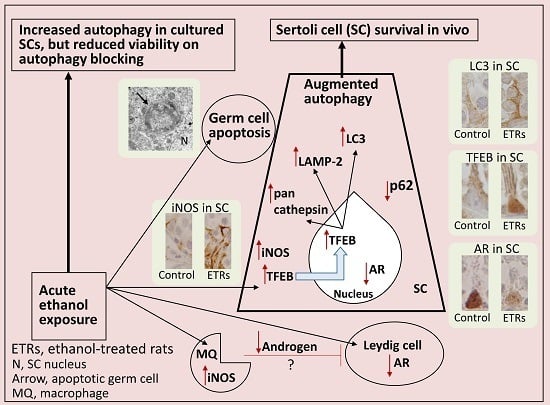
Upregulated Autophagy in Sertoli Cells of Ethanol-Treated Rats Is Associated with Induction of Inducible Nitric Oxide Synthase (iNOS), Androgen Receptor Suppression and Germ Cell Apoptosis
Abstract
:1. Introduction
2. Results
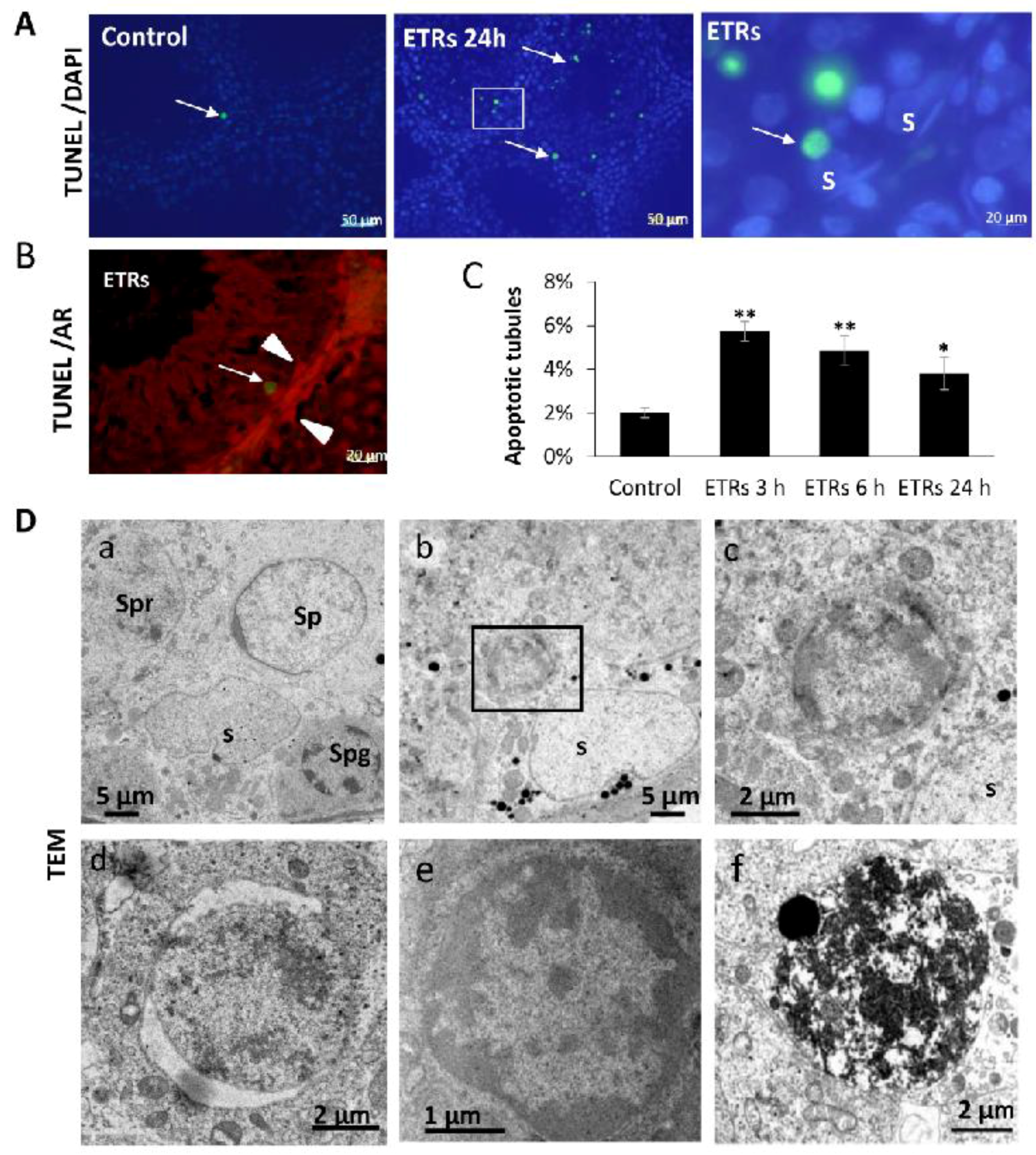
2.1. Ethanol Enhanced Apoptotic Germ Cell Death, but SCs Were Resistant to Apoptosis
2.2. Induction of iNOS and Suppression of AR Protein Levels in SCs and Interstitial Cells of ETRs
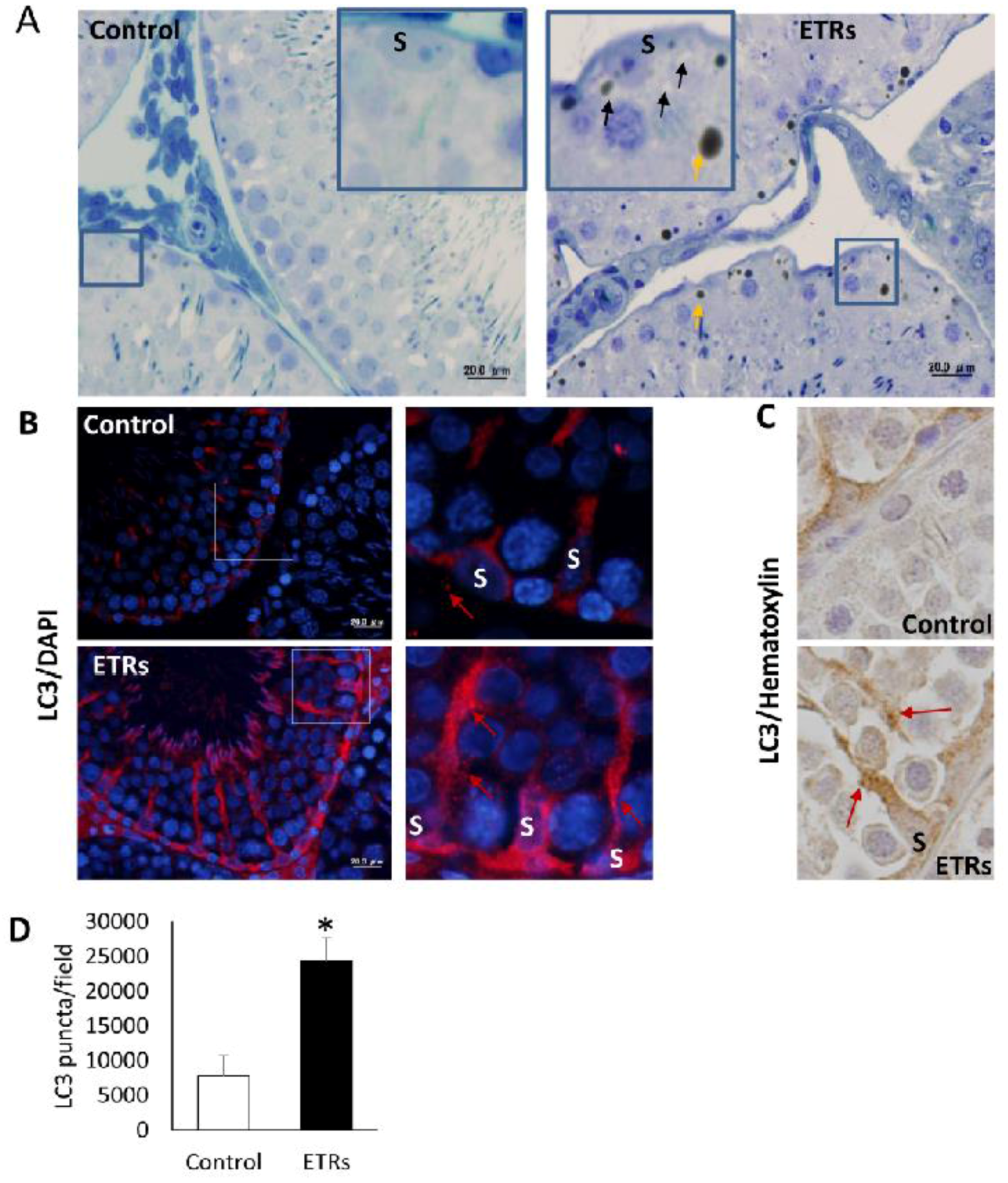
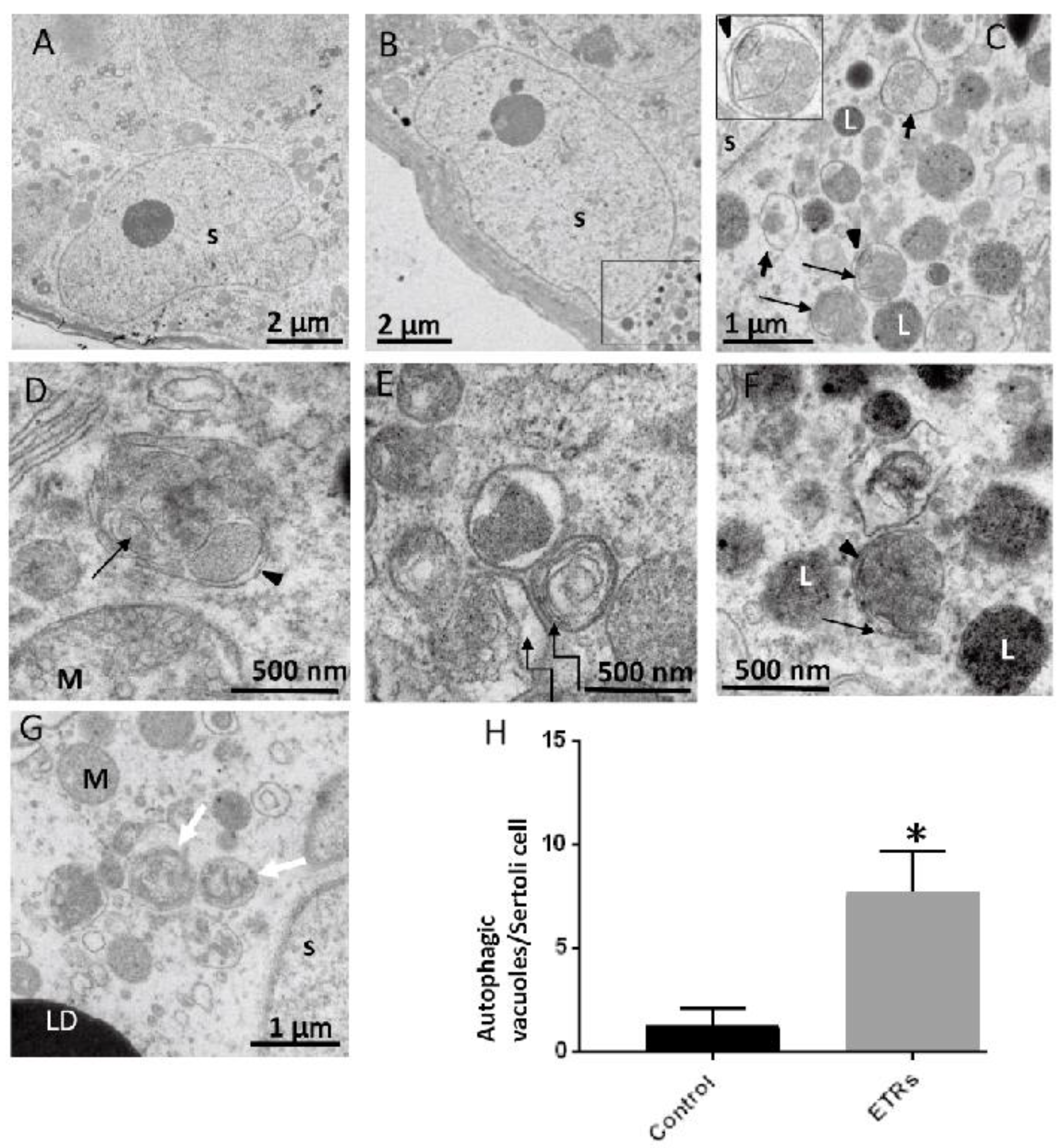
2.3. Upregulated Autophagic Response in ETR SCs: Light and TEM Observations
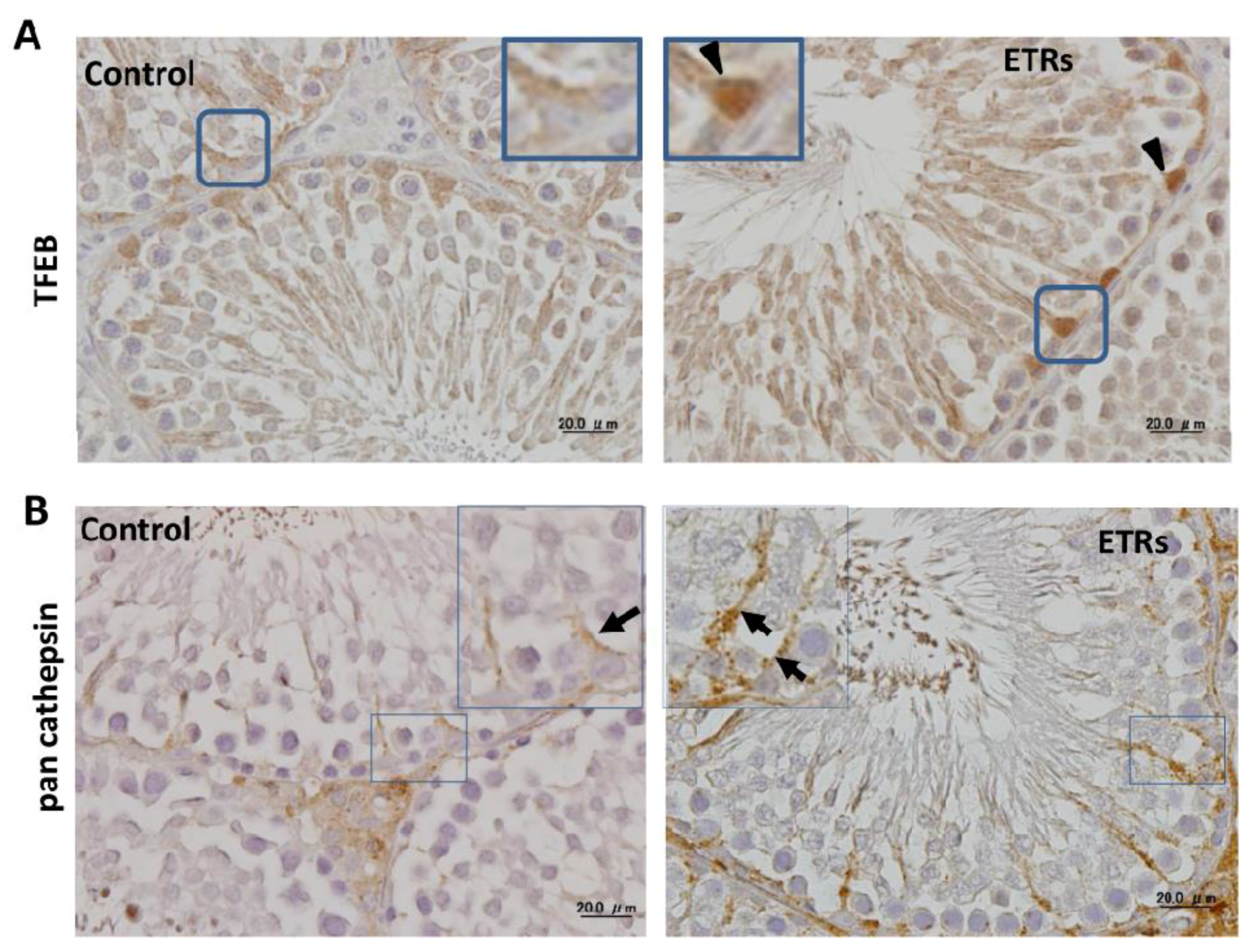
2.4. Ethanol-Induced Autophagy in SCs Is Associated with Upregulation and Nuclear Translocation of TFEB
2.5. Ethanol-Induced Autophagy in Cultured SCs as a Prosurvival Mechanism
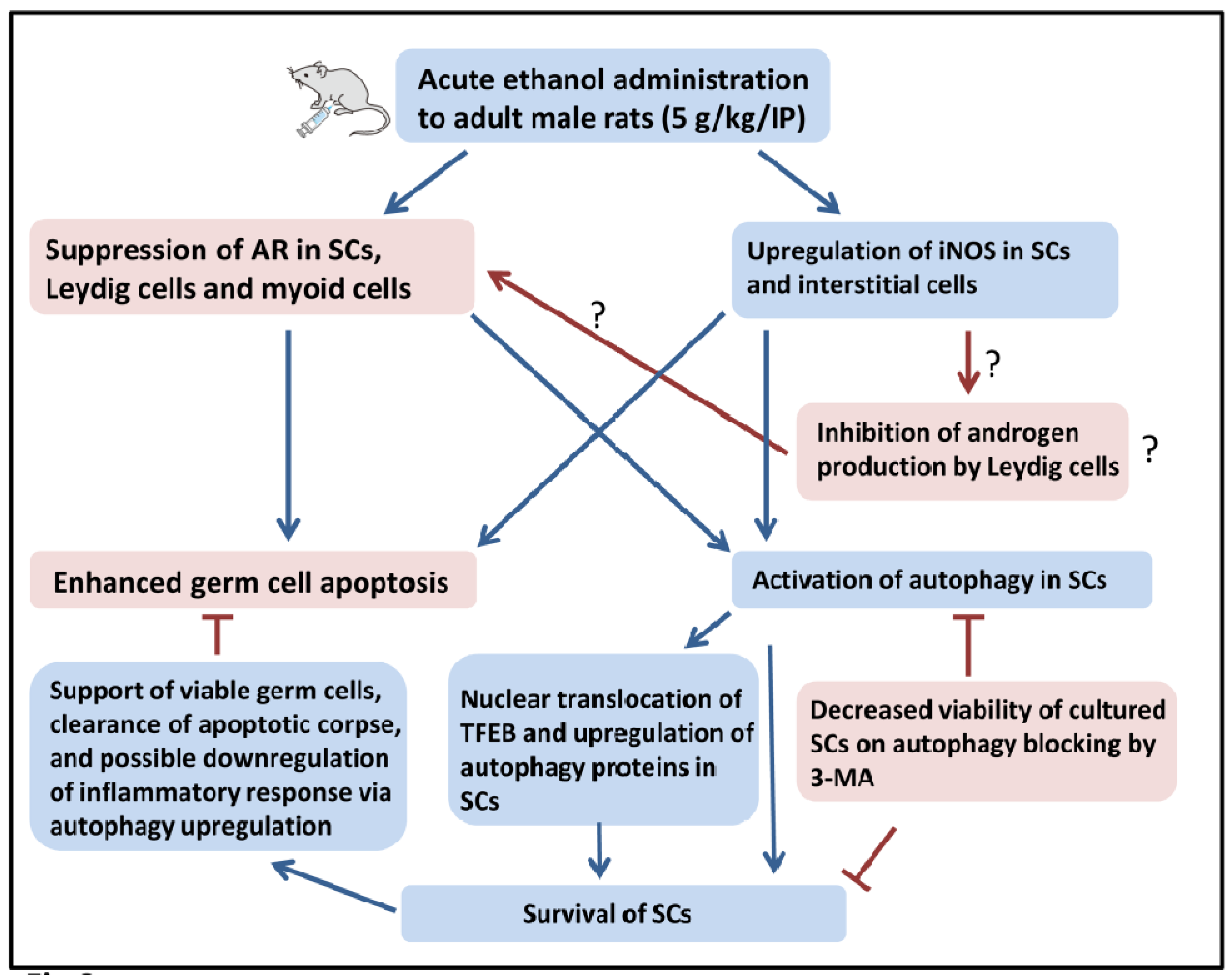
3. Discussion
4. Materials and Methods
4.1. Study Approval
4.2. Antibodies and Kits
4.3. Animal Experiment
4.4. Cell Culture and Treatment
4.5. Blocking Autophagy in Cultured SCs with 3-MA and Cell Viability Assay
4.6. Histopathological Analysis
4.7. TUNEL Assay and TUNEL/AR Double Labelling
4.8. Immunohistochemistry
4.9. Immunofluorescence Single and Double Labelling
4.10. Line Profile Plots for Co-Localization Analysis
4.11. TEM and Quantitative Analysis
4.12. Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- França, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Meneses, M.J; Silva, B.M.; Sousa, M.; Alves, M.G.; Oliveira, P.F. New insights on hormones and factors that modulate Sertoli cell metabolism. Histol. Histopathol. 2016, 31, 499–513. [Google Scholar] [PubMed]
- Oliveira, P.F.; Martins, A.D.; Moreira, A.C.; Cheng, C.Y.; Alves, M.G. The Warburg effect revisited-lesson from the Sertoli cell. Med. Res. Rev. 2015, 35, 126–151. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, H.; Skalli, M.; Chatelain, P.G.; Avallet, O.; Saez, J.M. The paracrine role of Sertoli cells on Leydig cell function. Cell Biol. Toxicol. 1992, 8, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Sanberg, P.R.; Borlongan, C.V.; Othberg, A.; Saporta, S.; Freeman, T.B.; Cameron, D.F. Testis-derived Sertoli cells have a trophic effect on dopamine neurons and alleviate hemiparkinsonism in rats. Nat. Med. 1997, 3, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Long, C.R.; Dufour, J.M. Genetically engineered immune privileged Sertoli cells: A new road to cell based gene therapy. Spermatogenesis 2012, 2, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell. 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Ito, Y.; Otsuki, Y. The autophagic response to alcohol toxicity: The missing layer. J. Hepatol. 2013, 59, 398. [Google Scholar] [CrossRef] [PubMed]
- Sica, V.; Galluzzi, L.; Bravo-San Pedro, J.M.; Izzo, V.; Maiuri, M.C.; Kroemer, G. Organelle-specific initiation of Autophagy. Mol. Cell 2015, 59, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, J.; Fan, S.; Liang, C.Z. Autophagy: A stumbling block of androgen inhibition to treat benign prostatic hyperplasia or prostate cancer. Asian J. Androl. 2016, 18, 654–656. [Google Scholar] [PubMed]
- Eid, N.; Ito, Y.; Maemura, K.; Otsuki, Y. Elevated autophagic sequestration of mitochondria and lipid droplets in steatotic hepatocytes of chronic ethanol treated rats: An immunohistochemical and electron microscopic study. J. Mol. Histol. 2013, 44, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Betsuyaku, T.; Eid, N.; Ito, Y.; Tanaka, Y.; Otsuki, Y.; Kondo, Y. Ethanol enhances thymocyte apoptosis and autophagy in macrophages of rat thymi. Histol. Histopathol. 2016, 27, 11861. [Google Scholar]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Impey, S.; Kang, H.; di Ronza, A.; Pelz, C.; Sardiello, M.; Ballabio, A. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 2011, 20, 3852–3866. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; di Malta, C.; Polito, V.A.; Garcia, A.M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Thomes, P.G.; Trambly, C.S.; Fox, H.S.; Tuma, D.J.; Donohue, T.M., Jr. Acute and chronic ethanol administration differentially modulate hepatic autophagy and transcription factor EB. Alcohol. Clin. Exp. Res. 2015, 39, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Martina, J.A.; Diab, H.I.; Brady, O.A.; Puertollano, R. TFEB and TFE3 are novel components of the integrated stress response. EMBO J. 2016, 35, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, X.; Yu, L.; Yang, J.; Calvo, R.; Patnaik, S.; Hu, X.; Gao, Q.; Yang, M.; Lawas, M.; et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016, 7, 12109. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.Q.; Liao, T.T.; Yang, W.R.; Wang, Y.; Luo, H.Y.; Wang, X.Z. Heat stress-induced autophagy promotes lactate secretion in cultured immature boar Sertoli cells by inhibiting apoptosis and driving SLC2A3, LDHA, and SLC16A1 expression. Theriogenology 2017, 87, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Hu, C.; Quan, C.; Yu, T.; Huang, W.; Chen, W.; Tang, S.; Shi, Y.; Martin, F.L.; Yang, K. 4-Nonylphenol induces autophagy and attenuates mTOR-p70S6K/4EBP1 signaling by modulating AMPK activation in Sertoli cells. Toxicol. Lett. 2016, 267, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Yefimova, M.G.; Messaddeq, N.; Harnois, T.; Meunier, A.C.; Clarhaut, J.; Noblanc, A.; Weickert, J.L.; Cantereau, A.; Philippe, M.; Bourmeyster, N.; et al. A chimerical phagocytosis model reveals the recruitment by Sertoli cells of autophagy for the degradation of ingested illegitimate substrates. Autophagy 2013, 9, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, H.Z.; Xu, L.M.; Huang, Y.R.; Dai, H.L.; Kang, X.N. Testosterone regulates the autophagic clearance of androgen binding protein in rat Sertoli cells. Sci. Rep. 2015, 95, 8894. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.A.; Shibata, M.A.; Ito, Y.; Kusakabe, K.; Hammad, H.; Otsuki, Y. Involvement of Fas system and active caspases in apoptotic signalling in testicular germ cells of ethanol-treated rats. Int. J. Androl. 2002, 25, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Emanuele, M.A.; LaPaglia, N.; Kovacs, E.J.; Emanuele, N.V. Vitamin E prevents ethanol-induced inflammatory, hormonal, and cytotoxic changes in reproductive tissues. Endocrine 2007, 32, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Ito, Y.; Otsuki, Y. Involvement of inducible nitric oxide synthase in DNA fragmentation in various testicular germ cells of ethanol-treated rats. J. Men’s Health 2011, 8, S36–S40. [Google Scholar] [CrossRef]
- Eid, N.; Ito, Y.; Otsuki, Y. Enhanced mitophagy in Sertoli cells of ethanol-treated rats: Morphological evidence and clinical relevance. J. Mol. Histol. 2012, 43, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Rahimipour, M.; Talebi, A.R.; Anvari, M.; Sarcheshmeh, A.A.; Omidi, M. Effects of different doses of ethanol on sperm parameters, chromatin structure and apoptosis in adult mice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Rusyn, I.; Bataller, R. Alcohol and toxicity. J. Hepatol. 2013, 59, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Banerjee, A.; Jang, S.; Yoo, S.H.; Yun, J.W.; Gonzalez, F.J.; Keshavarzian, A.; Song, B.J. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic. Biol. Med. 2013, 65, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Ohsako, S.; Kanai, Y.; Kurohmaru, M. Single administration of butylparaben induces spermatogenic cell apoptosis in prepubertal rats. Acta Histochem. 2014, 116, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Ito, Y.; Horibe, A.; Otsuki, Y. Ethanol-induced mitophagy in liver is associated with activation of the PINK1-Parkin pathway triggered by oxidative DNA damage. Histol. Histopathol. 2016, 31, 1143–1159. [Google Scholar] [PubMed]
- Allan, D.J.; Harmon, B.V.; Roberts, S.A. Spermatogonial apoptosis has three morphologically recognizable phases and shows no circadian rhythm during normal spermatogenesis in the rat. Cell Prolif. 1992, 25, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, G.; Weber, S.; Volynets, V.; Spruss, A.; Bischoff, S.C.; Bergheim, I. Cinnamon extract protects against acute alcohol-induced liver steatosis in mice. J. Nutr. 2009, 139, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Pla, A.; Pascual, M.; Guerri, C. Autophagy constitutes a protective mechanism against ethanol toxicity in mouse astrocytes and neurons. PLoS ONE 2016, 11, e0153097. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.J.; Hardy, M.P.; Inigo, I.V.; Huhtaniemi, I.; Bardin, C.W.; Moo-Young, A.J. Effects of androgen on androgen receptor expression in rat testicular and epididymal cells: A quantitative immunohistochemical study. Biol. Reprod. 2000, 63, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, N.; Koeva, Y.; Bakalska, M.; Pavlova, E.; Nikolov, B.; Davidoff, M. Loss and recovery of androgen receptor protein expression in the adult rat testis following androgen withdrawal by ethane dimethanesulfonate. Folia Histochem. Cytobiol. 2006, 44, 81–86. [Google Scholar] [PubMed]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W. Effects of chronic ethanol intake on aromatization of androgens and concentration of estrogen and androgen receptors in rat liver. Toxicology 1990, 62, 285–295. [Google Scholar] [CrossRef]
- Vingren, J.L.; Koziris, L.P.; Gordon, S.E.; Kraemer, W.J.; Turner, R.T.; Westerlind, K.C. Chronic alcohol intake, resistance training, and muscle androgen receptor content. Med. Sci. Sports Exerc. 2005, 37, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Skuk, D.; Goulet, M.; Paradis, M.; Tremblay, J.P. Myopathy in a rhesus monkey with biopsy findings similar to human sporadic inclusion body myositis. Neuromuscul. Disord. 2013, 23, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Thomes, P.G.; Ehlers, R.A.; Trambly, C.S.; Clemens, D.L.; Fox, H.S.; Tuma, D.J.; Donohue, T.M., Jr. Multilevel regulation of autophagosome content by ethanol oxidation in HepG2 cells. Autophagy 2013, 9, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, H.W.; Youdim, M.B.; Rhyu, I.J.; Choe, K.M.; Oh, Y.J. Binding preference of p62 towards LC3-ll during dopaminergic neurotoxin-induced impairment of autophagic flux. Autophagy 2011, 7, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Ito, Y.; Otsuki, Y. Triggering of Parkin mitochondrial translocation in mitophagy: Implications for liver diseases. Front. Pharmacol. 2016, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Li, M.; Chen, X.; Ni, H.M.; Lin, C.W.; Gao, W.; Lu, B.; Stolz, D.B; Clemens, D.L.; Yin, X.M. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 2010, 139, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jeong, C.W.; Ku, J.H.; Kwak, C.; Kim, H.H. Inhibition of autophagy potentiates atorvastatin-induced apoptotic cell death in human bladder cancer cells in vitro. Int. J. Mol. Sci. 2014, 15, 8106–8121. [Google Scholar] [CrossRef] [PubMed]
- Weckman, A.; di leva, A.; Rotondo, F.; Syro, L.V.; Ortiz, L.D.; Kovacs, K.; Cusimano, M.D. Autophagy in the endocrine glands. J. Mol. Endocrinol. 2014, 52, 151–163. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, M.K.; Schlatt, S.; Gerdprasert, O.; Phillips, D.J.; de Kretser, D.M.; Hedger, M.P. Inducible nitric oxide synthase in the rat testis: Evidence for potential roles in both normal function and inflammation-mediated infertility. Biol. Reprod. 2000, 63, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Weissman, B.A.; Niu, E.; Ge, R.; Sottas, C.; Holmes, M.; Hutson, J.C.; Hardy, M.P. Paracrine modulation of androgen synthesis in rat leydig cells by nitric oxide. J. Androl. 2005, 26, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Yadav, S.K.; Prakash, J.; Westfall, S.; Ghosh, A.; Agarwal, N.K.; Singh, S.P. Increase in the expression of inducible nitric oxide synthase on exposure to bisphenol A: A possible cause for decline in steroidogenesis in male mice. Environ. Toxicol. Pharmacol. 2015, 39, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Wang, H.; Yang, H. Celastrol induces autophagy by targeting AR/miR-101 in prostate cancer cells. PLoS ONE 2015, 10, e0140745. [Google Scholar] [CrossRef] [PubMed]
- Chemes, H. The phagocytic function of Sertoli cells: A morphological, biochemical, and endocrinological study of lysosomes and acid phosphatase localization in the rat testis. Endocrinology 1986, 119, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Igdoura, S.A.; Morales, C.R.; Hermo, L. Differential expression of cathepsins B and D in testis and epididymis of adult rats. J. Histochem. Cytochem. 1995, 43, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Wright, W.W.; Zirkin, B.R.; Korah, N.; Mort, J.S.; Hermo, L. Expression and localization of cathepsin K in adult rat Sertoli cells. Biol. Reprod. 2004, 70, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, L.; Song, Z.; Lambert, J.C.; McClain, C.J.; Kang, Y.J. A critical involvement of oxidative stress in acute alcohol induced hepatic TNF-α production. Am. J. Pathol. 2003, 163, 1137–1146. [Google Scholar] [CrossRef]
- D’Souza El-Guindy, N.B.; Kovacs, E.J.; de Witte, P.; Spies, C.; Littleton, J.M.; de Villiers, W.J.; Lott, A.J.; Plackett, T.P.; Lanzke, N.; Meadows, G.G. Laboratory models available to study alcohol-induced organ damage and immune variations: Choosing the appropriate model. Alcohol. Clin. Exp. Res. 2010, 3, 1489–1511. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zhang, C.L.; Zhao, X.L.; Xie, K.Q.; Zeng, T. Inhibition of cytochrome P4502E1 by chlormethiazole attenuated acute ethanol-induced fatty liver. Chem. Biol. Interact. 2014, 222, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Nogales, F.; Rua, R.M.; Ojeda, M.L.; Murillo, M.L.; Carreras, O. Oral or intraperitoneal binge drinking and oxidative balance in adolescent rats. Chem. Res. Toxicol. 2014, 27, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Narabayashi, K.; Ito, Y.; Eid, N.; Maemura, K.; Inoue, T.; Takeuchi, T.; Otsuki, Y.; Higuchi, K. Indomethacin suppresses LAMP-2 expression and induces lipophagy and lipoapoptosis in rat enterocytes via the ER stress pathway. J. Gastroenterol. 2015, 50, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Corsetti, V.; Florenzano, F.; Atlante, A.; Bobba, A.; Nicolin, V.; Nori, S.L.; Calissano, P. Morphological and bioenergetic demands underlying the mitophagy in post-mitotic neurons: The pink-parkin pathway. Front. Aging Neurosci. 2014, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Benmeradi, N.; Payre, B.; Goodman, S.L. Easier and safer biological staining: High contrast uranyless staining of TEM grids using mPrep/g capsules. Microsc. Microanal. 2015, 21, 721–722. [Google Scholar] [CrossRef]
- Floriot, S.; Vesque, C.; Rodriguez, S.; Bourgain-Guglielmetti, F.; Karaiskou, A.; Gautier, M.; Duchesne, A.; Barbey, S.; Fritz, S.; Vasilescu, A.; et al. C-Nap1 mutation affects centriole cohesion and is associated with a Seckel-like syndrome in cattle. Nat. Commun. 2015, 23, 6894. [Google Scholar] [CrossRef] [PubMed]
- Teckman, J.H.; An, J.K.; Blomenkamp, K.; Schmidt, B.; Perlmutter, D. Mitochondrial autophagy and injury in the liver in α1-antitrypsin deficiency. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Muenzer, J.T.; Hawkins, W.G.; Davis, C.G.; Dixon, D.J.; McDunn, J.E.; Brackett, D.J.; Lerner, M.R.; Swanson, P.E.; Hotchkiss, R.S. Sepsis induces extensive autophagic vacuolization in hepatocytes: A clinical and laboratory-based study. Lab. Investig. 2009, 89, 549–561. [Google Scholar] [CrossRef] [PubMed]



| Primary Antibody | Company | Catalog Number | Host | Method | Dilution |
|---|---|---|---|---|---|
| LC3 | MBL | PM036 | Rabbit | IHC | 1:5000 |
| IF | 1:250 | ||||
| WB | 1:1000 | ||||
| p62/SQSTM1 | Novus | H00008878-M01 | Mouse | IF | 1:100 |
| WB | 1:500 | ||||
| pan cathepsin | Santa Cruz Biotechnology | sc-6499 | Goat | IHC | 1:100 |
| iNOS | abcam | ab15326 | Rabbit | IHC | 1:2 |
| IF | 1:2 | ||||
| iNOS | abcam | ab15323 | Rabbit | WB | 1:150 |
| LAMP2 | Santa Cruz Biotechnology | sc-5571 | Rabbit | WB | 1:250 |
| AR | Santa Cruz Biotechnology | sc-816 | Rabbit | IHC | 1:250 |
| IF | 1:250 | ||||
| WB | 1:250 | ||||
| TFEB | MyBioSource | MBS9125929 | Rabbit | IHC | 1:2000 |
| IF | 1:200 | ||||
| WB | 1:1000 | ||||
| FOXO3a | Cell signaling | 12829 | Rabbit | IHC | 1:1000 |
| IF | 1:400 | ||||
| WB | 1:1000 | ||||
| Actin | Santa Cruz Biotechnology | sc-1616 | Goat | WB | 1:1000 |
| Secondary Antibody | Company | Catalog Number | Host | Method | Dilution |
|---|---|---|---|---|---|
| AlexaFluor 488 | Thermofisher | A11001 (anti-Mouse) | Goat | IF | 1:250 |
| AlexaFluor 594 | Thermofisher | A11012 (anti-Rabbit) | Goat | IF | 1:250 |
| EnVision + Dual link | DAKO | K4063 | IHC | 1:1 | |
| EnVision+ Single 15 nm gold-conjugated | DAKO Aurion | K4002 815.011 | Goat | IHC IEM | 1:1 1:25 |
| Goat immunoglobulins | DAKO | E0466 | Rabbit | IHC | 1:250 |
| Rabbit immunoglobulins | DAKO | E0432 | Goat | IHC | 1:250 |
| donkey anti-goat IgG-HRP | Santa Cruz Biotechnology | SC-2304 | Donkey | WB | 1:10,000 |
| donkey anti-mouse IgG-HRP | Santa Cruz Biotechnology | SC-2096 | Donkey | WB | 1:10,000 |
| donkey anti-rabbit IgG-HRP | Santa Cruz Biotechnology | SC-2077 | Donkey | WB | 1:10,000 |
| Name of Kit | Company | Catalog Number |
|---|---|---|
| Vectastain ABC Standard Kit | Vector | PK-4000 |
| ImmPACT DAB | Vector | SK-4105 |
| In Situ Cell Death (TUNEL) Detection Kit | Roche Diagnotics | 11684817910 |
| Western Lightning ECL Pro | PerkinElmer | NEL120001EA |
| VectaFluor™ R.T.U. DyLight® 488 | Vector | DI-3788 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horibe, A.; Eid, N.; Ito, Y.; Hamaoka, H.; Tanaka, Y.; Kondo, Y. Upregulated Autophagy in Sertoli Cells of Ethanol-Treated Rats Is Associated with Induction of Inducible Nitric Oxide Synthase (iNOS), Androgen Receptor Suppression and Germ Cell Apoptosis. Int. J. Mol. Sci. 2017, 18, 1061. https://doi.org/10.3390/ijms18051061
Horibe A, Eid N, Ito Y, Hamaoka H, Tanaka Y, Kondo Y. Upregulated Autophagy in Sertoli Cells of Ethanol-Treated Rats Is Associated with Induction of Inducible Nitric Oxide Synthase (iNOS), Androgen Receptor Suppression and Germ Cell Apoptosis. International Journal of Molecular Sciences. 2017; 18(5):1061. https://doi.org/10.3390/ijms18051061
Chicago/Turabian StyleHoribe, Akio, Nabil Eid, Yuko Ito, Hitomi Hamaoka, Yoshihisa Tanaka, and Yoichi Kondo. 2017. "Upregulated Autophagy in Sertoli Cells of Ethanol-Treated Rats Is Associated with Induction of Inducible Nitric Oxide Synthase (iNOS), Androgen Receptor Suppression and Germ Cell Apoptosis" International Journal of Molecular Sciences 18, no. 5: 1061. https://doi.org/10.3390/ijms18051061