Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications—Focus on Prostate and Breast Cancer
Abstract
:1. Introduction
2. Nanoparticles for Prostate and Breast Cancer
2.1. Iron Oxide Nanoparticles
2.2. Gadolinium, Manganese, Gold, Silver, and Platinum Nanoparticles
2.3. Quantum and Cornell Dots
2.4. Carbon Based Nanoparticles
2.5. Liposomes
2.6. Polymer Based Nanoparticles
2.7. Solid Lipid Nanoparticles
3. Multimodal Imaging Options for Prostate and Breast Cancer
3.1. Computed Tomography and Tomosynthesis
3.2. Positron Emission and Single Photon Emission Computed Tomography
3.3. Magnetic Resonance Imaging
3.4. Ultrasound Imaging
3.5. Cherenkov Luminescence Imaging
3.6. Photoacoustic Imaging
3.7. Optical Imaging
3.8. Electron Microscopy
4. Multimodal Therapy Options for Prostate and Breast Cancer
4.1. Chemotherapy
4.2. Gene Therapy
4.3. Photon Activation Therapy
4.4. Photodynamic Therapy
4.5. Photothermal Therapy
4.6. Radioimmunotherapy
4.7. Neutron Capture Therapy
4.8. Magnetic Therapy
5. The Prostate Cancer Case
5.1. Background Statistics
5.2. Diagnosis, Staging, Recurrence, and Metastases
6. Theranostic Nanoparticles for Prostate and Breast Cancer
Conflicts of Interest
References
- Li, L.; Tong, R.; Li, M.; Kohane, D.S. Self-assembled gemcitabine-gadolinium nanoparticles for magnetic resonance imaging and cancer therapy. Acta Biomater. 2016, 33, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.Y.; Theek, B.; Storm, G.; Kiessling, F.; Lammers, T. Recent progress in nanomedicine: Therapeutic, diagnostic and theranostic applications. Curr. Opin. Biotechnol. 2013, 24, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Qian, W.P.; Wang, L.; Wang, Y.A.; Staley, C.A.; Satpathy, M.; Nie, S.; Mao, H.; Yang, L. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano 2013, 7, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Hu, J.; Zhu, Y.; Zou, R.; Chen, Z.; Yang, S.; Li, R.; Su, Q.; Han, Y.; Liu, X. Sub-10 nm Fe3O4@Cu2-xS core-shell nanoparticles for dual-modal imaging and photothermal therapy. J. Am. Chem. Soc. 2013, 135, 8571–8577. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Theranostic nanoshells: From probe design to imaging and treatment of cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Rong, P.; Lin, J.; Li, W.; Yan, X.; Zhang, M.G.; Nie, L.; Niu, G.; Lu, J.; Wang, W.; et al. Triphase interface synthesis of plasmonic gold bellflowers as near-infrared light mediated acoustic and thermal theranostics. J. Am. Chem. Soc. 2014, 136, 8307–8313. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. Engl. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, G.; Hu, J.; Wang, X.; Zhu, M.; Liu, S. Hyperbranched self-immolative polymers (HSIPS) for programmed payload delivery and ultrasensitive detection. J. Am. Chem. Soc. 2015, 137, 11645–11655. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and perspectives. Angew. Chem. Int. Ed. Engl. 2009, 48, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Polymer-controlled drug delivery systems. Acc. Chem. Res. 1993, 26, 537–542. [Google Scholar] [CrossRef]
- Tong, R.; Chiang, H.H.; Kohane, D.S. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc. Natl. Acad. Sci. USA 2013, 110, 19048–19053. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Gadde, S.; Pfirschke, C.; Engblom, C.; Sprachman, M.M.; Kohler, R.H.; Yang, K.S.; Laughney, A.M.; Wojtkiewicz, G.; Kamaly, N.; et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 2015, 7, 314ra183. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.A.; Fenton, M.E.; Salesky, J.S.; Murphey, M.D. Magnetic resonance imaging of benign soft tissue neoplasms in adults. Radiol. Clin. N. Am. 2011, 49, 1197–1217. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, X.; Qian, Y.; Hu, X.; Liu, S. Multifunctional pH-disintegrable micellar nanoparticles of asymmetrically functionalized β-cyclodextrin-based star copolymer covalently conjugated with doxorubicin and DOTA-Gd moieties. Biomaterials 2012, 33, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-raman nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Kielar, F.; Tei, L.; Terreno, E.; Botta, M. Large relaxivity enhancement of paramagnetic lipid nanoparticles by restricting the local motions of the Gd(III) chelates. J. Am. Chem. Soc. 2010, 132, 7836–7837. [Google Scholar] [CrossRef] [PubMed]
- Mi, P.; Kokuryo, D.; Cabral, H.; Kumagai, M.; Nomoto, T.; Aoki, I.; Terada, Y.; Kishimura, A.; Nishiyama, N.; Kataoka, K. Hydrothermally synthesized pegylated calcium phosphate nanoparticles incorporating Gd-DTPA for contrast enhanced MRI diagnosis of solid tumors. J. Control. Release 2014, 174, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Mi, P.; Cabral, H.; Kokuryo, D.; Rafi, M.; Terada, Y.; Aoki, I.; Saga, T.; Takehiko, I.; Nishiyama, N.; Kataoka, K. Gd-DTPA-loaded polymer-metal complex micelles with high relaxivity for MR cancer imaging. Biomaterials 2013, 34, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.C.; Williams, K.J.; Fisher, E.A.; Fayad, Z.A. Recombinant hdl-like nanoparticles: A specific contrast agent for MRI of atherosclerotic plaques. J. Am. Chem. Soc. 2004, 126, 16316–16317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, Y.; Liu, T.; Hu, X.; Zhang, G.; You, Y.; Liu, S. Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and mr imaging. Biomaterials 2011, 32, 6595–6605. [Google Scholar] [CrossRef] [PubMed]
- Fossheim, S.L.; Fahlvik, A.K.; Klaveness, J.; Muller, R.N. Paramagnetic liposomes as MRI contrast agents: Influence of liposomal physicochemical properties on the in vitro relaxivity. Magn. Reson. Imaging 1999, 17, 83–89. [Google Scholar] [CrossRef]
- Perrier, M.; Gallud, A.; Ayadi, A.; Kennouche, S.; Porredon, C.; Gary-Bobo, M.; Larionova, J.; Goze-Bac, C.; Zanca, M.; Garcia, M.; et al. Investigation of cyano-bridged coordination nanoparticles Gd(3+)/[Fe(Cn)6](3-)/d-mannitol as T1-weighted MRI contrast agents. Nanoscale 2015, 7, 11899–11903. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, G.; Li, Y.; Wang, X.; Liu, S. Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals. J. Am. Chem. Soc. 2015, 137, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Song, Y.; Hong, B.J.; MacRenaris, K.W.; Mastarone, D.J.; O’Halloran, T.V.; Meade, T.J.; Nguyen, S.T. Modular polymer-caged nanobins as a theranostic platform with enhanced magnetic resonance relaxivity and pH-responsive drug release. Angew. Chem. Int. Ed. Engl. 2010, 49, 9960–9964. [Google Scholar] [CrossRef] [PubMed]
- Budd, G.T. Let me do more than count the ways: What circulating tumor cells can tell us about the biology of cancer. Mol. Pharm. 2009, 6, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Moller, B. Predicting the future burden of cancer. Nat. Rev. Cancer 2006, 6, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Danila, D.C.; Fleisher, M.; Scher, H.I. Circulating tumor cells as biomarkers in prostate cancer. Clin. Cancer Res. 2011, 17, 3903–3912. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, T.R. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med. J. Aust. 1869, 14, 146–147. [Google Scholar]
- Pantel, K.; Alix-Panabieres, C.; Riethdorf, S. Cancer micrometastases. Nat. Rev. Clin. Oncol. 2009, 6, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Massague, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Sagalowsky, A.; Clifford, E.; Beitsch, P.; Saboorian, H.; Euhus, D.; Meng, S.; Morrison, L.; Tucker, T.; Lane, N.; et al. Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin. Cancer Res. 2002, 8, 2073–2084. [Google Scholar] [PubMed]
- Sleijfer, S.; Gratama, J.W.; Sieuwerts, A.M.; Kraan, J.; Martens, J.W.; Foekens, J.A. Circulating tumour cell detection on its way to routine diagnostic implementation? Eur. J. Cancer 2007, 43, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F.; Smerage, J. Is there a role for circulating tumor cells in the management of breast cancer? Clin. Cancer Res. 2008, 14, 3646–3650. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Riethdorf, S. Pathology: Are circulating tumor cells predictive of overall survival? Nat. Rev. Clin. Oncol. 2009, 6, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Doyle, G.V.; Terstappen, L.W. Significance of circulating tumor cells detected by the cellsearch system in patients with metastatic breast colorectal and prostate cancer. J. Oncol. 2010, 2010, 617421. [Google Scholar] [CrossRef] [PubMed]
- Swaby, R.F.; Cristofanilli, M. Circulating tumor cells in breast cancer: A tool whose time has come of age. BMC Med. 2011, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Hekimian, K.; Meisezahl, S.; Trompelt, K.; Rabenstein, C.; Pachmann, K. Epithelial cell dissemination and readhesion: Analysis of factors contributing to metastasis formation in breast cancer. ISRN Oncol. 2012, 2012, 601810. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.M.; Moreno, J.G.; Zweitzig, D.R.; Gross, S.; Gomella, L.G.; Terstappen, L.W. Multigene reverse transcription-PCR profiling of circulating tumor cells in hormone-refractory prostate cancer. Clin. Chem. 2004, 50, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Swennenhuis, J.F.; Olmos, D.; Reid, A.H.; Vickers, E.; A’Hern, R.; Levink, R.; Coumans, F.; Moreira, J.; Riisnaes, R.; et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009, 69, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.; Doggen, C.J.; Attard, G.; de Bono, J.S.; Terstappen, L.W. All circulating EpCam+CK+CD45-objects predict overall survival in castration-resistant prostate cancer. Ann. Oncol. 2010, 21, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radiolabeled mesoporous silica nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, G. Single-walled carbon nanohorns and their applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Badea, I. Nanodiamonds as novel nanomaterials for biomedical applications: Drug delivery and imaging systems. Int. J. Nanomed. 2013, 8, 203–220. [Google Scholar]
- Clift, M.J.; Stone, V. Quantum dots: An insight and perspective of their biological interaction and how this relates to their relevance for clinical use. Theranostics 2012, 2, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Wilson, K.M.; Murray, P.; Fernig, D.G.; Levy, R. Long-term tracking of cells using inorganic nanoparticles as contrast agents: Are we there yet? Chem. Soc. Rev. 2012, 41, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Chung, H.J.; Park, T.G. Nanomaterials for cancer therapy and imaging. Mol. Cells 2011, 31, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Cheng, C.J.; Tietjen, G.T.; Saucier-Sawyer, J.K.; Saltzman, W.M. A holistic approach to targeting disease with polymeric nanoparticles. Nat. Rev. Drug Discov. 2015, 14, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, B.; Miskiel, S.; Markowska, J. Nanooncology in ovarian cancer treatment. Eur. J. Gynaecol. Oncol. 2016, 37, 161–163. [Google Scholar] [PubMed]
- Hu, J.J.; Xiao, D.; Zhang, X.Z. Advances in peptide functionalization on mesoporous silica nanoparticles for controlled drug release. Small 2016, 12, 3344–3359. [Google Scholar] [CrossRef] [PubMed]
- Mocan, L.; Matea, C.T.; Bartos, D.; Mosteanu, O.; Pop, T.; Mocan, T.; Iancu, C. Advances in cancer research using gold nanoparticles mediated photothermal ablation. Clujul Med. 2016, 89, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, X.; Mo, J.; Zhao, W. Drug delivery using nanoparticles for cancer stem-like cell targeting. Front. Pharmacol. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.G.; Marino, A.; Rocca, A.; Mattoli, V.; Ciofani, G. Barium titanate nanoparticles: Promising multitasking vectors in nanomedicine. Nanotechnology 2016, 27, 232001. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Sadreddini, S.; Rostamzadeh, D.; Ahmadi, M.; Jadidi-Niaragh, F.; Yousefi, M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed. Pharmacother. 2016, 80, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Santoso, M.R.; Yang, P.C. Magnetic nanoparticles for targeting and imaging of stem cells in myocardial infarction. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R.; et al. Nano carriers for drug transport across the blood brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; des Rieux, A.; Preat, V. Mechanisms of transport of polymeric and lipidic nanoparticles across the intestinal barrier. Adv. Drug Deliv. Rev. 2016, 106, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Matsumura, Y.; Kataoka, K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 2016, 107, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Ozpolat, B.; Ulubayram, K. Oligonucleotide-based theranostic nanoparticles in cancer therapy. Nanomedicine (lond.) 2016, 11, 1287–1308. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cai, T.; Xia, X.; Zhang, R.; Cai, Y.; Chiba, P. Nanoparticle delivery of anticancer drugs overcomes multidrug resistance in breast cancer. Drug Deliv. 2016, 23, 3350–3357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tian, M.; Li, C. Copper-based nanomaterials for cancer imaging and therapy. Bioconjug. Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mousa, S.A. Lipid nanoparticles and their application in nanomedicine. Curr. Pharm. Biotechnol. 2016, 17, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Jun Wei, L.; Gan, S.H. Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.D.; Yang, W.X. Engineered nanoparticles induce cell apoptosis: Potential for cancer therapy. Oncotarget 2016, 7, 40882–40903. [Google Scholar] [CrossRef] [PubMed]
- Shabestari Khiabani, S.; Farshbaf, M.; Akbarzadeh, A.; Davaran, S. Magnetic nanoparticles: Preparation methods, applications in cancer diagnosis and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2016, 45, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Lemaster, J.E.; Jokerst, J.V. What is new in nanoparticle-based photoacoustic imaging? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 9, e1404. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Chen, X.; Du, X.S.; Zhang, J.L.; Liu, G.; Zhang, W.G. Application of iron oxide nanoparticles in glioma imaging and therapy: From bench to bedside. Nanoscale 2016, 8, 7808–7826. [Google Scholar] [CrossRef] [PubMed]
- Fathi Karkan, S.; Mohammadhosseini, M.; Panahi, Y.; Milani, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, E.; Hosseini, A.; Davaran, S. Magnetic nanoparticles in cancer diagnosis and treatment: A review. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Radenkovic, D.; Kobayashi, H.; Remsey-Semmelweis, E.; Seifalian, A.M. Quantum dot nanoparticle for optimization of breast cancer diagnostics and therapy in a clinical setting. Nanomedicine 2016, 12, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Pratt, E.C.; Shaffer, T.M.; Grimm, J. Nanoparticles and radiotracers: Advances toward radionanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 872–890. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, L.; Leggio, A.; Sisci, D.; Ando, S.; Morelli, C. Mesoporous silica nanoparticles in cancer therapy: Relevance of the targeting function. Mini Rev. Med. Chem. 2016, 16, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Magne, N.; Vallard, A.; Guy, J.B.; Rodriguez-Lafrasse, C.; Deutsch, E.; Chargari, C. Nanoparticles in radiation oncology: From bench-side to bedside. Cancer Lett. 2016, 375, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Naim, M.; Aziz, M.; Yadav, N. Unique roles of nanotechnology in medicine and cancer-II. Indian J. Cancer 2015, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.M.; Fu, C.P.; Fang, J.Z.; Xu, X.D.; Wei, X.H.; Tang, W.J.; Jiang, X.Q.; Zhang, L.M. Hyaluronan-modified superparamagnetic iron oxide nanoparticles for bimodal breast cancer imaging and photothermal therapy. Int. J. Nanomed. 2017, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Oddo, L.; Cerroni, B.; Domenici, F.; Bedini, A.; Bordi, F.; Chiessi, E.; Gerbes, S.; Paradossi, G. Next generation ultrasound platforms for theranostics. J. Colloid Interface Sci. 2017, 491, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Dadras, P.; Atyabi, F.; Irani, S.; Ma’mani, L.; Foroumadi, A.; Mirzaie, Z.H.; Ebrahimi, M.; Dinarvand, R. Formulation and evaluation of targeted nanoparticles for breast cancer theranostic system. Eur. J. Pharm. Sci. 2017, 97, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Kaol, G.D.; Tsourkas, A.; et al. Theranostic application of mixed gold and superparamagnetic iron oxide nanoparticle micelles in glioblastoma multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Multhoff, G. Recent developments of magnetic nanoparticles for theranostics of brain tumor. Curr. Drug Metab. 2016, 17, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, A.; Sadighian, S.; Rostamizadeh, K.; Firuzi, O.; Hamidi, M.; Mohammadi-Samani, S.; Miri, R. Design, preparation, and in vitro characterization of a trimodally-targeted nanomagnetic onco-theranostic system for cancer diagnosis and therapy. Int. J. Pharm. 2016, 500, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, Z.; Saei, A.A.; Hajipour, M.J.; Raoufi, M.; Vermesh, O.; Mahmoudi, M. Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges. Nanomedicine 2016, 12, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.; Kraitchman, D.L. Iron oxide mr contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Alwi, R.; Telenkov, S.; Mandelis, A.; Leshuk, T.; Gu, F.; Oladepo, S.; Michaelian, K. Silica-coated super paramagnetic iron oxide nanoparticles (SPION) as biocompatible contrast agent in biomedical photoacoustics. Biomed. Opt. Express 2012, 3, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Bohmer, N.; Jordan, A. Caveolin-1 and CDC42 mediated endocytosis of silica-coated iron oxide nanoparticles in HeLa cells. Beilstein J. Nanotechnol. 2015, 6, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Nyalosaso, J.L.; Rascol, E.; Pisani, C.; Dorandeu, C.; Dumail, X.; Maynadier, M.; Gary-Bobo, M.; Kee Him, J.L.; Bron, P.; Garcia, M.; et al. Synthesis, decoration, and cellular effects of magnetic mesoporous silica nanoparticles. RSC Adv. 2016, 6, 7275–7283. [Google Scholar] [CrossRef]
- Winter, A.; Engels, S.; Kowald, T.; Paulo, T.S.; Gerullis, H.; Chavan, A.; Wawroschek, F. Magnetic sentinel lymph node detection in prostate cancer after intraprostatic injection of superparamagnetic iron oxide nanoparticles. Aktuelle Urol. 2017. [Google Scholar] [CrossRef]
- Sabnis, S.; Sabnis, N.A.; Raut, S.; Lacko, A.G. Superparamagnetic reconstituted high-density lipoprotein nanocarriers for magnetically guided drug delivery. Int. J. Nanomed. 2017, 12, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.; Johnson, N.R.; Boya, V.K.; Chowdhury, P.; Othman, S.F.; Khalilzad-Sharghi, V.; Hafeez, B.B.; Ganju, A.; Khan, S.; Behrman, S.W.; et al. PSMA targeted docetaxel-loaded superparamagnetic iron oxide nanoparticles for prostate cancer. Colloids Surf. B Biointerfaces 2016, 144, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, Y.; Chen, Y.; Liu, W.; Jiang, J.; Guan, W.; Zhang, Z.; Duan, Y. In vivo molecular MRI imaging of prostate cancer by targeting psma with polypeptide-labeled superparamagnetic iron oxide nanoparticles. Int. J. Mol. Sci. 2015, 16, 9573–9587. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Kim, D.; Lee, I.H.; So, J.S.; Jeong, Y.Y.; Jon, S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small 2011, 7, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Jo, H.; Song, K.; Cho, M.; Chun, Y.S.; Jon, S.; Kim, W.J.; Ban, C. Dual-aptamer-based delivery vehicle of doxorubicin to both PSMA (+) and PSMA (−) prostate cancers. Biomaterials 2011, 32, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Ananthanarayanan, P.; Aziz, S.K.; Rai, S.; Mutalik, S.; Sadashiva, S.R. Enhanced effect of geldanamycin nanocomposite against breast cancer cells growing in vitro and as xenograft with vanquished normal cell toxicity. Toxicol. Appl. Pharmacol. 2017, 320, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Shaik, A.P.; Shaik, A.S.; Majwal, A.A.; Faraj, A.A. Blocking IL4-α receptor using polyethylene glycol functionalized superparamagnetic iron oxide nanocarriers to inhibit breast cancer cell proliferation. Cancer Res. Treat. 2016, 49, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Chiappi, M.; Conesa, J.J.; Pereiro, E.; Sorzano, C.O.; Rodriguez, M.J.; Henzler, K.; Schneider, G.; Chichon, F.J.; Carrascosa, J.L. Cryo-soft X-ray tomography as a quantitative three-dimensional tool to model nanoparticle:Cell interaction. J. Nanobiotechnol. 2016, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Stapf, M.; Pompner, N.; Teichgraber, U.; Hilger, I. Heterogeneous response of different tumor cell lines to methotrexate-coupled nanoparticles in presence of hyperthermia. Int. J. Nanomed. 2016, 11, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Almaki, J.H.; Nasiri, R.; Idris, A.; Majid, F.A.; Salouti, M.; Wong, T.S.; Dabagh, S.; Marvibaigi, M.; Amini, N. Synthesis, characterization and in vitro evaluation of exquisite targeting SPIONs-PEG-HER in HER2+ human breast cancer cells. Nanotechnology 2016, 27, 105601. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Stephen, Z.R.; Veiseh, O.; Arami, H.; Wang, T.; Lai, V.P.; Park, J.O.; Ellenbogen, R.G.; Disis, M.L.; Zhang, M. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional spions. ACS Nano 2012, 6, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Lentschig, M.G.; Reimer, P.; Rausch-Lentschig, U.L.; Allkemper, T.; Oelerich, M.; Laub, G. Breath-hold gadolinium-enhanced MR angiography of the major vessels at 1.0 t: Dose-response findings and angiographic correlation. Radiology 1998, 208, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Koretsky, A.P. Manganese ion enhances t1-weighted MRI during brain activation: An approach to direct imaging of brain function. Magn. Reson. Med. 1997, 38, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Xie, J. Development of manganese-based nanoparticles as contrast probes for magnetic resonance imaging. Theranostics 2012, 2, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Lee, J.H.; Aoki, I.; Koretsky, A.P. Manganese-enhanced magnetic resonance imaging (MEMRI): Methodological and practical considerations. NMR Biomed. 2004, 17, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Koretsky, A.P.; Silva, A.C. Manganese-enhanced magnetic resonance imaging (MEMRI). NMR Biomed. 2004, 17, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Paratala, B.S.; Jacobson, B.D.; Kanakia, S.; Francis, L.D.; Sitharaman, B. Physicochemical characterization, and relaxometry studies of micro-graphite oxide, graphene nanoplatelets, and nanoribbons. PLoS ONE 2012, 7, e38185. [Google Scholar] [CrossRef] [PubMed]
- Harisinghani, M.G.; Jhaveri, K.S.; Weissleder, R.; Schima, W.; Saini, S.; Hahn, P.F.; Mueller, P.R. MRI contrast agents for evaluating focal hepatic lesions. Clin. Radiol. 2001, 56, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi Murph, S.; Jacobs, S.; Liu, J.; Hu, T.; Siegfired, M.; Serkiz, S.; Hudson, J. Manganese–gold nanoparticles as an MRI positive contrast agent in mesenchymal stem cell labeling. J. Nanopart. Res. 2012, 14, 658. [Google Scholar] [CrossRef]
- Cai, X.; Gao, W.; Ma, M.; Wu, M.; Zhang, L.; Zheng, Y.; Chen, H.; Shi, J. Nanoparticles: A prussian blue-based core-shell hollow-structured mesoporous nanoparticle as a smart theranostic agent with ultrahigh pH-responsive longitudinal relaxivity (adv. Mater. 41/2015). Adv. Mater. 2015, 27, 6382–6389. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Hosseini-Nami, S.; Kamrava, S.K.; Ghaznavi, H.; Shakeri-Zadeh, A. Folate-conjugated gold nanoparticle as a new nanoplatform for targeted cancer therapy. J. Cancer Res. Clin. Oncol. 2016, 142, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Gossai, N.P.; Naumann, J.A.; Li, N.S.; Zamora, E.A.; Gordon, D.J.; Piccirilli, J.A.; Gordon, P.M. Drug conjugated nanoparticles activated by cancer cell specific mRNA. Oncotarget 2016, 7, 38243–38256. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Moyano, D.F.; Parnsubsakul, A.; Papadopoulos, A.; Wang, L.S.; Landis, R.F.; Das, R.; Rotello, V.M. Ultra-stable biofunctionalizable gold nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14096–14101. [Google Scholar] [CrossRef] [PubMed]
- Ramya, A.N.; Joseph, M.M.; Nair, J.B.; Karunakaran, V.; Narayanan, N.; Maiti, K.K. New insight of tetraphenylethylene-based raman signatures for targeted SERS nanoprobe construction toward prostate cancer cell detection. ACS Appl. Mater. Interfaces 2016, 8, 10220–10225. [Google Scholar] [CrossRef] [PubMed]
- Spaliviero, M.; Harmsen, S.; Huang, R.; Wall, M.A.; Andreou, C.; Eastham, J.A.; Touijer, K.A.; Scardino, P.T.; Kircher, M.F. Detection of lymph node metastases with SERRS nanoparticles. Mol. Imaging Biol. 2016, 18, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Moeendarbari, S.; Tekade, R.; Mulgaonkar, A.; Christensen, P.; Ramezani, S.; Hassan, G.; Jiang, R.; Oz, O.K.; Hao, Y.; Sun, X. Theranostic nanoseeds for efficacious internal radiation therapy of unresectable solid tumors. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.C.; Hsieh, H.Y.; Lu, K.Y.; Wang, S.Y.; Mi, F.L. EGCG/gelatin-doxorubicin gold nanoparticles enhance therapeutic efficacy of doxorubicin for prostate cancer treatment. Nanomedicine (Lond.) 2016, 11, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Morshed, R.A.; Muroski, M.E.; Dai, Q.; Wegscheid, M.L.; Auffinger, B.; Yu, D.; Han, Y.; Zhang, L.; Wu, M.; Cheng, Y.; et al. Cell penetrating peptide-modified gold nanoparticles for the delivery of doxorubicin to brain metastatic breast cancer. Mol. Pharm. 2016, 13, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Cui, L.; Bristow, R.G.; Allen, C. Dual action enhancement of gold nanoparticle radiosensitization by pentamidine in triple negative breast cancer. Radiat. Res. 2016, 185, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.; Christoforou, N.; Lee, S. Optimization of anti-cancer drugs and a targeting molecule on multifunctional gold nanoparticles. Nanotechnology 2016, 27, 185704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Feng, B.; Yu, H.; Wang, D.; Wang, T.; Liu, J.; Meng, Q.; Wang, S.; Zhang, P.; Zhang, Z.; et al. Cisplatin prodrug-conjugated gold nanocluster for fluorescence imaging and targeted therapy of the breast cancer. Theranostics 2016, 6, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Lu, Y.; Jeong, J.J.; Cai, Z.; Tong, L.; Alwarda, R.; Pignol, J.P.; Winnik, M.A.; Reilly, R.M. Stability and biodistribution of thiol-functionalized and 177Lu-labeled metal chelating polymers bound to gold nanoparticles. Biomacromolecules 2016, 17, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Determination of the minimum temperature required for selective photothermal destruction of cancer cells with the use of immunotargeted gold nanoparticles. Photochem. Photobiol. 2006, 82, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Yu, Y.; Zhang, H.; He, Q. Structural-engineering rationales of gold nanoparticles for cancer theranostics. Adv. Mater. 2016, 28, 8567–8585. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Bioconjugated gold nanoparticles enhance cellular uptake: A proof of concept study for siRNA delivery in prostate cancer cells. Int. J. Pharm. 2016, 509, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Stuchinskaya, T.; Moreno, M.; Cook, M.J.; Edwards, D.R.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates. Photochem. Photobiol. Sci. 2011, 10, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Nativo, P.; Smith, J.A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Liu, T.Y.; Chen, P.J.; Chang, P.H.; Chen, S.Y. A high-sensitivity and low-power theranostic nanosystem for cell sers imaging and selectively photothermal therapy using anti-EGFR-conjugated reduced graphene oxide/mesoporous silica/aunps nanosheets. Small 2016, 12, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Pelaz, B.; del Pino, P.; Carril, M.; Escudero, A.; Parak, W.J.; Soliman, M.G.; Zhang, Q.; Carrillo-Carrion, C. Gold-based nanomaterials for applications in nanomedicine. Top. Curr. Chem. 2016, 370, 169–202. [Google Scholar] [PubMed]
- Conde, J.; de la Fuente, J.M.; Baptista, P.V. Nanomaterials for reversion of multidrug resistance in cancer: A new hope for an old idea? Front. Pharmacol. 2013, 4, 134. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ghosh, P.; Rotello, V.M. Multi-functional gold nanoparticles for drug delivery. Adv. Exp. Med. Biol. 2007, 620, 48–56. [Google Scholar] [PubMed]
- Conde, J.; Tian, F.; Hernandez, Y.; Bao, C.; Cui, D.; Janssen, K.P.; Ibarra, M.R.; Baptista, P.V.; Stoeger, T.; de la Fuente, J.M. In vivo tumor targeting via nanoparticle-mediated therapeutic siRNA coupled to inflammatory response in lung cancer mouse models. Biomaterials 2013, 34, 7744–7753. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Larguinho, M.; Cordeiro, A.; Raposo, L.R.; Costa, P.M.; Santos, S.; Diniz, M.S.; Fernandes, A.R.; Baptista, P.V. Gold-nanobeacons for gene therapy: Evaluation of genotoxicity, cell toxicity and proteome profiling analysis. Nanotoxicology 2014, 8, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Rosa, J.; de la Fuente, J.M.; Baptista, P.V. Gold-nanobeacons for simultaneous gene specific silencing and intracellular tracking of the silencing events. Biomaterials 2013, 34, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.M.; Baptista, P.V. Anti-cancer precision theranostics: A focus on multifunctional gold nanoparticles. Expert Rev. Mol. Diagn. 2014, 14, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, F.; Yang, X.; Ning, B.; Harp, M.G.; Culp, S.H.; Hu, S.; Huang, P.; Nie, L.; Chen, J.; et al. Gold nanoparticle coated carbon nanotube ring with enhanced raman scattering and photothermal conversion property for theranostic applications. J. Am. Chem. Soc. 2016, 138, 7005–7015. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Qi, C.; Maynadier, M.; Cattoen, X.; Wong Chi Man, M.; Raehm, L.; Mongin, O.; Blanchard-Desce, M.; Garcia, M.; Gary-Bobo, M.; et al. Multifunctional gold-mesoporous silica nanocomposites for enhanced two-photon imaging and therapy of cancer cells. Front. Mol. Biosci. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.; Maynadier, M.; Mongin, O.; Hugues, V.; Blanchard-Desce, M.; Chaix, A.; Cattoen, X.; Wong Chi Man, M.; Gallud, A.; Gary-Bobo, M.; et al. Enhanced two-photon fluorescence imaging and therapy of cancer cells via gold@bridged silsesquioxane nanoparticles. Small 2015, 11, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Yu, J.H.; Kim, I.; Nam, Y.S. Genetically programmed clusters of gold nanoparticles for cancer cell-targeted photothermal therapy. ACS Appl. Mater. Interfaces 2015, 7, 22578–22586. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Mancilla, N.; Ferro-Flores, G.; Santos-Cuevas, C.; Ocampo-Garcia, B.; Luna-Gutierrez, M.; Azorin-Vega, E.; Isaac-Olive, K.; Camacho-Lopez, M.; Torres-Garcia, E. Multifunctional targeted therapy system based on (99M)TC/(177) Lu-labeled gold nanoparticles-TAT(49–57)-lys(3)-bombesin internalized in nuclei of prostate cancer cells. J. Labelled Comp. Radiopharm. 2013, 56, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Szlachcic, A.; Pala, K.; Zakrzewska, M.; Jakimowicz, P.; Wiedlocha, A.; Otlewski, J. FGF1-gold nanoparticle conjugates targeting FGFR efficiently decrease cell viability upon NIR irradiation. Int. J. Nanomed. 2012, 7, 5915–5927. [Google Scholar]
- Van de Broek, B.; Devoogdt, N.; D’Hollander, A.; Gijs, H.L.; Jans, K.; Lagae, L.; Muyldermans, S.; Maes, G.; Borghs, G. Specific cell targeting with nanobody conjugated branched gold nanoparticles for photothermal therapy. ACS Nano 2011, 5, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Lukianova-Hleb, E.Y.; Oginsky, A.O.; Samaniego, A.P.; Shenefelt, D.L.; Wagner, D.S.; Hafner, J.H.; Farach-Carson, M.C.; Lapotko, D.O. Tunable plasmonic nanoprobes for theranostics of prostate cancer. Theranostics 2011, 1, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Singh, A.K.; Khan, S.A.; Senapati, D.; Yu, H.; Ray, P.C. Gold nano-popcorn-based targeted diagnosis, nanotherapy treatment, and in situ monitoring of photothermal therapy response of prostate cancer cells using surface-enhanced raman spectroscopy. J. Am. Chem. Soc. 2010, 132, 18103–18114. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Ko, Y.T. Lipid-coated gold nanocomposites for enhanced cancer therapy. Int. J. Nanomed. 2015, 10, 33–45. [Google Scholar]
- Banu, H.; Sethi, D.K.; Edgar, A.; Sheriff, A.; Rayees, N.; Renuka, N.; Faheem, S.M.; Premkumar, K.; Vasanthakumar, G. Doxorubicin loaded polymeric gold nanoparticles targeted to human folate receptor upon laser photothermal therapy potentiates chemotherapy in breast cancer cell lines. J. Photochem. Photobiol. B 2015, 149, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Mkandawire, M.M.; Lakatos, M.; Springer, A.; Clemens, A.; Appelhans, D.; Krause-Buchholz, U.; Pompe, W.; Rodel, G.; Mkandawire, M. Induction of apoptosis in human cancer cells by targeting mitochondria with gold nanoparticles. Nanoscale 2015, 7, 10634–10640. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tseng, Y.T.; Suo, G.; Chen, L.; Yu, J.; Chiu, W.J.; Huang, C.C.; Lin, C.H. Photothermal therapeutic response of cancer cells to aptamer-gold nanoparticle-hybridized graphene oxide under nir illumination. ACS Appl. Mater. Interfaces 2015, 7, 5097–5106. [Google Scholar] [CrossRef] [PubMed]
- Lechtman, E.; Mashouf, S.; Chattopadhyay, N.; Keller, B.M.; Lai, P.; Cai, Z.; Reilly, R.M.; Pignol, J.P. A Monte Carlo-based model of gold nanoparticle radiosensitization accounting for increased radiobiological effectiveness. Phys. Med. Biol. 2013, 58, 3075–3087. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Coulter, J.A.; Hounsell, A.R.; Butterworth, K.T.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J.; et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Butler, H.J.; Fogarty, S.W.; Kerns, J.G.; Martin-Hirsch, P.L.; Fullwood, N.J.; Martin, F.L. Gold nanoparticles as a substrate in bio-analytical near-infrared surface-enhanced Raman spectroscopy. Analyst 2015, 140, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Kalmodia, S.; Harjwani, J.; Rajeswari, R.; Yang, W.; Barrow, C.J.; Ramaprabhu, S.; Krishnakumar, S.; Elchuri, S.V. Synthesis and characterization of surface-enhanced Raman-scattered gold nanoparticles. Int. J. Nanomed. 2013, 8, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, J.; Guo, J.; Cai, W.; Liu, B.; Wang, Z.; Sun, Z. Surface-enhanced Raman spectroscopy investigation on human breast cancer cells. Chem. Cent. J. 2013, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles using the extract of -antiproliferative effect against prostate cancer cells. Cancer Nanotechnol. 2013, 4, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Yu, H.; Wu, D.; Ma, H.; Li, H.; Du, B.; Wei, Q. Label-free electrochemical immunosensor for prostate-specific antigen based on silver hybridized mesoporous silica nanoparticles. Anal. Biochem. 2013, 434, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Minz, A.P.; Ashe, S.; Rauta, P.R.; Kumari, M.; Chopra, P.; Nayak, B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016, 470, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Karunamuni, R.; Naha, P.C.; Lau, K.C.; Al-Zaki, A.; Popov, A.V.; Delikatny, E.J.; Tsourkas, A.; Cormode, D.P.; Maidment, A.D. Development of silica-encapsulated silver nanoparticles as contrast agents intended for dual-energy mammography. Eur. Radiol. 2016, 26, 3301–3309. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.A.; Ali, M.A.; Chen, S.M.; Li, Y.; Al-Hemaid, F.M.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Lee, J. Silver nanoparticles synthesized from adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf. B Biointerfaces 2016, 141, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Jannathul Firdhouse, M.; Lalitha, P. Apoptotic efficacy of biogenic silver nanoparticles on human breast cancer MCF-7 cell lines. Prog. Biomater. 2015, 4, 113–121. [Google Scholar]
- Casanas Pimentel, R.G.; Robles Botero, V.; San Martin Martinez, E.; Gomez Garcia, C.; Hinestroza, J.P. Soybean agglutinin-conjugated silver nanoparticles nanocarriers in the treatment of breast cancer cells. J. Biomater. Sci. Polym. Ed. 2016, 27, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Swanner, J.; Mims, J.; Carroll, D.L.; Akman, S.A.; Furdui, C.M.; Torti, S.V.; Singh, R.N. Differential cytotoxic and radiosensitizing effects of silver nanoparticles on triple-negative breast cancer and non-triple-negative breast cells. Int. J. Nanomed. 2015, 10, 3937–3953. [Google Scholar]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by bacillus tequilensis and calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed. 2015, 10, 4203–4222. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.A.; Graham, E.; MacNeill, C.M.; Young, M.; Donati, G.; Wailes, E.M.; Jones, B.T.; Levi-Polyachenko, N.H. Differential response of MCF-7, MDA-MB-231, and MCF-10a cells to hyperthermia, silver nanoparticles and silver nanoparticle-induced photothermal therapy. Int. J. Hyperth. 2014, 30, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-mediated synthesis of silver nanoparticles: Their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, A.; Zhu, H.; Woo, J.; Zabinyakov, N.; Sharma, S.; Gimzewski, J.K. Biophysical and morphological effects of nanodiamond/nanoplatinum solution (DPV576) on metastatic murine breast cancer cells in vitro. Nanotechnology 2014, 25, 465101. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Liu, Y.L.; Xu, J.Q.; Lv, S.W.; Guo, S.; Huang, W.H. Real-time monitoring of H2O2 release from single cells using nanoporous gold microelectrodes decorated with platinum nanoparticles. Analyst 2015, 140, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wu, D.; Zhang, Y.; Ma, H.; Li, H.; Du, B.; Wei, Q.; Ju, H. Ultrasensitive electrochemical immunosensors for multiplexed determination using mesoporous platinum nanoparticles as nonenzymatic labels. Anal. Chim. Acta 2014, 807, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Basu, S.; Soni, S.; Pandey, A.; Roy, B.; Oh, M.S.; Chin, K.T.; Paraskar, A.S.; Sarangi, S.; Connor, Y.; et al. Cholesterol-tethered platinum II-based supramolecular nanoparticle increases antitumor efficacy and reduces nephrotoxicity. Proc. Natl. Acad. Sci. USA 2012, 109, 11294–11299. [Google Scholar] [CrossRef] [PubMed]
- Teow, Y.; Valiyaveettil, S. Active targeting of cancer cells using folic acid-conjugated platinum nanoparticles. Nanoscale 2010, 2, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Spain, E.; Gilgunn, S.; Sharma, S.; Adamson, K.; Carthy, E.; O’Kennedy, R.; Forster, R.J. Detection of prostate specific antigen based on electrocatalytic platinum nanoparticles conjugated to a recombinant SCFV antibody. Biosens. Bioelectron. 2016, 77, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, B.; Chen, G.; Tang, D. Redox and catalysis ‘all-in-one’ infinite coordination polymer for electrochemical immunosensor of tumor markers. Biosens. Bioelectron. 2015, 64, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Huo, S.; Zhang, X.; Liu, J.; Tan, A.; Li, S.; Jin, S.; Xue, X.; Zhao, Y.; Ji, T.; et al. Neuropilin-1-targeted gold nanoparticles enhance therapeutic efficacy of platinum(IV) drug for prostate cancer treatment. ACS Nano 2014, 8, 4205–4220. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Sillerud, L.O. Paclitaxel-loaded iron platinum stealth immunomicelles are potent MRI imaging agents that prevent prostate cancer growth in a psma-dependent manner. Int. J. Nanomed. 2012, 7, 4341–4352. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Huber, D.L.; Monson, T.C.; Ali, A.M.; Bisoffi, M.; Sillerud, L.O. Multifunctional iron platinum stealth immunomicelles: Targeted detection of human prostate cancer cells using both fluorescence and magnetic resonance imaging. J. Nanopart. Res. 2011, 13, 4717–4729. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.H.; Brown, P.R.; Bulovic, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, A.; Li, L.; Wang, D.; Zhu, T.; Xu, J.; Yang, C.; Li, Y. Bright, multicoloured light-emitting diodes based on quantum dots. Nat. Photonics 2007, 1, 717–722. [Google Scholar] [CrossRef]
- Fang, M.; Peng, C.W.; Pang, D.W.; Li, Y. Quantum dots for cancer research: Current status, remaining issues, and future perspectives. Cancer Biol. Med. 2012, 9, 151–163. [Google Scholar] [PubMed]
- Jamieson, T.; Bakhshi, R.; Petrova, D.; Pocock, R.; Imani, M.; Seifalian, A.M. Biological applications of quantum dots. Biomaterials 2007, 28, 4717–4732. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.Z. Applications of quantum dots in biology: An overview. Methods Mol. Biol. 2005, 303, 1–17. [Google Scholar] [PubMed]
- Barar, J.; Omidi, Y. Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. Bioimpacts 2014, 4, 3–14. [Google Scholar] [PubMed]
- Choi, H.S.; Frangioni, J.V. Nanoparticles for biomedical imaging: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 291–310. [Google Scholar] [PubMed]
- Ow, H.; Larson, D.R.; Srivastava, M.; Baird, B.A.; Webb, W.W.; Wiesner, U. Bright and stable core-shell fluorescent silica nanoparticles. Nano Lett. 2005, 5, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.; Ow, H.; Vishwasrao, H.; Heikal, A.; Wiesner, U.; Webb, W. Silica nanoparticle architecture determines radiative properties of encapsulated fluorophores. Chem. Mater. 2008, 20, 2677–2684. [Google Scholar] [CrossRef]
- Burns, A.A.; Vider, J.; Ow, H.; Herz, E.; Penate-Medina, O.; Baumgart, M.; Larson, S.M.; Wiesner, U.; Bradbury, M. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 2009, 9, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.L.; Cheng, Z.; Lee, S.W.; Bentolila, L.A.; Iyer, G.; Rao, J.; Chen, X.; Wu, A.M.; Weiss, S.; Gambhir, S.S. Micropet-based biodistribution of quantum dots in living mice. J. Nucl. Med. 2007, 48, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gonen, M.; Kalaigian, H.; Schoder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Mewada, A.; Pandey, S.; Bhori, M.; Singh, K.; Sharon, M.; Sharon, M. Milk-derived multi-fluorescent graphene quantum dot-based cancer theranostic system. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Li, F.; Zhang, W.; Shen, Y.; Zhou, D.; Guo, S. A multifunctional poly(curcumin) nanomedicine for dual-modal targeted delivery, intracellular responsive release, dual-drug treatment and imaging of multidrug resistant cancer cells. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 2954–2962. [Google Scholar] [CrossRef] [PubMed]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Hussein, M.Z.; Alitheen, N.B.; Abu, N. Folic acid targeted MN:Zns quantum dots for theranostic applications of cancer cell imaging and therapy. Int. J. Nanomed. 2016, 11, 413–428. [Google Scholar]
- Lin, Z.; Ma, Q.; Fei, X.; Zhang, H.; Su, X. A novel aptamer functionalized cuins2 quantum dots probe for daunorubicin sensing and near infrared imaging of prostate cancer cells. Anal. Chim. Acta 2014, 818, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shaffer, T.M.; Das, S.; Perez-Medina, C.; Mulder, W.J.; Grimm, J. Near-infrared quantum dot and 89Zr dual-labeled nanoparticles for in vivo Cerenkov imaging. Bioconjug. Chem. 2017, 28, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, C.; Kostarelos, K.; Prato, M.; Bianco, A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim. Biophys. Acta 2006, 1758, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef] [PubMed]
- Serpell, C.J.; Kostarelos, K.; Davis, B.G. Can carbon nanotubes deliver on their promise in biology? Harnessing unique properties for unparalleled applications. ACS Cent. Sci. 2016, 2, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomedicine 2016, 12, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Sitharaman, B. Multifunctional fullerene- and metallofullerene-based nanobiomaterials. Nano Life 2013, 3, 1342003. [Google Scholar] [CrossRef]
- Ruggiero, A.; Villa, C.H.; Bander, E.; Rey, D.A.; Bergkvist, M.; Batt, C.A.; Manova-Todorova, K.; Deen, W.M.; Scheinberg, D.A.; McDevitt, M.R. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl. Acad. Sci. USA 2010, 107, 12369–12374. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Bianco, A.; Prato, M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat. Nanotechnol. 2009, 4, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Petibone, D.; Xu, Y.; Mahmood, M.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A.S. Toxicity and efficacy of carbon nanotubes and graphene: The utility of carbon-based nanoparticles in nanomedicine. Drug Metab. Rev. 2014, 46, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Gass, M.; Muller, K.; Skepper, J.N.; Midgley, P.A.; Welland, M. Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotechnol. 2007, 2, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj, J.; Szwarc, H.; Moussa, F. Toxicity studies of carbon nanotubes. Adv. Exp. Med. Biol. 2007, 620, 181–204. [Google Scholar] [PubMed]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.; Seaton, A.; Stone, V.; Brown, S.; Macnee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Corredor, C.; Hou, W.; Klein, S.; Moghadam, B.; Goryll, M.; Doudrick, K.; Westerhoff, P.; Posner, J. Disruption of model cell membranes by carbon nanotubes. Carbon 2013, 60, 67–75. [Google Scholar] [CrossRef]
- Yoshida, M.; Goto, H.; Hirose, Y.; Zhao, X.; Osawa, E. Prediction of favorable isomeric structures for the c100 to c120 giant fullerenes. An application of the phason line criteria. Electron. J. Theor. Chem. 1996, 1, 163–171. [Google Scholar] [CrossRef]
- Castro Nava, A.; Cojoc, M.; Peitzsch, C.; Cirillo, G.; Kurth, I.; Fuessel, S.; Erdmann, K.; Kunhardt, D.; Vittorio, O.; Hampel, S.; et al. Development of novel radiochemotherapy approaches targeting prostate tumor progenitor cells using nanohybrids. Int. J. Cancer 2015, 137, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Heydari-Bafrooei, E.; Shamszadeh, N.S. Electrochemical bioassay development for ultrasensitive aptasensing of prostate specific antigen. Biosens. Bioelectron. 2017, 91, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Youn, Y.S.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; Kim, J.O. Graphene oxide-wrapped pegylated liquid crystalline nanoparticles for effective chemo-photothermal therapy of metastatic prostate cancer cells. Colloids Surf. B Biointerfaces 2016, 143, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.H.; Kuo, S.H.; Lin, T.Y.; Lin, C.W.; Fang, P.Y.; Yang, H.W. An electrochemical biosensor to simultaneously detect vegf and psa for early prostate cancer diagnosis based on graphene oxide/ssdna/plla nanoparticles. Biosens. Bioelectron. 2017, 89, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cheng, J.; Chen, Y.; Yu, S.; Liu, F.; Sun, Y.; Chen, Y.; Ran, H. Phase-transition nanodroplets for real-time photoacoustic/ultrasound dual-modality imaging and photothermal therapy of sentinel lymph node in breast cancer. Sci. Rep. 2017, 7, 45213. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Srivastava, I.; Tripathi, I.; Daza, E.; Ostadhossein, F.; Pan, D. Macromolecularly “caged” carbon nanoparticles for intracellular trafficking via switchable photoluminescence. J. Am. Chem. Soc. 2017, 139, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Y.; Ming, J.; Liu, J.; Zhong, L.; Liang, Q.; Fan, L.; Jiang, J. Evaluation of the tracing effect of carbon nanoparticle and carbon nanoparticle-epirubicin suspension in axillary lymph node dissection for breast cancer treatment. World J. Surg. Oncol. 2016, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, Q.; Chen, G.; Lu, J.; Zeng, Y.; Chen, X.; Yan, J. Sentinel lymph node detection using carbon nanoparticles in patients with early breast cancer. PLoS ONE 2015, 10, e0135714. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Ohoka, A.; Kolmodin, N.J.; Pan, D. Next generation carbon nanoparticles for efficient gene therapy. Mol. Pharm. 2015, 12, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D. Lipid bilayers and biomembranes. Annu. Rev. Biochem. 1972, 41, 753–776. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Thorek, D.L.; Ramos, N.N.; Pinkse, M.W.; Wolterbeek, H.T.; Carlin, S.D.; Beattie, B.J.; Lewis, J.S. 89Zr-labeled paramagnetic octreotide-liposomes for PET-MR imaging of cancer. Pharm. Res. 2013, 30, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Laverman, P.; Brouwers, A.H.; Dams, E.T.; Oyen, W.J.; Storm, G.; van Rooijen, N.; Corstens, F.H.; Boerman, O.C. Preclinical and clinical evidence for disappearance of long-circulating characteristics of polyethylene glycol liposomes at low lipid dose. J. Pharmacol. Exp. Ther. 2000, 293, 996–1001. [Google Scholar] [PubMed]
- Yeh, C.Y.; Hsiao, J.K.; Wang, Y.P.; Lan, C.H.; Wu, H.C. Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer. Biomaterials 2016, 99, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Jin, C.S.; Huang, H.; Ding, L.; Zhang, Z.; Chen, J.; Zheng, G. Nanoparticle-enabled, image-guided treatment planning of target specific rnai therapeutics in an orthotopic prostate cancer model. Small 2014, 10, 3072–3082. [Google Scholar] [CrossRef] [PubMed]
- Yaari, Z.; da Silva, D.; Zinger, A.; Goldman, E.; Kajal, A.; Tshuva, R.; Barak, E.; Dahan, N.; Hershkovitz, D.; Goldfeder, M.; et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun. 2016, 7, 13325. [Google Scholar] [CrossRef] [PubMed]
- Rizzitelli, S.; Giustetto, P.; Faletto, D.; Delli Castelli, D.; Aime, S.; Terreno, E. The release of doxorubicin from liposomes monitored by MRI and triggered by a combination of us stimuli led to a complete tumor regression in a breast cancer mouse model. J. Control. Release 2016, 230, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Miller-Kleinhenz, J.M.; Bozeman, E.N.; Yang, L. Targeted nanoparticles for image-guided treatment of triple-negative breast cancer: Clinical significance and technological advances. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 797–816. [Google Scholar] [CrossRef] [PubMed]
- Rizzitelli, S.; Giustetto, P.; Cutrin, J.C.; Delli Castelli, D.; Boffa, C.; Ruzza, M.; Menchise, V.; Molinari, F.; Aime, S.; Terreno, E. Sonosensitive theranostic liposomes for preclinical in vivo MRI-guided visualization of doxorubicin release stimulated by pulsed low intensity non-focused ultrasound. J. Control. Release 2015, 202, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, C.S.; Moshkelani, D.; Zhang, H. Thermosensitive liposome formulated indocyanine green for near-infrared triggered photodynamic therapy: In vivo evaluation for triple-negative breast cancer. Pharm. Res. 2015, 32, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, L.; Zhu, D.; Song, C. Design of multifunctional magnetic iron oxide nanoparticles/mitoxantrone-loaded liposomes for both magnetic resonance imaging and targeted cancer therapy. Int. J. Nanomed. 2014, 9, 4055–4066. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Kulkarni, S.A.; Raju, A.; Feng, S.S. Theranostic liposomes of TPGS coating for targeted co-delivery of docetaxel and quantum dots. Biomaterials 2012, 33, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, H.; Dobroff, A.S.; Driessen, W.H.; Cristini, V.; Brinker, L.M.; Staquicini, F.I.; Cardo-Vila, M.; D’Angelo, S.; Ferrara, F.; Proneth, B.; et al. Integrated nanotechnology platform for tumor-targeted multimodal imaging and therapeutic cargo release. Proc. Natl. Acad. Sci. USA 2016, 113, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Zhang, K.; Tam, Y.Y.; Quick, J.; Tam, Y.K.; Lin, P.J.; Chen, S.; Liu, Y.; Nair, J.K.; Zlatev, I.; et al. A glu-urea-lys ligand-conjugated lipid nanoparticle/siRNA system inhibits androgen receptor expression in vivo. Mol. Ther. Nucleic Acids 2016, 5, e348. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, C.C.; Li, J.; Roy, S.; Wu, Q.; King, M.R. Two-stage nanoparticle delivery of piperlongumine and tumor necrosis factor-related apoptosis-inducing ligand (Trail) anti-cancer therapy. Technology 2016, 4, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.R.; Gangadharappa, H.V.; Hani, U.; Osmani, R.A.; Vaghela, R.; Kulkarni, P.K.; Venkata, K.S. Current perspectives on novel drug delivery systems and therapies for management of prostate cancer: An inclusive review. Curr. Drug Targets 2016. [Google Scholar] [CrossRef]
- Majzoub, R.N.; Wonder, E.; Ewert, K.K.; Kotamraju, V.R.; Teesalu, T.; Safinya, C.R. Rab11 and lysotracker markers reveal correlation between endosomal pathways and transfection efficiency of surface-functionalized cationic liposome-DNA nanoparticles. J. Phys. Chem. B 2016, 120, 6439–6453. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhang, R.; Chen, Z.; Zhu, L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J. Control. Release 2014, 196, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Zheng, S.; Han, J.; Le, V.H.; Park, J.O.; Park, S. Nanohybrid magnetic liposome functionalized with hyaluronic acid for enhanced cellular uptake and near-infrared-triggered drug release. Colloids Surf. B Biointerfaces 2017, 154, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Sneider, A.; Jadia, R.; Piel, B.; VanDyke, D.; Tsiros, C.; Rai, P. Engineering remotely triggered liposomes to target triple negative breast cancer. Oncomedicine 2017, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, R.; Pichler, M.; Kanlikilicer, P.; Ivan, C.; Bayraktar, E.; Kahraman, N.; Aslan, B.; Oguztuzun, S.; Ulasli, M.; Arslan, A.; et al. Microrna 603 acts as a tumor suppressor and inhibits triple-negative breast cancer tumorigenesis by targeting elongation factor 2 kinase. Oncotarget 2016, 8, 11641–11658. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.S.; Silva, J.O.; Monteiro, L.O.; Leite, E.A.; Cassali, G.D.; Rubello, D.; Cardoso, V.N.; Ferreira, L.A.; Oliveira, M.C.; de Barros, A.L. Doxorubicin-loaded nanocarriers: A comparative study of liposome and nanostructured lipid carrier as alternatives for cancer therapy. Biomed. Pharmacother. 2016, 84, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Alaarg, A.; Jordan, N.Y.; Verhoef, J.J.; Metselaar, J.M.; Storm, G.; Kok, R.J. Docosahexaenoic acid liposomes for targeting chronic inflammatory diseases and cancer: An in vitro assessment. Int. J. Nanomed. 2016, 11, 5027–5040. [Google Scholar] [CrossRef] [PubMed]
- Amiri, B.; Ebrahimi-Far, M.; Saffari, Z.; Akbarzadeh, A.; Soleimani, E.; Chiani, M. Preparation, characterization and cytotoxicity of silibinin-containing nanoniosomes in T47D human breast carcinoma cells. Asian Pac. J. Cancer Prev. 2016, 17, 3835–3838. [Google Scholar] [PubMed]
- Qian, R.C.; Cao, Y.; Long, Y.T. Binary system for microrna-targeted imaging in single cells and photothermal cancer therapy. Anal. Chem. 2016, 88, 8640–8647. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Dan, Z.; He, X.; Zhang, Z.; Yu, H.; Yin, Q.; Li, Y. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 2016, 10, 7738–7748. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; He, B.; Pan, D.; Luo, K.; Yi, Q.; Gu, Z. Anti-cancer efficacy of paclitaxel loaded in PH triggered liposomes. J. Biomed. Nanotechnol. 2016, 12, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Kreuter, J. Drug delivery to the central nervous system by polymeric nanoparticles: What do we know? Adv. Drug Deliv. Rev. 2014, 71, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Rossin, R.; Pan, D.; Qi, K.; Turner, J.L.; Sun, X.; Wooley, K.L.; Welch, M.J. 64Cu-labeled folate-conjugated shell cross-linked nanoparticles for tumor imaging and radiotherapy: Synthesis, radiolabeling, and biologic evaluation. J. Nucl. Med. 2005, 46, 1210–1218. [Google Scholar] [PubMed]
- Salmaso, S.; Caliceti, P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J. Drug Deliv. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.C.; Adams, Y.E.; Tomalia, D.; Mercer-Smith, J.A.; Lavallee, D.K. Using starburst dendrimers as linker molecules to radiolabel antibodies. Bioconjug. Chem. 1990, 1, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.; Naylor, A.; Goddard, W. Starburst dendrimers: Molecular-level control of size, shape, surface-chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Sk, U.H.; Kojima, C. Dendrimers for theranostic applications. Biomol. Concepts 2015, 6, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mejia, D.; Maysinger, D.; Kakkar, A. Design and synthesis of multifunctional traceable dendrimers for visualizing drug delivery. RSC Adv. 2014, 4, 19242–19245. [Google Scholar] [CrossRef]
- Sharma, A.; Khatchadourian, A.; Khanna, K.; Sharma, R.; Kakkar, A.; Maysinger, D. Multivalent niacin nanoconjugates for delivery to cytoplasmic lipid droplets. Biomaterials 2011, 32, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Brechbiel, M.; Kozak, R.; Gansow, O. Metal-chelate-dendrimer-antibody constructs for use in radioimmunotherapy and imaging. Bioorg. Med. Chem. Lett. 1994, 4, 449–454. [Google Scholar] [CrossRef]
- Wangler, C.; Moldenhauer, G.; Saffrich, R.; Knapp, E.M.; Beijer, B.; Schnolzer, M.; Wangler, B.; Eisenhut, M.; Haberkorn, U.; Mier, W. Pamam structure-based multifunctional fluorescent conjugates for improved fluorescent labelling of biomacromolecules. Chemistry 2008, 14, 8116–8130. [Google Scholar] [CrossRef]
- Ling, Y.; Wei, K.; Luo, Y.; Gao, X.; Zhong, S. Dual docetaxel/superparamagnetic iron oxide loaded nanoparticles for both targeting magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 7139–7150. [Google Scholar] [CrossRef]
- Abbasi, A.Z.; Prasad, P.; Cai, P.; He, C.; Foltz, W.D.; Amini, M.A.; Gordijo, C.R.; Rauth, A.M.; Wu, X.Y. Manganese oxide and docetaxel co-loaded fluorescent polymer nanoparticles for dual modal imaging and chemotherapy of breast cancer. J. Control. Release 2015, 209, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.N.; Lavan, D.A.; Langer, R. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cai, P.; Li, J.; Zhang, T.; Lin, L.; Abbasi, A.Z.; Henderson, J.T.; Rauth, A.M.; Wu, X.Y. Blood-brain barrier-penetrating amphiphilic polymer nanoparticles deliver docetaxel for the treatment of brain metastases of triple negative breast cancer. J. Control. Release 2017, 246, 98–109. [Google Scholar] [CrossRef]
- Pramanik, A.; Laha, D.; Dash, S.K.; Chattopadhyay, S.; Roy, S.; Das, D.K.; Pramanik, P.; Karmakar, P. An in vivo study for targeted delivery of copper-organic complex to breast cancer using chitosan polymer nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 327–337. [Google Scholar] [CrossRef]
- Zhou, Z.; Munyaradzi, O.; Xia, X.; Green, D.; Bong, D. High-capacity drug carriers from common polymer amphiphiles. Biomacromolecules 2016, 17, 3060–3066. [Google Scholar] [CrossRef] [PubMed]
- Danafar, H.; Sharafi, A.; Kheiri Manjili, H.; Andalib, S. Sulforaphane delivery using MPEG-PCL co-polymer nanoparticles to breast cancer cells. Pharm. Dev. Technol. 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H.; Faramarzi, H.; Dolatabadi, J.E.; Omidfar, K. Drug targeting using solid lipid nanoparticles. Chem. Phys. Lipids 2014, 181, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H. Preparation of solid lipid nanoparticles as drug carriers for levothyroxine sodium with in vitro drug delivery kinetic characterization. Mol. Biol. Rep. 2014, 41, 3521–3527. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242–4282. [Google Scholar] [CrossRef] [PubMed]
- Uner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Zur Muhlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Jenning, V.; Thunemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Radaic, A.; de Paula, E.; de Jesus, M.B. Factorial design and development of solid lipid nanoparticles (SLN) for gene delivery. J. Nanosci. Nanotechnol. 2015, 15, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.H.; Rai, R.; Slipper, I.J.; Chowdhry, B.Z.; Lamprou, D.; Getti, G.; Douroumis, D. Delivery of retinoic acid to LNCaP human prostate cancer cells using solid lipid nanoparticles. Int. J. Pharm. 2015, 493, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Swami, R.; Singh, I.; Jeengar, M.K.; Naidu, V.G.; Khan, W.; Sistla, R. Adenosine conjugated lipidic nanoparticles for enhanced tumor targeting. Int. J. Pharm. 2015, 486, 287–296. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, M.B.; Radaic, A.; Hinrichs, W.L.; Ferreira, C.V.; de Paula, E.; Hoekstra, D.; Zuhorn, I.S. Inclusion of the helper lipid dioleoyl-phosphatidylethanolamine in solid lipid nanoparticles inhibits their transfection efficiency. J. Biomed. Nanotechnol. 2014, 10, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Tomasello, B.; Ruozi, B.; Renis, M.; Puglisi, G. Preparation and optimization of PIT solid lipid nanoparticles via statistical factorial design. Eur. J. Med. Chem. 2012, 49, 110–117. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, M.B.; Ferreira, C.V.; de Paula, E.; Hoekstra, D.; Zuhorn, I.S. Design of solid lipid nanoparticles for gene delivery into prostate cancer. J. Control. Release 2010, 148, e89–e90. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sharma, G.; Kushwah, V.; Thakur, K.; Ghoshal, G.; Singh, B.; Jain, S.; Shivhare, U.S.; Katare, O.P. Fabrication and functional attributes of lipidic nanoconstructs of lycopene: An innovative endeavour for enhanced cytotoxicity in MCF-7 breast cancer cells. Colloids Surf. B Biointerfaces 2017, 152, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Varas-Godoy, M.; Haidar, Z.S. Physicochemical characterization of chitosan-hyaluronan-coated solid lipid nanoparticles for the targeted delivery of paclitaxel: A proof-of-concept study in breast cancer cells. Nanomedicine (Lond.) 2017, 12, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, L.; Liu, B.; Chen, Z.; Li, C. Hyaluronic acid decorated pluronic p85 solid lipid nanoparticles as a potential carrier to overcome multidrug resistance in cervical and breast cancer. Biomed. Pharmacother. 2017, 86, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, T.; Yuan, M.; Wen, L.; Cheng, B.; Liu, N.; Huang, X.; Hong, Y.; Yuan, H.; Hu, F. MicroRNA-200c delivered by solid lipid nanoparticles enhances the effect of paclitaxel on breast cancer stem cell. Int. J. Nanomed. 2016, 11, 6713–6725. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, M.C.; Pereira, C.; Kreutzer, B.; Gouveia, L.F.; Silva-Lima, B.; Brito, A.M.; Videira, M. Evading p-glycoprotein mediated-efflux chemoresistance using solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.H.; Swanson, D.; Barqawi, A.B. Modalities for imaging of prostate cancer. Adv. Urol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P. Introduction to Nanomedicine and Nanobioengineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. 590. [Google Scholar]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Nebuloni, L.; Kuhn, G.A.; Muller, R. A comparative analysis of water-soluble and blood-pool contrast agents for in vivo vascular imaging with micro-ct. Acad. Radiol 2013, 20, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.; Ghaghada, K.; Moding, E.J.; Kirsch, D.G.; Badea, C.T. In vivo characterization of tumor vasculature using iodine and gold nanoparticles and dual energy micro-CT. Phys. Med. Biol. 2013, 58, 1683–1704. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Qin, X.; Zhang, G.; Lu, X.; Zhu, Y.; Zhang, H.; Dai, B.; Shi, G.; Ye, D. Oxidized low-density lipoprotein is associated with advanced-stage prostate cancer. Tumour Biol. 2015, 36, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.L.; Corbin, I.R.; Levitin, R.B.; Cao, W.; Mainprize, J.G.; Yaffe, M.J.; Zheng, G. In vitro assessment of poly-iodinated triglyceride reconstituted low-density lipoprotein: Initial steps toward ct molecular imaging. Acad. Radiol. 2010, 17, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Furuya, Y.; Sekine, Y.; Kato, H.; Miyazawa, Y.; Koike, H.; Suzuki, K. Low-density lipoprotein receptors play an important role in the inhibition of prostate cancer cell proliferation by statins. Prostate Int. 2016, 4, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Liu, Y.; Liu, J.; Yuan, Q.; He, Y.; Lu, L. Large-scale synthesis of Bi2S3 nanodots as a contrast agent for in vivo X-ray computed tomography imaging. Adv. Mater. 2011, 23, 4886–4891. [Google Scholar] [CrossRef] [PubMed]
- Carton, A.K.; Gavenonis, S.C.; Currivan, J.A.; Conant, E.F.; Schnall, M.D.; Maidment, A.D. Dual-energy contrast-enhanced digital breast tomosynthesis—A feasibility study. Br. J. Radiol. 2010, 83, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeong, Y.Y.; Jon, S. A drug-loaded aptamer-gold nanoparticle bioconjugate for combined ct imaging and therapy of prostate cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Lau, K.C.; Hsu, J.C.; Hajfathalian, M.; Mian, S.; Chhour, P.; Uppuluri, L.; McDonald, E.S.; Maidment, A.D.; Cormode, D.P. Gold silver alloy nanoparticles (GSAN): An imaging probe for breast cancer screening with dual-energy mammography or computed tomography. Nanoscale 2016, 8, 13740–13754. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Keliher, E.J.; Thurber, G.M.; Nahrendorf, M.; Weissleder, R. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconjug. Chem. 2009, 20, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.J.; Hawker, C.J.; Wooley, K.L. The advantages of nanoparticles for pet. J. Nucl. Med. 2009, 50, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Chrastina, A.; Schnitzer, J.E. Iodine-125 radiolabeling of silver nanoparticles for in vivo SPECT imaging. Int. J. Nanomed. 2010, 5, 653–659. [Google Scholar]
- Woodward, J.; Kennel, S.; Mirzadeh, S.; Dai, S.; Wall, J.; Richey, T.; Avanell, J.; Rondinone, A. In vivo SPECT/CT imaging and biodistribution using radioactive 125-CDTE/ZnS nanoparticles. Nanotechnology 2007, 18, 175103. [Google Scholar] [CrossRef]
- Wong, P.; Li, L.; Chea, J.; Delgado, M.K.; Crow, D.; Poku, E.; Szpikowska, B.; Bowles, N.; Channappa, D.; Colcher, D.; et al. Pet imaging of 64Cu-DOTA-SCFV-anti-psma lipid nanoparticles (LNPS): Enhanced tumor targeting over anti-PSMA SCFV or untargeted LNPS. Nucl. Med. Biol. 2017, 47, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sultan, D.; Detering, L.; Luehmann, H.; Liu, Y. Facile synthesis, pharmacokinetic and systemic clearance evaluation, and positron emission tomography cancer imaging of (6)(4)Cu-Au alloy nanoclusters. Nanoscale 2014, 6, 13501–13509. [Google Scholar] [CrossRef] [PubMed]
- Pressly, E.D.; Pierce, R.A.; Connal, L.A.; Hawker, C.J.; Liu, Y. Nanoparticle PET/CT imaging of natriuretic peptide clearance receptor in prostate cancer. Bioconjug. Chem. 2013, 24, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ma, Z.; Chen, F.; Dong, Q.; Hu, Q.; Bai, L.; Chen, J. Effective photothermal chemotherapy with docetaxel-loaded gold nanospheres in advanced prostate cancer. J. Drug Target. 2015, 23, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Behnam Azad, B.; Banerjee, S.R.; Pullambhatla, M.; Lacerda, S.; Foss, C.A.; Wang, Y.; Ivkov, R.; Pomper, M.G. Evaluation of a PSMA-targeted BNF nanoparticle construct. Nanoscale 2015, 7, 4432–4442. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lo, S.T.; Lim, J.; da Costa, V.C.; Ramezani, S.; Oz, O.K.; Pavan, G.M.; Annunziata, O.; Sun, X.; Simanek, E.E. Design, synthesis and biological assessment of a triazine dendrimer with approximately 16 paclitaxel groups and 8 PEG groups. Mol. Pharm. 2013, 10, 4452–4461. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Sanchez, A.N.; Ferro-Flores, G.; Ocampo-Garcia, B.E.; Morales-Avila, E.; de, M.R.F.; De Leon-Rodriguez, L.M.; Santos-Cuevas, C.L.; Medina, L.A.; Rojas-Calderon, E.L.; Camacho-Lopez, M.A. Lys3-bombesin conjugated to 99mTc-labelled gold nanoparticles for in vivo gastrin releasing peptide-receptor imaging. J. Biomed. Nanotechnol. 2010, 6, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Aanei, I.L.; ElSohly, A.M.; Farkas, M.E.; Netirojjanakul, C.; Regan, M.; Taylor Murphy, S.; O’Neil, J.P.; Seo, Y.; Francis, M.B. Biodistribution of antibody-MS2 viral capsid conjugates in breast cancer models. Mol. Pharm. 2016, 13, 3764–3772. [Google Scholar] [CrossRef] [PubMed]
- Perez-Medina, C.; Abdel-Atti, D.; Zhang, Y.; Longo, V.A.; Irwin, C.P.; Binderup, T.; Ruiz-Cabello, J.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.; et al. A modular labeling strategy for in vivo pet and near-infrared fluorescence imaging of nanoparticle tumor targeting. J. Nucl. Med. 2014, 55, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Black, K.C.; Luehmann, H.; Li, W.; Zhang, Y.; Cai, X.; Wan, D.; Liu, S.Y.; Li, M.; Kim, P.; et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano 2013, 7, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.C.; Xu, Z.; Guley, K.; Yuan, H.; Huang, L. Lipid-calcium phosphate nanoparticles for delivery to the lymphatic system and SPECT/CT imaging of lymph node metastases. Biomaterials 2014, 35, 4688–4698. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, A.; Price, M.C.; McDermott, S.; Harisinghani, M.G. Imaging on nodal staging of prostate cancer. Future Oncol. 2017, 13, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A.; Bulte, J.W. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011, 24, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, A.H.; Caruthers, S.D.; Keupp, J.; Wickline, S.A.; Lanza, G.M. Recent advances in fluorine magnetic resonance imaging with perfluorocarbon emulsions. Engineering (Beijing) 2015, 1, 475–489. [Google Scholar] [PubMed]
- Chen, H.; Song, M.; Tang, J.; Hu, G.; Xu, S.; Guo, Z.; Li, N.; Cui, J.; Zhang, X.; Chen, X.; et al. Ultrahigh (19)f loaded cu1.75s nanoprobes for simultaneous 19F magnetic resonance imaging and photothermal therapy. ACS Nano 2016, 10, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, G.; Jablonska, A.; Rose, L.; Walczak, P.; Janowski, M. Effect of MRI tags: Spio nanoparticles and 19F nanoemulsion on various populations of mouse mesenchymal stem cells. Acta Neurobiol. Exp. (Wars) 2015, 75, 144–159. [Google Scholar] [PubMed]
- Amiri, H.; Srinivas, M.; Veltien, A.; van Uden, M.J.; de Vries, I.J.; Heerschap, A. Cell tracking using 19F magnetic resonance imaging: Technical aspects and challenges towards clinical applications. Eur. Radiol. 2015, 25, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, R.J.; Frenkiel, T.A.; Newell, D.R.; Bauer, C.; Griffiths, J.R. 19F nuclear magnetic resonance imaging of drug distribution in vivo: The disposition of an antifolate anticancer drug in mice. Magn. Reson. Med. 1991, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Zhao, D.; Song, Y.; Constantinescu, A.; Mason, R.P.; Liu, H. Interplay of tumor vascular oxygenation and tumor PO2 observed using near-infrared spectroscopy, an oxygen needle electrode, and 19f mr po2 mapping. J. Biomed. Opt. 2003, 8, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yang, Y.; Bo, S.; Li, Y.; Chen, S.; Yang, Z.; Zheng, X.; Jiang, Z.X.; Zhou, X. Design and synthesis of fluorinated dendrimers for sensitive 19F MRI. J. Org. Chem. 2015, 80, 4443–4449. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Mizukami, S.; Sugihara, F.; Nakanishi, Y.; Yoshioka, Y.; Kikuchi, K. Multifunctional core-shell silica nanoparticles for highly sensitive 19F magnetic resonance imaging. Angew. Chem. Int. Ed. Engl. 2014, 53, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Bae, P.K.; Jung, J.; Lim, S.J.; Kim, D.; Kim, S.K.; Chung, B.H. Bimodal perfluorocarbon nanoemulsions for nasopharyngeal carcinoma targeting. Mol. Imaging Biol. 2013, 15, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, E.T.; Helfer, B.M.; O’Hanlon, C.F.; Schirda, C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn. Reson. Med. 2014, 72, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Park, W.; Kim, D.H. Silica-coated metal chelating-melanin nanoparticles as a dual-modal contrast enhancement imaging and therapeutic agent. ACS Appl. Mater. Interfaces 2017, 9, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Moghanaki, D.; Turkbey, B.; Vapiwala, N.; Ehdaie, B.; Frank, S.J.; McLaughlin, P.W.; Harisinghani, M. Advances in prostate cancer magnetic resonance imaging and positron emission tomography-computed tomography for staging and radiotherapy treatment planning. Semin. Radiat. Oncol. 2017, 27, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Jayapaul, J.; Arns, S.; Bunker, M.; Weiler, M.; Rutherford, S.; Comba, P.; Kiessling, F. In vivo evaluation of riboflavin receptor targeted fluorescent USPIO in mice with prostate cancer xenografts. Nano Res. 2016, 9, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, A.; Harisinghani, M.G.; Mahmood, U. Prostate cancer imaging and therapy: Potential role of nanoparticles. J. Nucl. Med. 2016, 57, 105S–110S. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.S.; Overchuk, M.; Cui, L.; Wilson, B.C.; Bristow, R.G.; Chen, J.; Zheng, G. Nanoparticle-enabled selective destruction of prostate tumor using MRI-guided focal photothermal therapy. Prostate 2016, 76, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Hurley, K.R.; Ring, H.L.; Etheridge, M.; Zhang, J.; Gao, Z.; Shao, Q.; Klein, N.D.; Szlag, V.M.; Chung, C.; Reineke, T.M.; et al. Predictable heating and positive MRI contrast from a mesoporous silica-coated iron oxide nanoparticle. Mol. Pharm. 2016, 13, 2172–2183. [Google Scholar] [CrossRef] [PubMed]
- Turino, L.N.; Ruggiero, M.R.; Stefania, R.; Cutrin, J.C.; Aime, S.; Geninatti Crich, S. Ferritin decorated PLGA/paclitaxel loaded nanoparticles endowed with an enhanced toxicity toward MCF-7 breast tumor cells. Bioconjug. Chem. 2017, 28, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.; Pirayesh Islamian, J. Breast cancer: Early diagnosis and effective treatment by drug delivery tracing. Nucl. Med. Rev. Cent. East. Eur. 2017, 20, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.H.; Wang, P.; Xiong, F.; Lu, H.Y.; Hu, H. Detection of human breast cancer cells using a 2-deoxy-d-glucose-functionalized superparamagnetic iron oxide nanoparticles. Cancer Biomark. 2017. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, M.; Shahbazi-Gahrouei, D.; Khoshfetrat, S.M.; Mehrgardi, M.A.; Aghaei, M. Aptamer-conjugated magnetic nanoparticles as targeted magnetic resonance imaging contrast agent for breast cancer. J. Med. Signals Sens. 2016, 6, 243–247. [Google Scholar] [PubMed]
- Zhang, L.; Varma, N.R.; Gang, Z.Z.; Ewing, J.R.; Arbab, A.S.; Ali, M.M. Targeting triple negative breast cancer with a small-sized paramagnetic nanoparticle. J. Nanomed. Nanotechnol. 2016, 7, 404. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Yang, B.Y.; Kim, Y.J.; Hong, M.K.; Lee, Y.S.; Lee, D.S.; Chung, J.K.; Jeong, J.M. Development of a complementary PET/MR dual-modal imaging probe for targeting prostate-specific membrane antigen (PSMA). Nanomedicine 2016, 12, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, D.; Liu, S.; Wang, M.; Moats, R.; Conti, P.S.; Li, Z. Integrin α2β1 targeted GdVO4:Eu ultrathin nanosheet for multimodal PET/MR imaging. Biomaterials 2014, 35, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Key, J.; Stigliano, C.; Landis, M.D.; Lee, D.Y.; Decuzzi, P. Positron emitting magnetic nanoconstructs for PET/MR imaging. Small 2014, 10, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhou, J.; Su, X.; Wang, Y.; Yan, X.; Jia, S.; Du, B. A smart responsive dual aptamers-targeted bubble-generating nanosystem for cancer triplex therapy and ultrasound imaging. Small 2017. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wan, C.; Du, J.; Li, H.; Liu, X.; Yang, H.; Li, F. Synthesis, characterization, and in vitro evaluation of targeted gold nanoshelled poly(d,l-lactide-co-glycolide) nanoparticles carrying anti p53 antibody as a theranostic agent for ultrasound contrast imaging and photothermal therapy. J. Biomater. Sci. Polym. Ed. 2017, 28, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Baghbani, F.; Moztarzadeh, F.; Mohandesi, J.A.; Yazdian, F.; Mokhtari-Dizaji, M. Novel alginate-stabilized doxorubicin-loaded nanodroplets for ultrasounic theranosis of breast cancer. Int. J. Biol. Macromol. 2016, 93, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Carugo, D.; Crake, C.; Owen, J.; de Saint Victor, M.; Seth, A.; Coussios, C.; Stride, E. Nanoparticle-loaded protein-polymer nanodroplets for improved stability and conversion efficiency in ultrasound imaging and drug delivery. Adv. Mater. 2015, 27, 5484–5492. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dai, Z.; Ke, H.; Qu, E.; Qi, X.; Zhang, K.; Wang, J. Contrast ultrasound-guided photothermal therapy using gold nanoshelled microcapsules in breast cancer. Eur. J. Radiol. 2014, 83, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Agemy, L.; Sugahara, K.N.; Kotamraju, V.R.; Gujraty, K.; Girard, O.M.; Kono, Y.; Mattrey, R.F.; Park, J.H.; Sailor, M.J.; Jimenez, A.I.; et al. Nanoparticle-induced vascular blockade in human prostate cancer. Blood 2010, 116, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, L.; Li, T.; Shen, X.; Yan, J.; Zuo, L.; Wu, C.; Liu, Y. Multifunctional PLGA nanobubbles as theranostic agents: Combining doxorubicin and P-GP siRNA co-delivery into human breast cancer cells and ultrasound cellular imaging. J. Biomed. Nanotechnol. 2015, 11, 2124–2136. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.P.; Wang, L.F.; Guo, Y.L.; Li, L.; Fan, X.Z.; Ding, J.; Huang, H.Y. Preparation of protamine cationic nanobubbles and experimental study of their physical properties and in vivo contrast enhancement. Ultrasound Med. Biol. 2013, 39, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Thorek, D.; Robertson, R.; Bacchus, W.A.; Hahn, J.; Rothberg, J.; Beattie, B.J.; Grimm, J. Cerenkov imaging —A new modality for molecular imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 163–173. [Google Scholar] [PubMed]
- Black, K.C.; Ibricevic, A.; Gunsten, S.P.; Flores, J.A.; Gustafson, T.P.; Raymond, J.E.; Samarajeewa, S.; Shrestha, R.; Felder, S.E.; Cai, T.; et al. In vivo fate tracking of degradable nanoparticles for lung gene transfer using pet and cerenkov imaging. Biomaterials 2016, 98, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.E.; Schiariti, M.P.; Grana, C.M.; Ferrari, M.; Cremonesi, M.; Boschi, F. Cerenkov and radioluminescence imaging of brain tumor specimens during neurosurgery. J. Biomed. Opt. 2016, 21, 50502. [Google Scholar] [CrossRef] [PubMed]
- Schwenck, J.; Fuchs, K.; Eilenberger, S.H.; Rolle, A.M.; Castaneda Vega, S.; Thaiss, W.M.; Maier, F.C. Fluorescence and Cerenkov luminescence imaging. Applications in small animal research. Nuklearmedizin 2016, 55, 63–70. [Google Scholar] [PubMed]
- Pandya, D.N.; Hantgan, R.; Budzevich, M.M.; Kock, N.D.; Morse, D.L.; Batista, I.; Mintz, A.; Li, K.C.; Wadas, T.J. Preliminary therapy evaluation of 225Ac-DOTA-c(RGDyK) demonstrates that cerenkov radiation derived from 225Ac daughter decay can be detected by optical imaging for in vivo tumor visualization. Theranostics 2016, 6, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, M.; Gotoh, K.; Hasegawa, K.; Kojima, A. Hybrid light imaging using cerenkov luminescence and liquid scintillation for preclinical optical imaging in vivo. Mol. Imaging Biol. 2016, 18, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, J.M.; Zhang, R.; Gladstone, D.J.; Williams, B.B.; Glaser, A.K.; Pogue, B.W.; Jarvis, L.A. Cherenkov imaging method for rapid optimization of clinical treatment geometry in total skin electron beam therapy. Med. Phys. 2016, 43, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, A.G.; Burger, I.A.; Sala, E.; Hricak, H.; Weber, W.A.; Vargas, H.A. Molecular imaging of prostate cancer. Radiographics 2016, 36, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.A.; Grimm, J.; O, F.D.; Sala, E.; Hricak, H. Molecular imaging of prostate cancer: Translating molecular biology approaches into the clinical realm. Eur. Radiol. 2015, 25, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, C.; Zhang, H.; Thorek, D.L.; Desai, P.; Zanzonico, P.B.; O’Donoghue, J.; Irwin, C.P.; Reiner, T.; Grimm, J.; Weber, W.A. Cerenkov luminescence imaging for radiation dose calculation of a (9)(0)Y-labeled gastrin-releasing peptide receptor antagonist. J. Nucl. Med. 2015, 56, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chi, C.; Liu, M.; Guo, H.; Zhang, Z.; Zeng, C.; Ye, J.; Wang, J.; Tian, J.; Yang, W.; et al. Nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging allows precise image-guided tumor-removal surgery. Nanomedicine 2017, 13, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Madru, R.; Tran, T.A.; Axelsson, J.; Ingvar, C.; Bibic, A.; Stahlberg, F.; Knutsson, L.; Strand, S.E. 68Ga-labeled superparamagnetic iron oxide nanoparticles (SPIONs) for multi-modality PET/MR/Cherenkov luminescence imaging of sentinel lymph nodes. Am. J. Nucl. Med. Mol. Imaging 2013, 4, 60–69. [Google Scholar] [PubMed]
- Hu, Z.; Zhao, M.; Qu, Y.; Zhang, X.; Zhang, M.; Liu, M.; Guo, H.; Zhang, Z.; Wang, J.; Yang, W.; et al. In vivo 3-dimensional radiopharmaceutical-excited fluorescence tomography. J. Nucl. Med. 2017, 58, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Yoon, G.; Lee, S.W.; Jeong, S.Y.; Ahn, B.C.; Lim, D.K.; Lee, J.; Jeon, Y.H. Combined positron emission tomography and Cerenkov luminescence imaging of sentinel lymph nodes using pegylated radionuclide-embedded gold nanoparticles. Small 2016, 12, 4894–4901. [Google Scholar] [CrossRef] [PubMed]
- Tanha, K.; Pashazadeh, A.M.; Pogue, B.W. Review of biomedical Cerenkov luminescence imaging applications. Biomed. Opt. Express 2015, 6, 3053–3065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kim, H.S.; Jin, T.; Yi, A.; Moon, W.K. Ultrasound-guided photoacoustic imaging for the selective detection of EGFR-expressing breast cancer and lymph node metastases. Biomed. Opt. Express 2016, 7, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Teng, Z.; Yao, H.; Wang, S.; Tian, Y.; Zhang, Y.; Liu, W.; Tian, W.; Zheng, L.; Lu, N.; et al. A multifunctional PB@mSiO2-PEG/DOX nanoplatform for combined photothermal-chemotherapy of tumor. ACS Appl. Mater. Interfaces 2016, 8, 17038–17046. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xia, X.; Li, C.; Song, Y.; Qin, C.; Zhang, Y.; Lan, X. Tyr as a multifunctional reporter gene regulated by the tet-on system for multimodality imaging: An in vitro study. Sci. Rep. 2015, 5, 15502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cui, H.; Fang, C.Y.; Cheng, K.; Yang, X.; Chang, H.C.; Forrest, M.L. Targeted nanodiamonds as phenotype-specific photoacoustic contrast agents for breast cancer. Nanomedicine (Lond.) 2015, 10, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Levi, J.; Sathirachinda, A.; Gambhir, S.S. A high-affinity, high-stability photoacoustic agent for imaging gastrin-releasing peptide receptor in prostate cancer. Clin. Cancer Res. 2014, 20, 3721–3729. [Google Scholar] [CrossRef] [PubMed]
- Dogra, V.S.; Chinni, B.K.; Valluru, K.S.; Joseph, J.V.; Ghazi, A.; Yao, J.L.; Evans, K.; Messing, E.M.; Rao, N.A. Multispectral photoacoustic imaging of prostate cancer: Preliminary ex vivo results. J. Clin. Imaging Sci. 2013, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Huang, S.W.; Day, K.C.; O’Donnell, M.; Agayan, R.R.; Day, M.A.; Kopelman, R.; Ashkenazi, S. Indocyanine-green-embedded pebbles as a contrast agent for photoacoustic imaging. J. Biomed. Opt. 2007, 12, 044020. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Favazza, C.; Wang, L.V. In vivo photoacoustic tomography of chemicals: High-resolution functional and molecular optical imaging at new depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Qian, W.; Shao, X.; Xie, Z.; Cheng, X.; Liu, S.; Cheng, Q.; Liu, B.; Wang, X. Plasmonic nanoparticles with quantitatively controlled bioconjugation for photoacoustic imaging of live cancer cells. Adv. Sci. (Weinh) 2016, 3, 1600237. [Google Scholar] [CrossRef] [PubMed]
- Olafsson, R.; Bauer, D.R.; Montilla, L.G.; Witte, R.S. Real-time, contrast enhanced photoacoustic imaging of cancer in a mouse window chamber. Opt. Express 2010, 18, 18625–18632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Cyclic arg-gly-asp-polyethyleneglycol-single-walled carbon nanotubes. In Molecular Imaging and Contrast Agent Database (Micad); Bethesda: Washington DC MD, USA, 2004. [Google Scholar]
- Xia, J.; Feng, G.; Xia, X.; Hao, L.; Wang, Z. Nh4hco3 gas-generating liposomal nanoparticle for photoacoustic imaging in breast cancer. Int. J. Nanomed. 2017, 12, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Biffi, S.; Petrizza, L.; Garrovo, C.; Rampazzo, E.; Andolfi, L.; Giustetto, P.; Nikolov, I.; Kurdi, G.; Danailov, M.B.; Zauli, G.; et al. Multimodal near-infrared-emitting plus silica nanoparticles with fluorescent, photoacoustic, and photothermal capabilities. Int. J. Nanomed. 2016, 11, 4865–4874. [Google Scholar]
- Hu, D.; Liu, C.; Song, L.; Cui, H.; Gao, G.; Liu, P.; Sheng, Z.; Cai, L. Indocyanine green-loaded polydopamine-iron ions coordination nanoparticles for photoacoustic/magnetic resonance dual-modal imaging-guided cancer photothermal therapy. Nanoscale 2016, 8, 17150–17158. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, X.; Liao, L.D.; Bandla, A.; Ling, J.M.; Liu, Y.H.; Thakor, N.; Bazan, G.C.; Liu, B. Encapsulated conjugated oligomer nanoparticles for real-time photoacoustic sentinel lymph node imaging and targeted photothermal therapy. Small 2016, 12, 4873–4880. [Google Scholar] [CrossRef] [PubMed]
- Pham, E.; Yin, M.; Peters, C.G.; Lee, C.R.; Brown, D.; Xu, P.; Man, S.; Jayaraman, L.; Rohde, E.; Chow, A.; et al. Preclinical efficacy of bevacizumab with crlx101, an investigational nanoparticle-drug conjugate, in treatment of metastatic triple-negative breast cancer. Cancer Res. 2016, 76, 4493–4503. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhang, Y.; Zhang, W.; Shan, X.; Yuan, Y.; Zhang, H.; Hou, L.; Zhang, Z. Tumor-targeted and multi-stimuli responsive drug delivery system for near-infrared light induced chemo-phototherapy and photoacoustic tomography. Acta Biomater. 2016, 38, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ahir, M.; Bhattacharya, S.; Karmakar, S.; Mukhopadhyay, A.; Mukherjee, S.; Ghosh, S.; Chattopadhyay, S.; Patra, P.; Adhikary, A. Tailored-CuO-nanowire decorated with folic acid mediated coupling of the mitochondrial-ROS generation and MIR425-PTEN axis in furnishing potent anti-cancer activity in human triple negative breast carcinoma cells. Biomaterials 2016, 76, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Zevon, M.; Ganapathy, V.; Kantamneni, H.; Mingozzi, M.; Kim, P.; Adler, D.; Sheng, Y.; Tan, M.C.; Pierce, M.; Riman, R.E.; et al. CXCR-4 targeted, short wave infrared (SWIR) emitting nanoprobes for enhanced deep tissue imaging and micrometastatic cancer lesion detection. Small 2015, 11, 6347–6357. [Google Scholar] [CrossRef] [PubMed]
- Ozel, T.; White, S.; Nguyen, E.; Moy, A.; Brenes, N.; Choi, B.; Betancourt, T. Enzymatically activated near infrared nanoprobes based on amphiphilic block copolymers for optical detection of cancer. Lasers Surg. Med. 2015. [Google Scholar] [CrossRef]
- Yuan, J.P.; Wang, L.W.; Qu, A.P.; Chen, J.M.; Xiang, Q.M.; Chen, C.; Sun, S.R.; Pang, D.W.; Liu, J.; Li, Y. Quantum dots-based quantitative and in situ multiple imaging on ki67 and cytokeratin to improve ki67 assessment in breast cancer. PLoS ONE 2015, 10, e0122734. [Google Scholar] [CrossRef] [PubMed]
- D’Angelis do, E.S.B.C.; Correa, J.R.; Medeiros, G.A.; Barreto, G.; Magalhaes, K.G.; de Oliveira, A.L.; Spencer, J.; Rodrigues, M.O.; Neto, B.A. Carbon dots (C-dots) from cow manure with impressive subcellular selectivity tuned by simple chemical modification. Chemistry 2015, 21, 5055–5060. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, X.; Guo, Y.; An, S.; Kuang, Y.; Ma, H.; He, X.; Jiang, C. Linear-dendritic copolymer composed of polyethylene glycol and all-trans-retinoic acid as drug delivery platform for paclitaxel against breast cancer. Bioconjug. Chem. 2015, 26, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Montecinos, V.P.; Morales, C.H.; Fischer, T.H.; Burns, S.; San Francisco, I.F.; Godoy, A.S.; Smith, G.J. Selective targeting of bioengineered platelets to prostate cancer vasculature: New paradigm for therapeutic modalities. J. Cell Mol. Med. 2015, 19, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, T.; Wu, Y.; Ma, X.; Yu, G.; Qi, J. In Vitro and in vivo imaging of prostate tumor using NAYF4: Yb, Er up-converting nanoparticles. Pathol. Oncol. Res. 2014, 20, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Laprise-Pelletier, M.; Lagueux, J.; Cote, M.F.; LaGrange, T.; Fortin, M.A. Low-dose prostate cancer brachytherapy with radioactive palladium-gold nanoparticles. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.M.; Mousa, S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Control. Release 2017, 248, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Bethu, M.S.; Pushpalatha, B.; Baki, V.B.; Aishwarya, S.; Rao, J.V.; Tartte, V. Biogenesis of silver nanoparticles using endophytic fungus pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomed. 2016, 11, 5683–5696. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Avan, A.; Ferns, G.A.; Pazoki-Toroudi, H. Enhanced detection sensitivity of prostate-specific antigen via PSA-conjugated gold nanoparticles based on localized surface plasmon resonance: GNP-coated anti-PSA/LSPR as a novel approach for the identification of prostate anomalies. Cancer Gene. Ther. 2016, 23, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Ghosh Ray, S.; Mandal, S. Development of bicalutamide-loaded PLGA nanoparticles: Preparation, characterization and in vitro evaluation for the treatment of prostate cancer. Artif. Cells Nanomed. Biotechnol. 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Singh, P.; Kim, Y.J.; Soshnikova, V.; Kang, J.; Markus, J.; Ahn, S.; Castro-Aceituno, V.; Mathiyalagan, R.; Chokkalingam, M.; et al. Biological synthesis of gold and silver chloride nanoparticles by glycyrrhiza uralensis and in vitro applications. Artif. Cells Nanomed. Biotechnol. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.J.; Koohpeima, F.; Mohammadi, H. A comparison inhibitory effects of cisplatin and MNPS-PEG-cisplatin on the adhesion capacity of bone metastatic breast cancer. Chem. Biol. Drug Des. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Xavier, R.J.; Arumugam, M. Facile synthesis of multifunctional silver nanoparticles using mangrove plant Excoecaria agallocha L. For its antibacterial, antioxidant and cytotoxic effects. J. Parasit. Dis. 2017, 41, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M. Targeted Therapies in Cancer; Nova Sciences Publishers: Hauppauge, NY, USA, 2014. [Google Scholar]
- Abramson, R. Overview of Targeted Therapies for Cancer. Available online: https://www.mycancergenome.org/content/molecular-medicine/overview-of-targeted-therapies-for-cancer/ (accessed on 13 June 2016).
- Collignon, J.; Lousberg, L.; Schroeder, H.; Jerusalem, G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer (Dove Med Press) 2016, 8, 93–107. [Google Scholar] [PubMed]
- Kontani, K.; Hashimoto, S.I.; Murazawa, C.; Norimura, S.; Tanaka, H.; Ohtani, M.; Fujiwara-Honjo, N.; Date, M.; Teramoto, K.; Houchi, H.; et al. Indication of metronomic chemotherapy for metastatic breast cancer: Clinical outcomes and responsive subtypes. Mol. Clin. Oncol. 2016, 4, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Bateman, J.C.; Carlton, H.N. The role of chemotherapy in the treatment of breast cancer. Surgery 1960, 47, 895–907. [Google Scholar] [PubMed]
- Nakano, M.; Shoji, S.; Higure, T.; Kawakami, M.; Tomonaga, T.; Terachi, T.; Uchida, T. Low-dose docetaxel, estramustine and prednisolone combination chemotherapy for castration-resistant prostate cancer. Mol. Clin. Oncol. 2016, 4, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Herbst, W.P. The present picture in chemotherapy in prostatic carcinoma. J. Urol. 1947, 57, 296–299. [Google Scholar] [PubMed]
- Albumin-bound paclitaxel (abraxane) for advanced breast cancer. Med. Lett. Drugs Ther. 2005, 47, 39–40.
- Barenholz, Y. Doxil—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Davidson, N.; Juozaityte, E.; Erdkamp, F.; Pluzanska, A.; Azarnia, N.; Lee, L.W. Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first-line therapy for metastatic breast cancer. Ann. Oncol. 2004, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of genexol-pm, a cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G. Nanomedicines for cancer therapy: An update of FDA approved and those under various stages of development. SOJ Pharm. Pharm. Sci. 2014, 1, 13. [Google Scholar]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, X.; Guo, S.; Lin, T.; Guo, H. Highly effective photothermal chemotherapy with pH-responsive polymer-coated drug-loaded melanin-like nanoparticles. Int. J. Nanomed. 2017, 12, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- Bakht, M.K.; Oh, S.W.; Hwang, D.W.; Lee, Y.S.; Youn, H.; Porter, L.A.; Cheon, G.J.; Kwak, C.; Lee, D.S.; Kang, K.W. The potential roles of radionanomedicine and radioexosomic in prostate cancer research and treatment. Curr. Pharm. Des. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.M.; Chen, K.Y.; Kamei, D.T. A transferrin variant as the targeting ligand for polymeric nanoparticles incorporated in 3-d PLGA porous scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Belz, J.; Castilla-Ojo, N.; Sridhar, S.; Kumar, R. Radiosensitizing silica nanoparticles encapsulating docetaxel for treatment of prostate cancer. Methods Mol. Biol. 2017, 1530, 403–409. [Google Scholar] [PubMed]
- Yan, J.; Wang, Y.; Jia, Y.; Liu, S.; Tian, C.; Pan, W.; Liu, X.; Wang, H. Co-delivery of docetaxel and curcumin prodrug via dual-targeted nanoparticles with synergistic antitumor activity against prostate cancer. Biomed. Pharmacother. 2017, 88, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Lin, J.N.; Hsieh, J.T.; Chou, S.C.; Lai, C.H.; Yun, E.J.; Lo, U.G.; Pong, R.C.; Lin, J.H.; Lin, Y.H. Nanoparticle targeting CD44-positive cancer cells for site-specific drug delivery in prostate cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 30722–30734. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Sudha, T.; Cui, H.; Mian, B.M.; Mousa, S.A. Anti-CD24 nano-targeted delivery of docetaxel for the treatment of prostate cancer. Nanomedicine 2017, 13, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Lee, R.J.; Sun, Y.; Cai, G.; Wang, J.; Wang, M.; Lu, J.; Meng, Q.; Teng, L.; Wang, D.; et al. Cabazitaxel-loaded human serum albumin nanoparticles as a therapeutic agent against prostate cancer. Int. J. Nanomed. 2016, 11, 3451–3459. [Google Scholar]
- Pirayesh Islamian, J.; Hatamian, M.; Aval, N.A.; Rashidi, M.R.; Mesbahi, A.; Mohammadzadeh, M.; Asghari Jafarabadi, M. Targeted superparamagnetic nanoparticles coated with 2-deoxy-d-gloucose and doxorubicin more sensitize breast cancer cells to ionizing radiation. Breast 2017, 33, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Wang, Y.; Liang, X.; Wusiman, Z.; Yin, Y.; Shen, Q. Synergistic inhibition of migration and invasion of breast cancer cells by dual docetaxel/quercetin-loaded nanoparticles via Akt/MMP-9 pathway. Int. J. Pharm. 2017, 523, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R. Exploring the genomes of cancer cells: Progress and promise. Science 2011, 331, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Merz, B. Gene therapy may have future role in cancer treatment. JAMA 1987, 257, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Kozielski, K.L.; Rui, Y.; Green, J.J. Non-viral nucleic acid containing nanoparticles as cancer therapeutics. Expert. Opin. Drug Deliv. 2016, 13, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Gogtay, N.J.; Sridharan, K. Therapeutic nucleic acids: Current clinical status. Br. J. Clin. Pharmacol. 2016, 82, 659–672. [Google Scholar]
- Yu, Y.; Yao, Y.; Yan, H.; Wang, R.; Zhang, Z.; Sun, X.; Zhao, L.; Ao, X.; Xie, Z.; Wu, Q. A tumor-specific microRNA recognition system facilitates the accurate targeting to tumor cells by magnetic nanoparticles. Mol. Ther. Nucleic Acids 2016, 5, e318. [Google Scholar] [CrossRef] [PubMed]
- Rejeeth, C.; Vivek, R. Comparison of two silica based nonviral gene therapy vectors for breast carcinoma: Evaluation of the p53 delivery system in balb/C mice. Artif. Cells Nanomed. Biotechnol. 2017, 45, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Rejeeth, C.; Salem, A. Novel luminescent silica nanoparticles (LSN): p53 Gene delivery system in breast cancer in vitro and in vivo. J. Pharm. Pharmacol. 2016, 68, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shen, X.; Chen, Y.; Zhang, C.; Yan, J.; Yang, H.; Wu, C.; Zeng, H.; Liu, Y. Polyetherimide-grafted Fe3O4@SiO2 nanoparticles as theranostic agents for simultaneous VEGF siRNA delivery and magnetic resonance cell imaging. Int. J. Nanomed. 2015, 10, 4279–4291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wei, J.; Dai, Q.; Wang, L.; Luo, J.; Cheang, T.; Wang, S. CaCo3/CaIP6 composite nanoparticles effectively deliver AKT1 small interfering rna to inhibit human breast cancer growth. Int. J. Nanomed. 2015, 10, 4255–4266. [Google Scholar]
- Nourbakhsh, M.; Jaafari, M.R.; Lage, H.; Abnous, K.; Mosaffa, F.; Badiee, A.; Behravan, J. Nanolipoparticles-mediated MDR1 siRNA delivery reduces doxorubicin resistance in breast cancer cells and silences MDR1 expression in xenograft model of human breast cancer. Iran J. Basic Med. Sci. 2015, 18, 385–392. [Google Scholar] [PubMed]
- Su, S.; Tian, Y.; Li, Y.; Ding, Y.; Ji, T.; Wu, M.; Wu, Y.; Nie, G. “Triple-punch” strategy for triple negative breast cancer therapy with minimized drug dosage and improved antitumor efficacy. ACS Nano 2015, 9, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Gao, W.; Liu, Y.; Qi, X.R. Therapeutic potential of targeted multifunctional nanocomplex co-delivery of siRNA and low-dose doxorubicin in breast cancer. Cancer Lett. 2015, 359, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.P.; Hung, C.M.; Hsu, Y.C.; Zhong, C.Y.; Wang, W.R.; Chang, C.C.; Lee, M.J. Suppression of breast cancer cell migration by small interfering RNA delivered by polyethylenimine-functionalized graphene oxide. Nanoscale Res. Lett. 2016, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Arami, S.; Rashidi, M.R.; Mahdavi, M.; Fathi, M.; Entezami, A.A. Synthesis and characterization of Fe3O4-PEG-LAC-chitosan-PEI nanoparticle as a survivin siRNA delivery system. Hum. Exp. Toxicol. 2017, 36, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Unsoy, G.; Gunduz, U. Targeted silencing of survivin in cancer cells by siRNA loaded chitosan magnetic nanoparticles. Expert Rev. Anticancer Ther. 2016, 16, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Cho, J.; Kwon, O.J.; Yun, C.O.; Choy, J.H. Biodegradable inorganic nanovector: Passive versus active tumor targeting in siRNA transportation. Angew. Chem. Int. Ed. Engl. 2016, 55, 4582–4586. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.W.; Massey, A.S.; McCaffrey, J.; McCrudden, C.M.; Coulter, J.A.; Dunne, N.J.; Robson, T.; McCarthy, H.O. Development of TMTP-1 targeted designer biopolymers for gene delivery to prostate cancer. Int. J. Pharm. 2016, 500, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Gao, S.; Duan, Y.; Han, H.; Li, L.; Yang, Y.; Li, Q. Delivery of dnazyme targeting aurora kinase a to inhibit the proliferation and migration of human prostate cancer. Int. J. Nanomed. 2015, 10, 5715–5727. [Google Scholar]
- Fitzgerald, K.A.; Guo, J.; Tierney, E.G.; Curtin, C.M.; Malhotra, M.; Darcy, R.; O’Brien, F.J.; O’Driscoll, C.M. The use of collagen-based scaffolds to simulate prostate cancer bone metastases with potential for evaluating delivery of nanoparticulate gene therapeutics. Biomaterials 2015, 66, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xue, X.; He, D.; Hsieh, J.T. A prostate cancer-targeted polyarginine-disulfide linked pei nanocarrier for delivery of microrna. Cancer Lett. 2015, 365, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Chiang, P.H.; Hsiao, W.C.; Chuang, C.C.; Chang, C.W. Redox-sensitive polymer/SPIO nanocomplexes for efficient magnetofection and mr imaging of human cancer cells. Langmuir 2015, 31, 6523–6531. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Nicolini, G.; Semperboni, S.; Requardt, H.; Le Duc, G.; Santini, C.; Pellei, M.; Bentivegna, A.; Dalpra, L.; Cavaletti, G.; et al. Synchrotron-based photon activation therapy effect on cisplatin pre-treated human glioma stem cells. Anticancer Res. 2014, 34, 5351–5355. [Google Scholar] [PubMed]
- Bakhshabadi, M.; Ghorbani, M.; Meigooni, A.S. Photon activation therapy: A Monte Carlo study on dose enhancement by various sources and activation media. Australas. Phys. Eng. Sci. Med. 2013, 36, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Nicolini, G.; Requardt, H.; Le Duc, G.; Cavaletti, G.; Bravin, A. The effect of photon activation therapy on cisplatin pre-treated human tumour cell lines: Comparison with conventional X-ray irradiation. J. Biol. Regul. Homeost. Agents 2013, 27, 477–485. [Google Scholar] [PubMed]
- Ceberg, C.; Jonsson, B.A.; Prezado, Y.; Pommer, T.; Nittby, H.; Englund, E.; Grafstrom, G.; Edvardsson, A.; Stenvall, A.; Stromblad, S.; et al. Photon activation therapy of RG2 glioma carrying fischer rats using stable thallium and monochromatic synchrotron radiation. Phys. Med. Biol. 2012, 57, 8377–8391. [Google Scholar] [CrossRef] [PubMed]
- Laster, B.H.; Dixon, D.W.; Novick, S.; Feldman, J.P.; Seror, V.; Goldbart, Z.I.; Kalef-Ezra, J.A. Photon activation therapy and brachytherapy. Brachytherapy 2009, 8, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Suortti, P.; Thomlinson, W. Medical applications of synchrotron radiation. Phys Med Biol 2003, 48, R1–R35. [Google Scholar] [CrossRef] [PubMed]
- Laster, B.H.; Thomlinson, W.C.; Fairchild, R.G. Photon activation of iododeoxyuridine: Biological efficacy of auger electrons. Radiat. Res. 1993, 133, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.W.; DeGraff, W.; Kinsella, T.J.; Mitchell, J.B. Evaluation of incorporated iododeoxyuridine cellular radiosensitization by photon activation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 1193–1197. [Google Scholar] [CrossRef]
- Fairchild, R.G.; Bond, V.P. Photon activation therapy. Strahlentherapie 1984, 160, 758–763. [Google Scholar] [PubMed]
- Choi, G.H.; Seo, S.J.; Kim, K.H.; Kim, H.T.; Park, S.H.; Lim, J.H.; Kim, J.K. Photon activated therapy (PAT) using monochromatic synchrotron X-rays and iron oxide nanoparticles in a mouse tumor model: Feasibility study of PAT for the treatment of superficial malignancy. Radiat. Oncol. 2012, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Keltner, L.; Christophersen, J.; Zheng, F.; Krouse, M.; Singhal, A.; Wang, S.S. New technology for deep light distribution in tissue for phototherapy. Cancer J. 2002, 8, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Sneider, A.; VanDyke, D.; Paliwal, S.; Rai, P. Remotely triggered nano-theranostics for cancer applications. Nanotheranostics (Syd.) 2017, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.J.; Chen, H.Y. Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, J.; Chelora, J.; Xiong, Y.; Kershaw, S.V.; Li, K.F.; Lo, P.K.; Cheah, K.W.; Rogach, A.L.; Zapien, J.A.; et al. Ruthenium(ii) complex incorporated uio-67 metal-organic framework nanoparticles for enhanced two-photon fluorescence imaging and photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 5699–5708. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, S.; Li, L.; Yao, S.Q.; Xu, Q.H. Highly efficient, conjugated-polymer-based nano-photosensitizers for selectively targeted two-photon photodynamic therapy and imaging of cancer cells. Chemistry 2015, 21, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Secret, E.; Maynadier, M.; Gallud, A.; Chaix, A.; Bouffard, E.; Gary-Bobo, M.; Marcotte, N.; Mongin, O.; El Cheikh, K.; Hugues, V.; et al. Two-photon excitation of porphyrin-functionalized porous silicon nanoparticles for photodynamic therapy. Adv. Mater. 2014, 26, 7643–7648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yu, K.; Li, L.; Zhang, T.; Guan, Z.; Gao, N.; Yuan, P.; Li, S.; Yao, S.Q.; Xu, Q.H.; et al. Gold nanorod enhanced two-photon excitation fluorescence of photosensitizers for two-photon imaging and photodynamic therapy. ACS Appl. Mater. Interfaces 2014, 6, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Basile, I.; Maynadier, M.; Vaillant, O.; Mongin, O.; Blanchard-Desce, M.; Morere, A.; et al. Mannose-functionalized mesoporous silica nanoparticles for efficient two-photon photodynamic therapy of solid tumors. Angew. Chem. Int. Ed. Engl. 2011, 50, 11425–11429. [Google Scholar] [CrossRef] [PubMed]
- Swartling, J.; Axelsson, J.; Ahlgren, G.; Kalkner, K.M.; Nilsson, S.; Svanberg, S.; Svanberg, K.; Andersson-Engels, S. System for interstitial photodynamic therapy with online dosimetry: First clinical experiences of prostate cancer. J. Biomed. Opt. 2010, 15, 058003. [Google Scholar] [CrossRef] [PubMed]
- Swartling, J.; Hoglund, O.V.; Hansson, K.; Sodersten, F.; Axelsson, J.; Lagerstedt, A.S. Online dosimetry for temoporfin-mediated interstitial photodynamic therapy using the canine prostate as model. J. Biomed. Opt. 2016, 21, 28002. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chatterjee, S.; Lisok, A.; Minn, I.; Pullambhatla, M.; Wharram, B.; Wang, Y.; Jin, J.; Bhujwalla, Z.M.; Nimmagadda, S.; et al. A psma-targeted theranostic agent for photodynamic therapy. J. Photochem. Photobiol. B 2017, 167, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tsui, B.; Ramamurthy, G.; Zhang, P.; Meyers, J.; Kenney, M.E.; Kiechle, J.; Ponsky, L.; Basilion, J.P. Theranostic agents for photodynamic therapy of prostate cancer by targeting prostate-specific membrane antigen. Mol. Cancer Ther. 2016, 15, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Guo, W.; Long, Q.; Ma, A.; Liu, Q.; Zhang, H.; Huang, Y.; Chandrasekaran, S.; Pan, C.; Lam, K.S.; et al. Hsp90 inhibitor encapsulated photo-theranostic nanoparticles for synergistic combination cancer therapy. Theranostics 2016, 6, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, O.; El Cheikh, K.; Warther, D.; Brevet, D.; Maynadier, M.; Bouffard, E.; Salgues, F.; Jeanjean, A.; Puche, P.; Mazerolles, C.; et al. Mannose-6-phosphate receptor: A target for theranostics of prostate cancer. Angew. Chem. Int. Ed. Engl. 2015, 54, 5952–5956. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, H.; Choi, Y. Theranostic nanoparticles for enzyme-activatable fluorescence imaging and photodynamic/chemo dual therapy of triple-negative breast cancer. Quant. Imaging Med. Surg. 2015, 5, 656–664. [Google Scholar] [PubMed]
- Wang, X.; Hu, J.; Wang, P.; Zhang, S.; Liu, Y.; Xiong, W.; Liu, Q. Analysis of the in vivo and in vitro effects of photodynamic therapy on breast cancer by using a sensitizer, sinoporphyrin sodium. Theranostics 2015, 5, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Kue, C.S.; Kamkaew, A.; Lee, H.B.; Chung, L.Y.; Kiew, L.V.; Burgess, K. Targeted PDT agent eradicates trkc expressing tumors via photodynamic therapy (PDT). Mol. Pharm. 2015, 12, 212–222. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, I.H. Nanotechnology in head and neck cancer: The race is on. Curr Oncol Rep 2010, 12, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, K.; Dumych, T.; Bilyy, R.; Turcheniuk, V.; Bouckaert, J.; Vovk, V.; Chopyak, V.; Zaitsev, V.; Mariot, P.; Prevarskaya, N.; et al. Plasmonic photothermal cancer therapy with gold nanorods/reduced graphene oxide core/shell nanocomposites. RSC Adv. 2016, 6, 1600–1610. [Google Scholar] [CrossRef]
- Peng, J.; Dong, M.; Ran, B.; Li, W.; Hao, Y.; Yang, Q.; Tan, L.; Shi, K.; Qian, Z. “One-for-all” type, biodegradable prussian blue/manganese dioxide hybrid nanocrystal for tri-modal imaging guided photothermal therapy and oxygen regulation of breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 13875–13886. [Google Scholar] [CrossRef] [PubMed]
- Cantu, T.; Walsh, K.; Pattani, V.P.; Moy, A.J.; Tunnell, J.W.; Irvin, J.A.; Betancourt, T. Conductive polymer-based nanoparticles for laser-mediated photothermal ablation of cancer: Synthesis, characterization, and in vitro evaluation. Int. J. Nanomed. 2017, 12, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Haberkorn, U.; Eder, M.; Kopka, K.; Babich, J.W.; Eisenhut, M. New strategies in prostate cancer: Prostate-specific membrane antigen (PSMA) ligands for diagnosis and therapy. Clin. Cancer Res. 2016, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bouchelouche, K.; Tagawa, S.T.; Goldsmith, S.J.; Turkbey, B.; Capala, J.; Choyke, P. PET/CT imaging and radioimmunotherapy of prostate cancer. Semin. Nucl. Med. 2011, 41, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Awada, G.; Gombos, A.; Aftimos, P.; Awada, A. Emerging drugs targeting human epidermal growth factor receptor 2 (Her2) in the treatment of breast cancer. Expert Opin. Emerg. Drugs 2016, 21, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Lluch, A.; Eroles, P.; Perez-Fidalgo, J.A. Emerging EGFR antagonists for breast cancer. Expert Opin. Emerg. Drugs 2014, 19, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Doddamane, I.; Butler, R.; Jhaveri, A.; Chung, G.G.; Cheng, D. Where does radioimmunotherapy fit in the management of breast cancer? Immunotherapy 2013, 5, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Evans-Axelsson, S.; Timmermand, O.V.; Bjartell, A.; Strand, S.E.; Elgqvist, J. Radioimmunotherapy for prostate cancer—Current status and future possibilities. Semin. Nucl. Med. 2016, 46, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Zolata, H.; Afarideh, H.; Davani, F.A. Triple therapy of HER2+ cancer using radiolabeled multifunctional iron oxide nanoparticles and alternating magnetic field. Cancer Biother. Radiopharm. 2016, 31, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Q.; Shen, S.; Xu, Y.; Huang, L. Nanotrastuzumab in combination with radioimmunotherapy: Can it be a viable treatment option for patients with HER2-positive breast cancer with brain metastasis? Med. Hypotheses 2016, 88, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Parslow, A.C.; Gan, H.K.; Scott, A.M. Antibody-mediated delivery of therapeutics for cancer therapy. Expert Opin. Drug Deliv. 2016, 13, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Parker, C.L.; McCallen, J.D.; Lai, S.K. Addressing challenges of heterogeneous tumor treatment through bispecific protein-mediated pretargeted drug delivery. J. Control. Release 2015, 220, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Cai, Z.; Lu, Y.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Radiation nanomedicine for egfr-positive breast cancer: Panitumumab-modified gold nanoparticles complexed to the beta-particle-emitter, 177Lu. Mol. Pharm. 2015, 12, 3963–3972. [Google Scholar] [CrossRef] [PubMed]
- Rasaneh, S.; Rajabi, H.; Johari Daha, F. Activity estimation in radioimmunotherapy using magnetic nanoparticles. Chin. J. Cancer Res. 2015, 27, 203–208. [Google Scholar] [PubMed]
- Bushman, J.; Vaughan, A.; Sheihet, L.; Zhang, Z.; Costache, M.; Kohn, J. Functionalized nanospheres for targeted delivery of paclitaxel. J. Control. Release 2013, 171, 315–321. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; Aerts, A.; Xavier, C.; Vaneycken, I.; Devoogdt, N.; Gijs, M.; Impens, N.; Baatout, S.; Ponsard, B.; Muyldermans, S.; et al. Development of 177Lu-nanobodies for radioimmunotherapy of her2-positive breast cancer: Evaluation of different bifunctional chelators. Contrast Media Mol. Imaging 2012, 7, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Xiong, C.Y.; Gruettner, C.; DeNardo, G.L.; DeNardo, S.J. Development of multivalent radioimmunonanoparticles for cancer imaging and therapy. Cancer Biother. Radiopharm. 2008, 23, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhian, K.; Aswath, N.; Thiruppathy, M.; Thirugnanamurthy, S. Boron neutron capture therapy—A literature review. J. Clin. Diagn. Res. 2016, 10, ZE01–ZE04. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.R.; Sahebkar, A.; Salehi, R.; Nahand, J.S.; Karimi, E.; Jaafari, M.R.; Mirzaei, H. Boron neutron capture therapy: Moving toward targeted cancer therapy. J. Cancer Res. Ther. 2016, 12, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Luderer, M.J.; de la Puente, P.; Azab, A.K. Advancements in tumor targeting strategies for boron neutron capture therapy. Pharm. Res. 2015, 32, 2824–2836. [Google Scholar] [CrossRef] [PubMed]
- Alberti, D.; Protti, N.; Franck, M.; Stefania, R.; Bortolussi, S.; Altieri, S.; Deagostino, A.; Aime, S.; Geninatti Crich, S. Theranostic nanoparticles loaded with imaging probes and rubrocurcumin for combined cancer therapy by folate receptor targeting. Chem. Med. Chem. 2017, 12, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.; Grunewald, C.; Blaickner, M.; Ziegner, M.; Schutz, C.; Iffland, D.; Hampel, G.; Nawroth, T.; Langguth, P. Cellular uptake and in vitro antitumor efficacy of composite liposomes for neutron capture therapy. Radiat. Oncol. 2015, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Heber, E.M.; Hawthorne, M.F.; Kueffer, P.J.; Garabalino, M.A.; Thorp, S.I.; Pozzi, E.C.; Monti Hughes, A.; Maitz, C.A.; Jalisatgi, S.S.; Nigg, D.W.; et al. Therapeutic efficacy of boron neutron capture therapy mediated by boron-rich liposomes for oral cancer in the hamster cheek pouch model. Proc. Natl. Acad. Sci. USA 2014, 111, 16077–16081. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, S.; Miyoshi, T.; Koganei, H.; El-Zaria, M.E.; Vinas, C.; Suzuki, M.; Ono, K.; Nakamura, H. Spermidinium closo-dodecaborate-encapsulating liposomes as efficient boron delivery vehicles for neutron capture therapy. Chem. Commun. (Camb.) 2014, 50, 12325–12328. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kawamoto, S.; Bernardo, M.; Brechbiel, M.W.; Knopp, M.V.; Choyke, P.L. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: Comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J. Control. Release 2006, 111, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Oyewumi, M.O.; Liu, S.; Moscow, J.A.; Mumper, R.J. Specific association of thiamine-coated gadolinium nanoparticles with human breast cancer cells expressing thiamine transporters. Bioconjug. Chem. 2003, 14, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.P.; Gazeau, F.; Wilhelm, C. Intracellular heating of living cells through neel relaxation of magnetic nanoparticles. Eur. Biophys. J. 2008, 37, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lei, S.L.; Lu, J.H.; He, Y.; Chen, Z.W.; Ren, L.; Zhou, X. Potential use of sers-assisted theranostic strategy based on Fe3O4/Au cluster/shell nanocomposites for bio-detection, MRI, and magnetic hyperthermia. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Stocke, N.A.; Sethi, P.; Jyoti, A.; Chan, R.; Arnold, S.M.; Hilt, J.Z.; Upreti, M. Toxicity evaluation of magnetic hyperthermia induced by remote actuation of magnetic nanoparticles in 3d micrometastasic tumor tissue analogs for triple negative breast cancer. Biomaterials 2017, 120, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Moorthy, M.S.; Manivasagan, P.; Bharathiraja, S.; Oh, J. Magnetic hyperthermia and pH-responsive effective drug delivery to the sub-cellular level of human breast cancer cells by modified coFe2O4 nanoparticles. Biochimie 2017, 133, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Niu, X.; Ma, K.; Huang, P.; Grothe, J.; Kaskel, S.; Zhu, Y. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small 2017, 13, 1602225. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dahring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.; Forman, D.; Bray, F. Globocan 2012 v1.0, Cancer Incidence and Mortality Worldwide: Iarc Cancerbase No. 11 [Internet]; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M.; Couper, M.P.; Zikmund-Fisher, B.J.; Levin, C.A.; McNaughton-Collins, M.; Helitzer, D.L.; VanHoewyk, J.; Barry, M.J. Prostate cancer screening decisions: Results from the national survey of medical decisions (decisions study). Arch. Intern. Med. 2009, 169, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Bill-Axelson, A.; Bratt, O. Words of wisdom. Re: Screening and prostate cancer mortality: Results of the european randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Eur. Urol. 2015, 67, 175. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. Eau guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Karnes, R.J.; Nguyen, P.L.; Ziegenfuss, J.Y.; Thompson, R.H.; Han, L.C.; Shah, N.D.; Smaldone, M.C.; Gross, C.P.; Frank, I.; et al. A national survey of radiation oncologists and urologists on recommendations of prostate-specific antigen screening for prostate cancer. BJU Int. 2014, 113, E106–E111. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S. Prostate cancer screening: Highlights from the 29th european association of urology congress Stockholm, Sweden, april 11–15, 2014. Rev. Urol. 2014, 16, 90–91. [Google Scholar] [PubMed]
- Schroder, F.H. Screening for prostate cancer: Current status of ERSPC and screening-related issues. Recent Results Cancer Res. 2014, 202, 47–51. [Google Scholar] [PubMed]
- Schroder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Maattanen, L.; Lilja, H.; et al. Screening and prostate cancer mortality: Results of the european randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef]
- Schroder, F.H. Erspc, plco studies and critique of cochrane review 2013. Recent Results Cancer Res. 2014, 202, 59–63. [Google Scholar] [PubMed]
- Vallabhajosula, S.; Nikolopoulou, A.; Jhanwar, Y.S.; Kaur, G.; Tagawa, S.T.; Nanus, D.M.; Bander, N.H.; Goldsmith, S.J. Radioimmunotherapy of metastatic prostate cancer with 177Lu-DOTA-HUJ591 anti prostate specific membrane antigen specific monoclonal antibody. Curr. Radiopharm. 2016, 9, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Van Rij, C.M.; Frielink, C.; Goldenberg, D.M.; Sharkey, R.M.; Lutje, S.; McBride, W.J.; Oyen, W.J.; Boerman, O.C. Pretargeted radioimmunotherapy of prostate cancer with an anti-TROP-2×-anti-HSG bispecific antibody and a 177Lu-labeled peptide. Cancer Biother. Radiopharm. 2014, 29, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Lin, W.Y.; Chen, M.C.; Lin, T.; Chao, C.H.; Hsu, F.N.; Lin, E.; Huang, C.Y.; Luo, T.Y.; Lin, H. Inhibitory effects of rhenium-188-labeled herceptin on prostate cancer cell growth: A possible radioimmunotherapy to prostate carcinoma. Int. J. Radiat. Biol. 2013, 89, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Van Rij, C.M.; Lutje, S.; Frielink, C.; Sharkey, R.M.; Goldenberg, D.M.; Franssen, G.M.; McBride, W.J.; Rossi, E.A.; Oyen, W.J.; Boerman, O.C. Pretargeted immuno-pet and radioimmunotherapy of prostate cancer with an anti-TROP-2× anti-HSG bispecific antibody. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Gao, D.W.; Feng, J.; He, J.; Seo, Y.; Tedesco, J.; Wolodzko, J.G.; Hasegawa, B.H.; Franc, B.L. Biodistributions of 177Lu- and 111In-labeled 7e11 antibodies to prostate-specific membrane antigen in xenograft model of prostate cancer and potential use of 111In-7e11 as a pre-therapeutic agent for 177Lu-7e11 radioimmunotherapy. Mol. Imaging Biol. 2009, 11, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.P.; Lee, S.T.; Lee, F.T.; Smyth, F.E.; Davis, I.D.; Brechbiel, M.W.; Scott, A.M. Therapeutic efficacy of 177Lu-CHX-A”-DTPA-hu3S193 radioimmunotherapy in prostate cancer is enhanced by EGFR inhibition or docetaxel chemotherapy. Prostate 2009, 69, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Morris, M.J.; Wills, E.A.; Schwartz, L.H.; Gonen, M.; Scher, H.I.; Larson, S.M.; Divgi, C.R. Antibody mass escalation study in patients with castration-resistant prostate cancer using 111In-j591: Lesion detectability and dosimetric projections for 90y radioimmunotherapy. J. Nucl. Med. 2008, 49, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Inoue, K.; Abe, M.; Nearman, J.; Baranowska-Kortylewicz, J. Pdgfrbeta and HIF-1α inhibition with imatinib and radioimmunotherapy of experimental prostate cancer. Cancer Biol. Ther. 2007, 6, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Schneider, D.; Biroc, S.L.; Parry, R.; Alicke, B.; Toy, P.; Xuan, J.A.; Sakamoto, C.; Wada, K.; Schulze, M.; et al. Targeting tomoregulin for radioimmunotherapy of prostate cancer. Cancer Res. 2005, 65, 2846–2853. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Goldsmith, S.J.; Kostakoglu, L.; Milowsky, M.I.; Nanus, D.M.; Bander, N.H. Radioimmunotherapy of prostate cancer using 90y- and 177Lu-labeled j591 monoclonal antibodies: Effect of multiple treatments on myelotoxicity. Clin. Cancer Res. 2005, 11, 7195s–7200s. [Google Scholar] [CrossRef] [PubMed]
- Richman, C.M.; Denardo, S.J.; O’Donnell, R.T.; Yuan, A.; Shen, S.; Goldstein, D.S.; Tuscano, J.M.; Wun, T.; Chew, H.K.; Lara, P.N.; et al. High-dose radioimmunotherapy combined with fixed, low-dose paclitaxel in metastatic prostate and breast cancer by using a muc-1 monoclonal antibody, m170, linked to indium-111/yttrium-90 via a cathepsin cleavable linker with cyclosporine to prevent human anti-mouse antibody. Clin. Cancer Res. 2005, 11, 5920–5927. [Google Scholar] [PubMed]
- DeNardo, S.J.; Richman, C.M.; Albrecht, H.; Burke, P.A.; Natarajan, A.; Yuan, A.; Gregg, J.P.; O’Donnell, R.T.; DeNardo, G.L. Enhancement of the therapeutic index: From nonmyeloablative and myeloablative toward pretargeted radioimmunotherapy for metastatic prostate cancer. Clin. Cancer Res. 2005, 11, 7187s–7194s. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Smith-Jones, P.M.; Navarro, V.; Goldsmith, S.J.; Bander, N.H. Radioimmunotherapy of prostate cancer in human xenografts using monoclonal antibodies specific to prostate specific membrane antigen (PSMA): Studies in nude mice. Prostate 2004, 58, 145–155. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, S.J.; DeNardo, G.L.; Yuan, A.; Richman, C.M.; O’Donnell, R.T.; Lara, P.N.; Kukis, D.L.; Natarajan, A.; Lamborn, K.R.; Jacobs, F.; et al. Enhanced therapeutic index of radioimmunotherapy (RIT) in prostate cancer patients: Comparison of radiation dosimetry for 1,4,7,10-tetraazacyclododecane-n,n′,n″,n‴-tetraacetic acid (DOTA)-peptide versus 2IT-DOTA monoclonal antibody linkage for rit. Clin. Cancer Res. 2003, 9, 3938S–3944S. [Google Scholar] [PubMed]
- O’Donnell, R.T.; DeNardo, S.J.; Miers, L.A.; Lamborn, K.R.; Kukis, D.L.; DeNardo, G.L.; Meyers, F.J. Combined modality radioimmunotherapy for human prostate cancer xenografts with taxanes and 90yttrium-DOTA-peptide-CHL6. Prostate 2002, 50, 27–37. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.T.; DeNardo, S.J.; Yuan, A.; Shen, S.; Richman, C.M.; Lara, P.N.; Griffith, I.J.; Goldstein, D.S.; Kukis, D.L.; Martinez, G.S.; et al. Radioimmunotherapy with 111In/90Y-2IT-BAD-m170 for metastatic prostate cancer. Clin. Cancer Res. 2001, 7, 1561–1568. [Google Scholar] [PubMed]
- McDevitt, M.R.; Barendswaard, E.; Ma, D.; Lai, L.; Curcio, M.J.; Sgouros, G.; Ballangrud, A.M.; Yang, W.H.; Finn, R.D.; Pellegrini, V.; et al. An alpha-particle emitting antibody ([213bi]j591) for radioimmunotherapy of prostate cancer. Cancer Res. 2000, 60, 6095–6100. [Google Scholar] [PubMed]
- Rydh, A.; Riklund-Ahlstrom, K.; Widmark, A.; Bergh, A.; Johansson, L.; Tavelin, B.; Nilsson, S.; Stigbrand, T.; Damber, J.E.; Hietala, S.O. Radioimmunotherapy of du-145 tumours in nude mice—A pilot study with e4, a novel monoclonal antibody against prostate cancer. Acta Oncol. 1999, 38, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Briers, E.; van den Bergh, R.; Bolla, M.; van Casteren, N.; Cornford, P.; Culine, S.; Joniau, S.; Lam, T.; et al. Guidelines on Prostate Cancer; European Association of Urology: Arnhem, the Netherlands, 2015; pp. 1–137. [Google Scholar]
- NCCN. NCCN guidelines on Prostate Cancer (version 1.2015). Available online: http://www.nccn.org (accessed on 9 March 2016).
- Edge, S.; Byrd, D.; Compton, C. AJCC Cancer Staging; Springer: New York, NY, USA, 2010. [Google Scholar]
- Powles, T.; Murray, I.; Brock, C.; Oliver, T.; Avril, N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur. Urol. 2007, 51, 1511–1520, discussion 1520–1511. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Pinto, P.A.; Choyke, P.L. Imaging techniques for prostate cancer: Implications for focal therapy. Nat. Rev. Urol. 2009, 6, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Blaufox, M.D. Nuclear medicine studies of the prostate, testes, and bladder. Semin. Nucl. Med. 2006, 36, 51–72. [Google Scholar] [CrossRef] [PubMed]
- DeGrado, T.R.; Coleman, R.E.; Wang, S.; Baldwin, S.W.; Orr, M.D.; Robertson, C.N.; Polascik, T.J.; Price, D.T. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: Initial findings in prostate cancer. Cancer Res. 2001, 61, 110–117. [Google Scholar] [PubMed]
- Watanabe, H.; Kanematsu, M.; Kondo, H.; Kako, N.; Yamamoto, N.; Yamada, T.; Goshima, S.; Hoshi, H.; Bae, K.T. Preoperative detection of prostate cancer: A comparison with 11C-choline pet, 18F-fluorodeoxyglucose pet and MR imaging. J. Magn. Reson. Imaging 2010, 31, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Akhurst, T.; Osman, I.; Nunez, R.; Macapinlac, H.; Siedlecki, K.; Verbel, D.; Schwartz, L.; Larson, S.M.; Scher, H.I. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology 2002, 59, 913–918. [Google Scholar] [CrossRef]
- Schoder, H.; Herrmann, K.; Gonen, M.; Hricak, H.; Eberhard, S.; Scardino, P.; Scher, H.I.; Larson, S.M. 2-18Ffluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin. Cancer Res. 2005, 11, 4761–4769. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.H.; Bouchelouche, K.; Hoilund-Carlsen, P.F.; Petersen, H.; Gerke, O.; Steffansen, S.I.; Marcussen, N.; Svolgaard, N.; Vach, W.; Geertsen, U.; et al. 18F fluoromethylcholine (FCH) positron emission tomography/computed tomography (PET/CT) for lymph node staging of prostate cancer: A prospective study of 210 patients. BJU Int. 2012, 110, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Brogsitter, C.; Zophel, K.; Kotzerke, J. 18F-choline, 11C-choline and 11C-acetate PET/CT: Comparative analysis for imaging prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Haider, M.; Ghai, S.; Sreeharsha, B. The expanding role of MRI in prostate cancer. AJR Am. J. Roentgenol. 2013, 201, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Souvatzoglou, M.; Eiber, M.; Takei, T.; Furst, S.; Maurer, T.; Gaertner, F.; Geinitz, H.; Drzezga, A.; Ziegler, S.; Nekolla, S.G.; et al. Comparison of integrated whole-body11C-choline pet/mr with PET/CT in patients with prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1486–1499. [Google Scholar] [CrossRef] [PubMed]
- Wetter, A.; Lipponer, C.; Nensa, F.; Heusch, P.; Rubben, H.; Altenbernd, J.C.; Schlosser, T.; Bockisch, A.; Poppel, T.; Lauenstein, T.; et al. Evaluation of the pet component of simultaneous 18F-choline PET/MRI in prostate cancer: Comparison with18F-choline PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Takei, T.; Souvatzoglou, M.; Mayerhoefer, M.E.; Furst, S.; Gaertner, F.C.; Loeffelbein, D.J.; Rummeny, E.J.; Ziegler, S.I.; Schwaiger, M.; et al. Performance of whole-body integrated 18F -FDG PET/MR in comparison to PET/CT for evaluation of malignant bone lesions. J. Nucl. Med. 2014, 55, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Van der Kwast, T.H.; Roobol, M.J. Defining the threshold for significant versus insignificant prostate cancer. Nat. Rev. Urol. 2013, 10, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Roobol, M.J.; Kattan, M.W.; van der Kwast, T.H.; de Koning, H.J.; Schroder, F.H. Prediction of indolent prostate cancer: Validation and updating of a prognostic nomogram. J. Urol. 2007, 177, 107–112, discussion 112. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Walsh, P.C.; Carmichael, M.; Brendler, C.B. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1C) prostate cancer. JAMA 1994, 271, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Chan, D.W.; Sokoll, L.J.; Walsh, P.C.; Cox, J.L.; Rittenhouse, H.; Wolfert, R.; Carter, H.B. Nonpalpable stageT1C prostate cancer: Prediction of insignificant disease using free/total prostate specific antigen levels and needle biopsy findings. J. Urol. 1998, 160, 2407–2411. [Google Scholar] [CrossRef]
- Bastian, P.J.; Carter, B.H.; Bjartell, A.; Seitz, M.; Stanislaus, P.; Montorsi, F.; Stief, C.G.; Schroder, F. Insignificant prostate cancer and active surveillance: From definition to clinical implications. Eur. Urol. 2009, 55, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Oon, S.F.; Watson, R.W.; O’Leary, J.J.; Fitzpatrick, J.M. Epstein criteria for insignificant prostate cancer. BJU Int. 2011, 108, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. Eau guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Briers, J.; van den Berg, D. Guidelines on prostate cancer. Eur. Urol. 2015. [Google Scholar]
- Pepe, P.; Fraggetta, F.; Galia, A.; Panella, P.; Pennisi, M.; Colecchia, M.; Aragona, F. Preoperative findings, pathological stage PSA recurrence in men with prostate cancer incidentally detected at radical cystectomy: Our experience in 242 cases. Int. Urol. Nephrol. 2014, 46, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Picchio, M.; Messa, C.; Landoni, C.; Gianolli, L.; Sironi, S.; Brioschi, M.; Matarrese, M.; Matei, D.V.; De Cobelli, F.; Del Maschio, A.; et al. Value of 11C-choline-positron emission tomography for re-staging prostate cancer: A comparison with 18F-fluorodeoxyglucose-positron emission tomography. J. Urol. 2003, 169, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Uphoff, J.; Henke, R.P.; Wawroschek, F. First results of 11C-choline PET/CT-guided secondary lymph node surgery in patients with psa failure and single lymph node recurrence after radical retropubic prostatectomy. Urol. Int. 2010, 84, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Fuccio, C.; Castellucci, P.; Schiavina, R.; Santi, I.; Allegri, V.; Pettinato, V.; Boschi, S.; Martorana, G.; Al-Nahhas, A.; Rubello, D.; et al. Role of 11C-choline PET/CT in the restaging of prostate cancer patients showing a single lesion on bone scintigraphy. Ann. Nucl. Med. 2010, 24, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Cimitan, M.; Bortolus, R.; Morassut, S.; Canzonieri, V.; Garbeglio, A.; Baresic, T.; Borsatti, E.; Drigo, A.; Trovo, M.G. 18F-fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at psa relapse: Experience in 100 consecutive patients. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Flamini, E.; Mercatali, L.; Sacanna, E.; Serra, P.; Amadori, D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer 2010, 116, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Helyar, V.; Mohan, H.K.; Barwick, T.; Livieratos, L.; Gnanasegaran, G.; Clarke, S.E.; Fogelman, I. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Herranz-Blanco, B.; Shahbazi, M.A.; Correia, A.R.; Balasubramanian, V.; Kohout, T.; Hirvonen, J.; Santos, H.A. PH-switch nanoprecipitation of polymeric nanoparticles for multimodal cancer targeting and intracellular triggered delivery of doxorubicin. Adv. Healthc. Mater. 2016, 5, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Vu-Quang, H.; Vinding, M.S.; Nielsen, T.; Ullisch, M.G.; Nielsen, N.C.; Kjems, J. Theranostic tumor targeted nanoparticles combining drug delivery with dual near infrared and f magnetic resonance imaging modalities. Nanomedicine 2016, 12, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ma, H.; Hou, B.; Zhao, H.; Ji, Y.; Jiang, R.; Hu, X.; Lu, X.; Zhang, L.; Tang, Y.; et al. Engineering lysosome-targeting bodipy nanoparticles for photoacoustic imaging and photodynamic therapy under near-infrared light. ACS Appl. Mater. Interfaces 2016, 8, 12039–12047. [Google Scholar] [CrossRef] [PubMed]
- Detappe, A.; Lux, F.; Tillement, O. Pushing radiation therapy limitations with theranostic nanoparticles. Nanomedicine (Lond.) 2016, 11, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Huang, W.C.; Lin, Y.W.; Yu, T.W.; Chen, H.H.; Lin, S.C.; Chiang, W.H.; Chiu, H.C. Active tumor permeation and uptake of surface charge-switchable theranostic nanoparticles for imaging-guided photothermal/chemo combinatorial therapy. Theranostics 2016, 6, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, F.; Tian, R.; Zhang, L.; Fu, G.; Yang, L.; Zhu, L. Nanotubes-embedded indocyanine green-hyaluronic acid nanoparticles for photoacoustic-imaging-guided phototherapy. ACS Appl. Mater. Interfaces 2016, 8, 5608–5617. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, B.; Mashinchian, O.; Mousavi, T.; Karimi, R.; Kharrazi, S.; Amani, A. Harnessing the cancer radiation therapy by lanthanide-doped zinc oxide based theranostic nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Gurka, M.K.; Pender, D.; Chuong, P.; Fouts, B.L.; Sobelov, A.; McNally, M.W.; Mezera, M.; Woo, S.Y.; McNally, L.R. Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. J. Control. Release 2016, 231, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Andreou, C.; Pal, S.; Rotter, L.; Yang, J.; Kircher, M.F. Molecular imaging in nanotechnology and theranostics. Mol. Imaging Biol. 2017, 19, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Shi, X.; Shen, M. Aqueous-phase synthesis of iron oxide nanoparticles and composites for cancer diagnosis and therapy. Adv. Colloid Interface Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.S.; Yang, X.; Zhou, Z.; Yang, Z.; Jacobson, O.; Liu, Y.; Yang, A.; Niu, G.; Song, J.; Yang, H.H.; et al. Yolk-shell nanostructure: An ideal architecture to achieve harmonious integration of magnetic-plasmonic hybrid theranostic platform. Adv. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, F.; Yu, F.; Sun, W.; Song, M.; Chen, X.; Zhang, X.; Sun, X. Croconaine nanoparticles with enhanced tumor accumulation for multimodality cancer theranostics. Biomaterials 2017, 129, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 dye encapsulated in CRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 12217–12226. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.; Crake, C.; Lee, J.Y.; Carugo, D.; Beguin, E.; Khrapitchev, A.A.; Browning, R.J.; Sibson, N.; Stride, E. A versatile method for the preparation of particle-loaded microbubbles for multimodality imaging and targeted drug delivery. Drug Deliv. Transl. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharreddy, P.; Huang, C.; Busi, S.; Rajkumari, J.; Tai, M.H.; Liu, G. Green synthesized nanomaterials as theranostic platforms for cancer treatment: Principles, challenges and the road ahead. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiao, R.; Tang, N.; Lu, Z.; Wang, H.; Zhang, Z.; Xue, X.; Huang, Z.; Zhang, S.; Zhang, G.; et al. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials 2017, 127, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ansari, C.; Tikhomirov, G.A.; Hong, S.H.; Falconer, R.A.; Loadman, P.M.; Gill, J.H.; Castaneda, R.; Hazard, F.K.; Tong, L.; Lenkov, O.D.; et al. Development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy. Small 2014, 10, 417, 566–575. [Google Scholar] [CrossRef]
- Kiess, A.P.; Banerjee, S.R.; Mease, R.C.; Rowe, S.P.; Rao, A.; Foss, C.A.; Chen, Y.; Yang, X.; Cho, S.Y.; Nimmagadda, S.; et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 241–268. [Google Scholar] [PubMed]
- Hou, Y.; Qiao, R.; Fang, F.; Wang, X.; Dong, C.; Liu, K.; Liu, C.; Liu, Z.; Lei, H.; Wang, F.; et al. Nagdf4 nanoparticle-based molecular probes for magnetic resonance imaging of intraperitoneal tumor xenografts in vivo. ACS Nano 2013, 7, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Franci, C.; Zhou, J.; Jiang, Z.; Modrusan, Z.; Good, Z.; Jackson, E.; Kouros-Mehr, H. Biomarkers of residual disease, disseminated tumor cells, and metastases in the MMTV-PYMT breast cancer model. PLoS ONE 2013, 8, e58183. [Google Scholar] [CrossRef] [PubMed]
- FDA. Fda Drug Safety Communication: Fda Strengthens Warnings and Changes Prescribing Instructions to Decrease the Risk of Serious Allergic Reactions with Anemia Drug Feraheme (Ferumoxytol). Available online: http://www.fda.gov/Drugs/DrugSafety/ucm440138.htm (accessed on 17 June 2016).



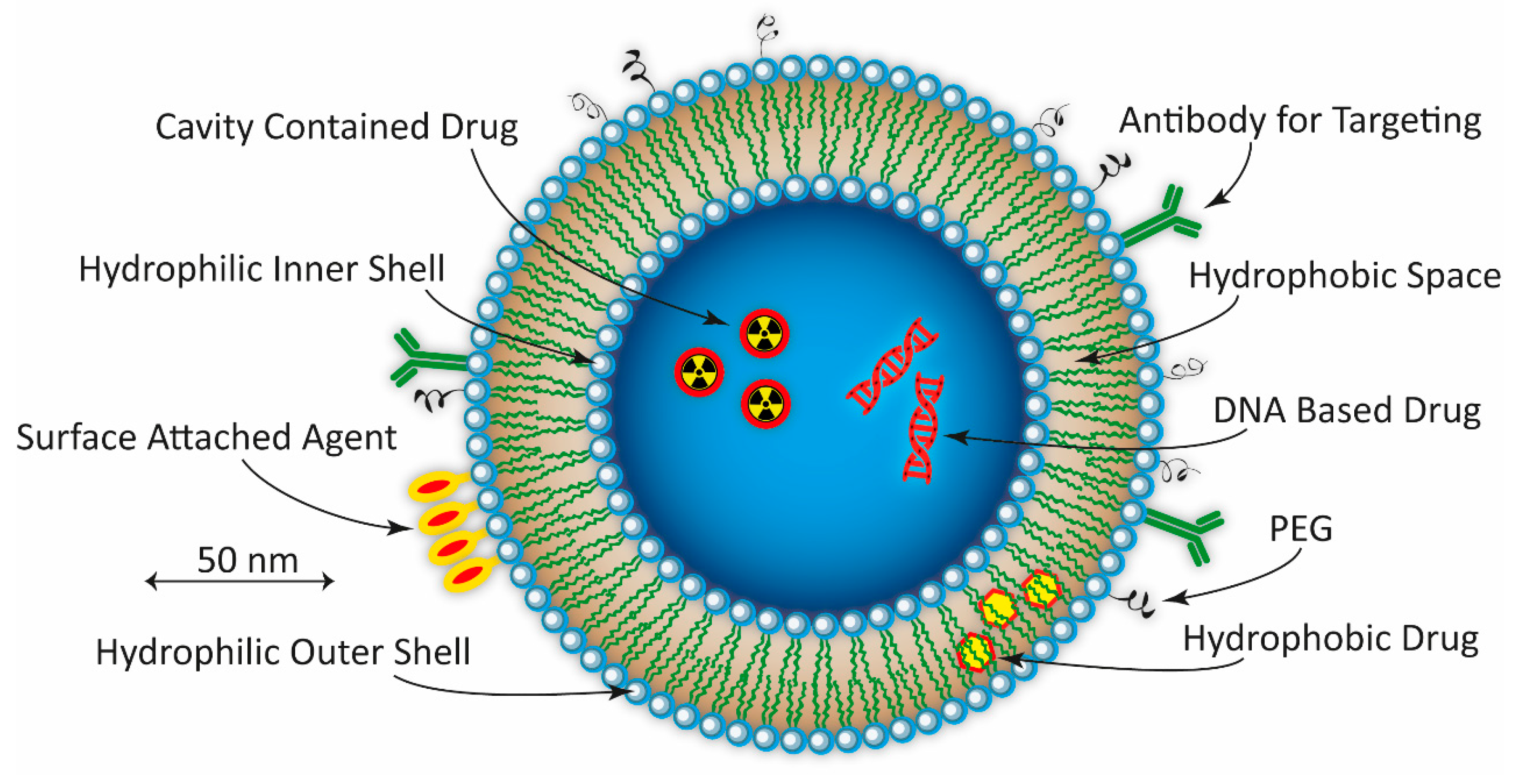


| Cancer | Specific Indication | Nanoparticle | Drug | Product | Phase | Company |
|---|---|---|---|---|---|---|
| PCa | US enhancement imaging | Phospholipid microbubbles | - | SonoVue® | Phase III | Bracco Diagnostics Inc. |
| Metastatic CRPC | Polymeric | Docetaxel | BIND-014 (Accurin™) | Phase II | Pfizer Inc/BIND Therapeutics Inc. | |
| Hormone refractive PCa | Albumin-based NP | Paclitaxel | Abraxane® | Phase II | Celgene Corporation | |
| Androgen independant PCa | Liposome | Doxorubicin | Doxil® | Phase II | Janssen Pharmaceuticals Inc. | |
| - | Iron NP | Iron NP | Magnablate | Phase I/0 | University College London Hospitals | |
| BC | Metastatic BC | Liposome | Doxorubicin | Myocet™ | Approved | Teva UK Ltd. |
| Metastatic BC | Albumin-based NP | Paclitaxel | Abraxane™ | Approved | Celgene Corporation | |
| - | Micelle (polymeric) | Paclitaxel | Genexol-PM™ | Approved | Samyang Pharmaceuticals Co. | |
| US enhancement imaging | Lipid microspheres | - | Definity® | Approved | Lantheus Medical Imaging Inc. | |
| Metastatic BC | Liposome | Paclitaxel | LIPUSU® | Phase IV | Nanjing Luye Sike Pharmaceutical Co., Ltd. | |
| Metastatic BC | Polymeric conjugate | Irinotecan | NKTR-102 | Phase III | Nektar Therapeutics | |
| Refractory chest wall BC | Liposome | Doxorubicin | ThermoDox™ | Phase II/I | Celsion Co. | |
| - | Micelle (polymeric) | Paclitaxel | NK105 | Phase III | NanoCarrier Co., Ltd. | |
| Advanced/metastatic BC | HER2-liposome | Doxorubicin | -MM-302 | Phase III/II/I | Merrimack Pharmaceuticals Inc. | |
| Tripple-negative metastatic BC | Liposome | Doxorubicin | Doxil® | Phase II | Janssen Pharmaceuticals Inc. | |
| - | Liposome | Doxorubicin | Caelyx® | Phase II | Janssen-Cilag Ltd. | |
| Tripple-negative metastatic BC | Liposome | Paclitaxel | EndoTAG-1 | Phase II | MediGene AG | |
| Metastatic | Liposome | Paclitaxel | LEP-ETU | Phase II | Insys Therapeutics Inc. | |
| Advanced recurrent/metastatic BC | Liposome | Mitoxantrone | Mitoxantrone HCL Liposome | Phase II | CSPC ZhongQi Pharmaceutical Technology | |
| Metastatic BC | Micelle (polymeric) | Paclitaxel | Nanoxel™ | Phase I | Samyang Pharmaceuticals Co. | |
| US enhancement imaging | Phospholipid microbubbles | - | SonoVue® | Pilot | Bracco Imaging Inc. | |
| Metastatic/locally recurrent | Micelle (polymeric) | Paclitaxel | Cynviloq | Not provided | Sorrento Therapeutics Inc. | |
| Solid cancers | Advanced tumors | Liposome | Curcumin | Lipocurc | Phase II/I | SignPath Pharma Inc. |
| Advanced tumors | Micelle | Gemcitabine/Cisplatin | NC-6004 Nanoplatin | Phase II/I | NanoCarrier Co., Ltd. | |
| Advanced tumors | Cyclodextrin-based NP | Docetaxel | CRLX301 | Phase II/I | Cerulean Pharma Inc. | |
| - | Micelle (polymeric) | Docetaxel/Taxotere | Docetaxel-PM DOPNP201 | Phase I | Samyang Pharmaceuticals Co. | |
| - | Micelle | Docetaxel | CriPec | Phase I | Cristal Therapeutics | |
| Advanced tumors | Micelle (polymeric) | Cisplatin/paclitaxel | NC-4016 DACH-Platin micelle | Phase I | NanoCarrier Co Ltd/MD Anderson Cancer Center | |
| - | Liposome | Eribulin mesylate | Halaven E7389-LF | Phase I | Eisai Co., Ltd. | |
| - | Liposome | Mitomycin-C | Promitil® | Phase I | LipoMedix Pharmaceuticals Inc. | |
| Refractory/recurrent tumors | Liposome | Topotecan, docetaxel, CP | SGT-53 | Phase I | SynerGene Therapeutics Inc. | |
| - | Liposome | RB94 plasmid DNA | SGT-94 | Phase I | SynerGene Therapeutics Inc. | |
| Advanced tumors | Liposome | 188Re-BMEDA | 188Re-BMEDA | Phase I | INER, Taiwan | |
| Advanced tumors | Albumin-based NP | Thiocolchicine | ABI-011 | Phase I | NantBioScience Inc. | |
| - | Lipid | DsiRNA | DCR-MYC | Phase I | Dicerna Pharmaceuticals Inc. | |
| Advanced recurrent tumors | Liposome | siRNA | siRNA-EphA2-DOPC | Phase I | MD Anderson Cancer Center | |
| Advanced/refractory tumors | Liposome | Cisplatin | LiPlaCis | Phase I | Oncology Venture/LiPlasome Pharma A/S | |
| Advanced solid tumors | Polymeric | AZD2811, Irinotecan | AZD2811 (Accurin™) | Phase I | AztraZeneca/BIND Therapeutics Inc. |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgqvist, J. Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications—Focus on Prostate and Breast Cancer. Int. J. Mol. Sci. 2017, 18, 1102. https://doi.org/10.3390/ijms18051102
Elgqvist J. Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications—Focus on Prostate and Breast Cancer. International Journal of Molecular Sciences. 2017; 18(5):1102. https://doi.org/10.3390/ijms18051102
Chicago/Turabian StyleElgqvist, Jörgen. 2017. "Nanoparticles as Theranostic Vehicles in Experimental and Clinical Applications—Focus on Prostate and Breast Cancer" International Journal of Molecular Sciences 18, no. 5: 1102. https://doi.org/10.3390/ijms18051102





