biology to novel therapeutic strategies. Cancer Biol. Ther. 2016, 17, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.A.; de Francesco, F.; Tirino, V.; Cataldo, C.; Rossano, F.; Nicoletti, G.; D’Andrea, F. Effects of a new centrifugation method on adipose cell viability for autologous fat grafting. Aesthet. Plast. Surg. 2011, 35, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Clauser, L.; Ferroni, L.; Gardin, C.; Tieghi, R.; Galiè, M.; Elia, G.; Piattelli, A.; Pinton, P.; Bressan, E.; Zavan, B. Selective augmentation of stem cell populations in structural fat grafts for maxillofacial surgery. PLoS ONE 2014, 9, e110796. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Su, Y.; Zhang, D.; Song, Y.; Liu, B.; Yu, Z.; Guo, S.; Yi, C. Identification of the Centrifuged lipoaspirate fractions suitable for postgrafting survival. Plast. Reconstr. Surg. 2016, 137, 67e–76e. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statemen. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- D’Andrea, F.; de Francesco, F.; Ferraro, G.A.; Desiderio, V.; Tirino, V.; de Rosa, A.; Papaccio, G. Large-scale production of human adipose tissue from stem cells: A new tool for regenerative medicine and tissue banking. Tissue Eng. Part C Methods 2008, 14, 233–242. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Tirino, V.; Desiderio, V.; Ferraro, G.; D’Andrea, F.; Giuliano, M.; Libondi, G.; Pirozzi, G.; de Rosa, A.; Papaccio, G. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS ONE 2009, 4, e6537. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.A.; de Francesco, F.; Nicoletti, G.; Paino, F.; Desiderio, V.; Tirino, V.; D’Andrea, F. Human adipose CD34+ CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. J. Cell Biochem. 2013, 114, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Human adipose stem cells: From bench to bedside. Tissue Eng. Part B Rev. 2015, 21, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Illouz, Y.G.; Sterodimas, A. Autologous fat transplantation to the breast: A personal technique with 25 years of experience. Aesthet. Plast. Surg. 2009, 33, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Cecchinato, F.; Karlsson, J.; Ferroni, L.; Gardin, C.; Galli, S.; Wennerberg, A.; Zavan, B.; Andersson, M.; Jimbo, R. Osteogenic potential of human adipose-derived stromal cells on 3-dimensional mesoporous TiO2 coating with magnesium impregnation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Conde-Green, A.; de Amorim, N.F.G.; Pitanguy, I. Influence of decantation, washing, and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: A comparative study. J. Plast. Reconstr. Aesthet. 2010, 63, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Kakagia, D.; Pallua, N. Autologous fat grafting: In search of the optimal technique. Surg. Innov. 2014, 21, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Choi, T.; Yeo, H.; Kim, J.; Han, K. The effect of centrifugation condition on mature adipocytes and adipose stem cell viability. Ann. Plast. Surg. 2014, 72, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, E.C.; Albano, N.J.; Hazen, A. Roll, Spin, Wash or Filter? Processing of lipoaspirate for autologous fat grafting: An updated evidence-based review of the literature. Plast. Reconstr. Surg. 2015, 136, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Miconi, G.; Cinque, B.; la Torre, C.; Lombardi, F.; Zoccali, G.; Orsini, G.; Leocata, P.; Giuliani, M.; Cifone, M.G. In vitro evaluation of different methods of handling human liposuction aspirate and their effect on adipocytes and adipose derived stem cells. J. Cell Physiol. 2015, 230, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Kolle, S.F.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.L.; et al. Enrichment of autologous fat grafts with ex vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthet. Plast. Surg. 2008, 32, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kosowski, T.R.; Rigotti, G.; Khouri, R.K. Tissue-engineered autologous breast regeneration with Brava®-assisted fat grafting. Clin. Plast. Surg. 2015, 42, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Khouri, R.K.; Khouri, R.K., Jr.; Rigotti, G.; Marchi, A.; Cardoso, E.; Rotemberg, S.C.; Biggs, T.M. Aesthetic applications of Brava-assisted megavolume fat grafting to the breasts: A 9-year, 476 patient, multicenter experience. Plast. Reconstr. Surg. 2014, 133, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Khouri, R.K., Jr.; Biggs, T.M. Fat grafting & the philosopher’s stone. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, e17–e18. [Google Scholar] [PubMed]
- Derby, B.M.; Dai, H.; Reichensperger, J.; Cox, L.; Harrison, C.; Cosenza, N.; Yang, M.; Bueno, R.A.; Neumeister, M.W. Adipose-derived stem cell to epithelial stem cell transdifferentiation: A mechanism to potentially improve understanding of fat grafting’s impact on skin rejuvenation. Aesthet. Surg. J. 2013, 34, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Araki, J.; Doi, K.; Kuno, S.; Kinoshita, K.; Mineda, K.; Kanayama, K.; Yoshimura, K. Normobaric hyperoxygenation enhances initial survival, regeneration, and final retention in fat grafting. Plast. Reconstr. Surg. 2014, 134, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.F.; Jung, S.N. The immunomodulatory effects of mesenchymal stem cells in prevention or treatment of excessive scars. Stem Cells Int. 2016, 2016, 6937976. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Orlandi, A.; Scioli, M.G.; di Pasquali, C.; Bocchini, I.; Curcio, C.B.; Floris, M.; Fiaschetti, V.; Floris, R.; Cervelli, V. A comparative translational study: The combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl. Med. 2012, 1, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Purpura, V.; Bondioli, E.; Graziano, A.; Trovato, L.; Melandri, D.; Ghetti, M.; Marchesini, A.; Cusella de Angelis, M.G.; Benedetti, L.; Ceccarelli, G.; et al. Tissue characterization after new disaggregation method for skin micro-grafts generation. J. Vis. Exp. 2016, 4, e53579. [Google Scholar] [CrossRef] [PubMed]
- Trovato, L.; Monti, M.; del Fante, C.; Cervio, M.; Lampinem, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A new medical device rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. J. Cell Physiol. 2015, 230, 2299–2303. [Google Scholar] [CrossRef] [PubMed]


| MHDL Group | ||||||||||||
| CD14 (%) | CD29 (%) | CD31 (%) | CD34 (%) | CD44 (%) | CD45 (%) | CD73 (%) | CD90 (%) | CD105 (%) | CD117 (%) | CD133 (%) | CD34/CD90 (%) | |
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Average | 0% | 42% | 6% | 33% | 5% | 0% | 45% | 62% | 28% | 10% | 8% | 85% |
| Minimum | 0% | 26% | 4% | 23% | 2% | 0% | 37% | 53% | 13% | 3% | 4% | 78% |
| Maximum | 0% | 48% | 10% | 38% | 8% | 0% | 53% | 76% | 34% | 16% | 14% | 90% |
| LDL Group | ||||||||||||
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Average | 0% | 15% | 3% | 12% | 2% | 0% | 20% | 31% | 12% | 4% | 6% | 63% |
| Minimum | 0% | 10% | 1% | 8% | 1% | 0% | 10% | 23% | 7% | 1% | 2% | 59% |
| Maximum | 0% | 25% | 5% | 20% | 5% | 0% | 40% | 44% | 26% | 8% | 10% | 65% |
| Cluster of Differentiation | MHDL Group (%) | LDL Group (%) | p-Value |
|---|---|---|---|
| CD14 | 0 | 0 | |
| CD29 | 42 | 15 | p < 0.001 |
| CD31 | 6 | 3 | p = 0.005 |
| CD34 | 33 | 12 | p < 0.0005 |
| CD44 | 5 | 2 | p = 0.005 |
| CD45 | 0 | 0 | |
| CD73 | 45 | 20 | p < 0.0005 |
| CD90 | 62 | 31 | p < 0.0005 |
| CD105 | 28 | 12 | p < 0.001 |
| CD117 | 10 | 4 | p < 0.001 |
| CD133 | 8 | 6 | p = 0.005 |
| CD34+CD90+ | 85 | 63 | p < 0.0005 |
| Gene | Function |
|---|---|
| SOX2 | SOX2 is a transcriptional activator that identity neural stem cells. In addition to regulating the progression of neurogenesis, this group of activators is also active in post-mitotic neurons. |
| WNT3A | WNTs are a family of lipid-modified secreted glycoproteins involved on stem cell niches. |
| END | (endoglin or CD 105): human endoglin is an RGD-containing transmembrane glycoprotein identified in vascular endothelial cells. |
| CD44 | A ubiquitous multistructural and multifunctional cell surface adhesion molecule involved in cell-cell and cell-matrix interactions. CD44 also participates in the uptake and intracellular degradation of HA, it is present in mesenchymal stem cells. |
| FUT4 | A myeloid α1,3-fucosyltransferase responsible for the fucosylation of adhesive interactions between selectins and their ligands, and it plays an essential role in hematopoietic cell lines. |
| COLL1 | Collagen type I is present in the extracellular matrix and it is produced by mesenchymal stem cells. |
| CTNNB1 | The β-catenin gene is involved in WNT signaling. The WNT factor family plays numerous roles in embryonic development and stem cell biology. WNT signaling is transduced by the FZD family of receptors. |
| hbEGF | Heparin-binding epidermal growth factor-like growth factor: a member of the EGF family of growth factors, which interact with the EGF receptor to exert mitogenic activity in various cell types. Recent studies indicate that HB-EGF contributes to neuronal survival and the proliferation of glial/stem cells. |
| KRTLG | Also know as Stem Cell Factor (SCF): a dimeric molecule that exerts its biological functions by binding to and activating the receptor tyrosine kinase c-Kit. Signaling from c-Kit is crucial for normal hematopoiesis, pigmentation, fertility, gut motility, and some aspects of the nervous system. |
| MMP2 | Matrix metalloproteinases 2: a protein involved in the migration of hematopoietic stem cells through the blood and across the endothelial vasculature to different organs and to their bone marrow (BM) niches, or, in other words, during the homing process. |
| VIM | Vimentin: a type III intermediate filament protein that is expressed in mesenchymal cells. |
| Patients Characteristics | ||
| No. of cases | 25 | |
| Sex | 25 Female, 0 Male | |
| Age (years) | 52.8 | |
| Surgical procedure * | ||
| group A group B group C | 7 cases 10 cases 8 cases | |
| Radiotherapy (RT) | 10 cases | |
| Site of liposuctions | ||
| Thights Thights and abdomen | 10 cases 15 cases | |
| Number of section | 3.4 ± 1.9 | |
| Average volume | 245.5 ± 18 cc | |
| Interval between sessions | 3.9 ± 1.3 months | |
| Clinical Assessment before Treatment | Clinical Assessment Post Fat Grafting | |
| RT | Fibrosis, atrophy, retraction, ulcers, ulcers with implant exposure, telangectasia, itching | Remission of symptoms of RT therapy |
| No-RT ** | ||
| Volume (respect controlateral) | 4 | |
| Contour | 5 | |
| Breast implant (contracture, wrinkling, extrusion) | 4 | |
| Pliability | 5 | |
| Thickness | 5 | |
| Overall result | 23 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Francesco, F.; Guastafierro, A.; Nicoletti, G.; Razzano, S.; Riccio, M.; Ferraro, G.A. The Selective Centrifugation Ensures a Better In Vitro Isolation of ASCs and Restores a Soft Tissue Regeneration In Vivo. Int. J. Mol. Sci. 2017, 18, 1038. https://doi.org/10.3390/ijms18051038
De Francesco F, Guastafierro A, Nicoletti G, Razzano S, Riccio M, Ferraro GA. The Selective Centrifugation Ensures a Better In Vitro Isolation of ASCs and Restores a Soft Tissue Regeneration In Vivo. International Journal of Molecular Sciences. 2017; 18(5):1038. https://doi.org/10.3390/ijms18051038
Chicago/Turabian StyleDe Francesco, Francesco, Antonio Guastafierro, Gianfranco Nicoletti, Sergio Razzano, Michele Riccio, and Giuseppe A. Ferraro. 2017. "The Selective Centrifugation Ensures a Better In Vitro Isolation of ASCs and Restores a Soft Tissue Regeneration In Vivo" International Journal of Molecular Sciences 18, no. 5: 1038. https://doi.org/10.3390/ijms18051038







