Natural Killer Cells Response to IL-2 Stimulation Is Distinct between Ascites with the Presence or Absence of Malignant Cells in Ovarian Cancer Patients
Abstract
:1. Introduction
2. Results
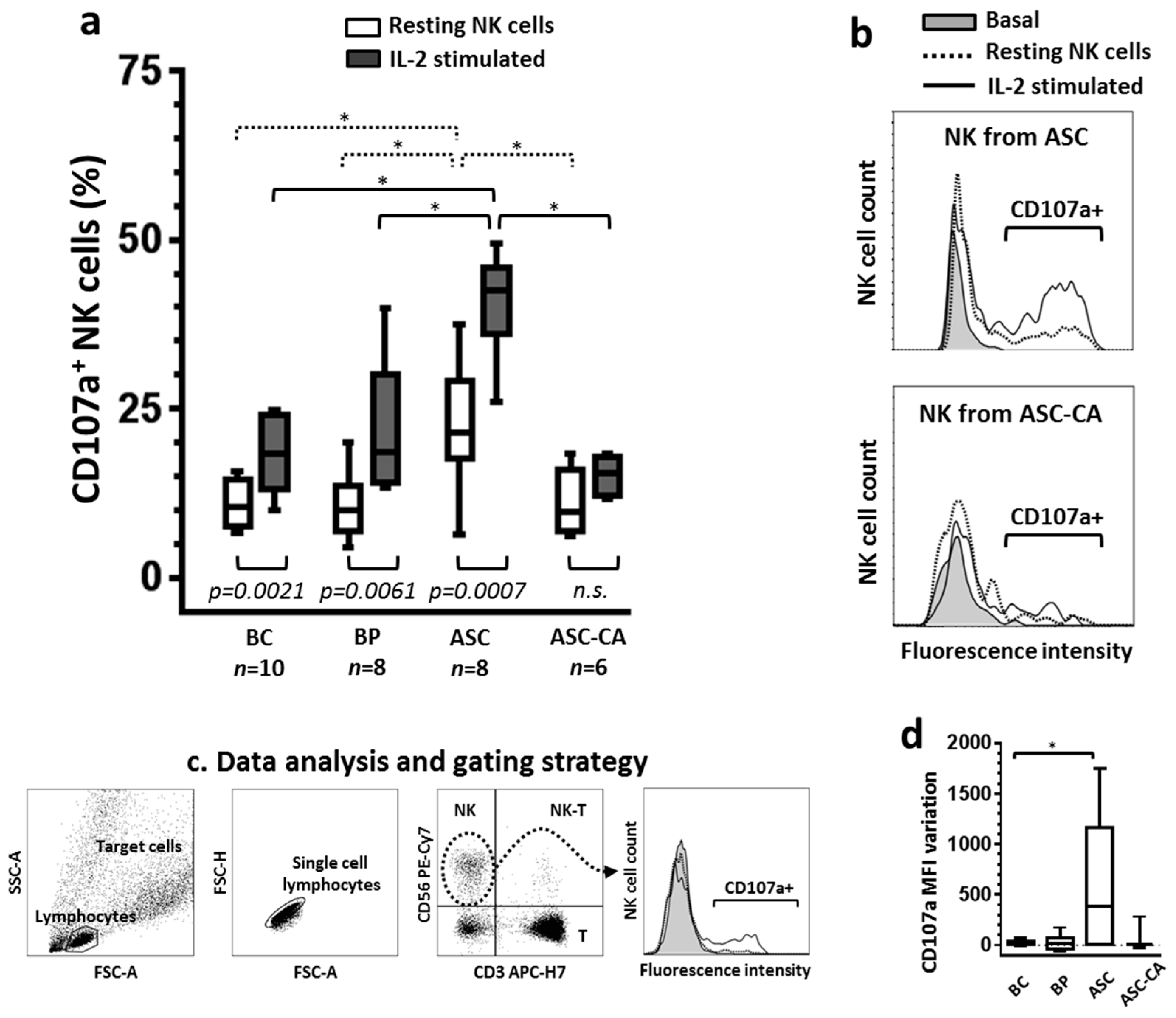
2.1. Degranulation of NK Cells and Their Response to IL-2
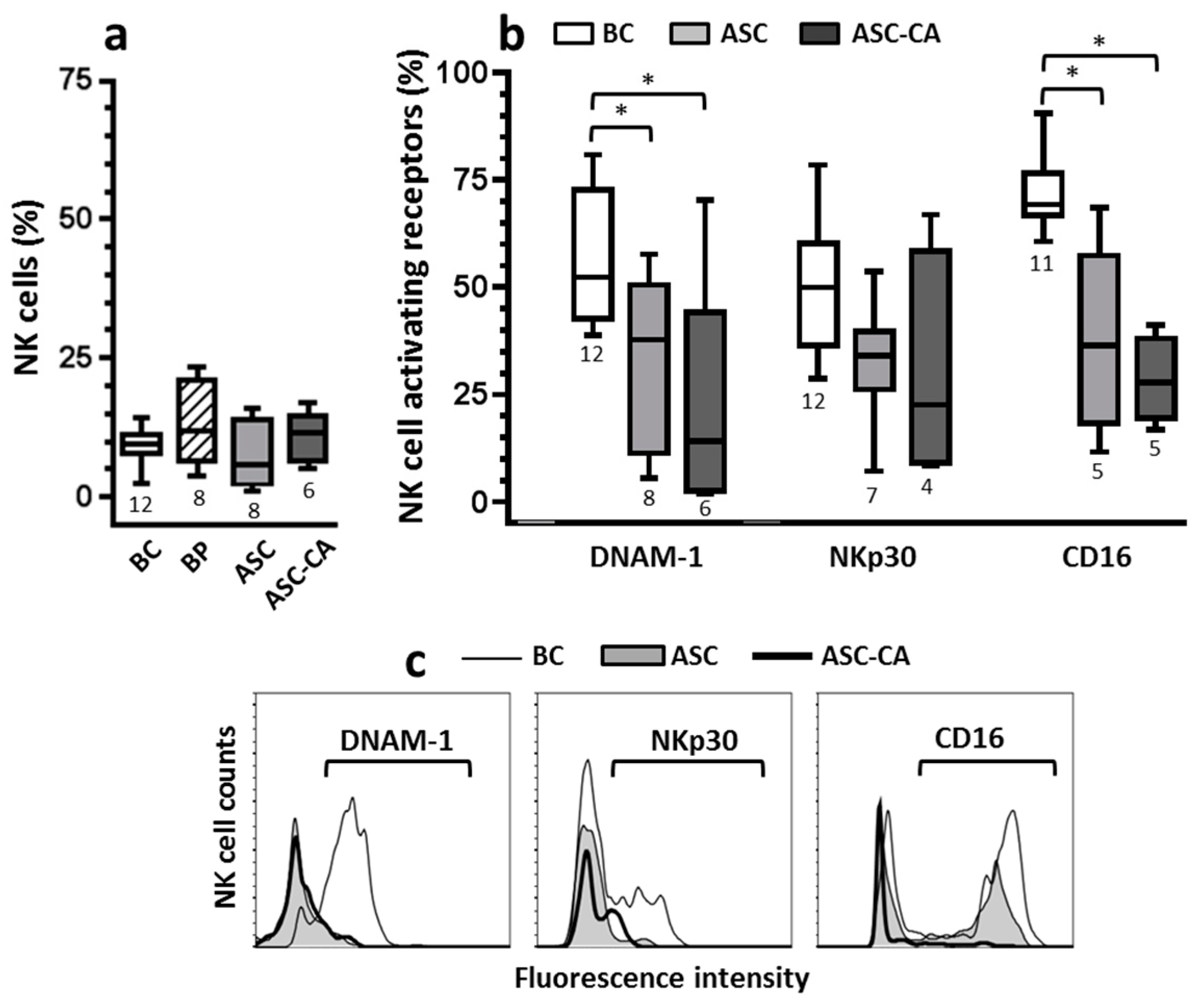
2.2. Expression of Activating Receptors on NK Cells
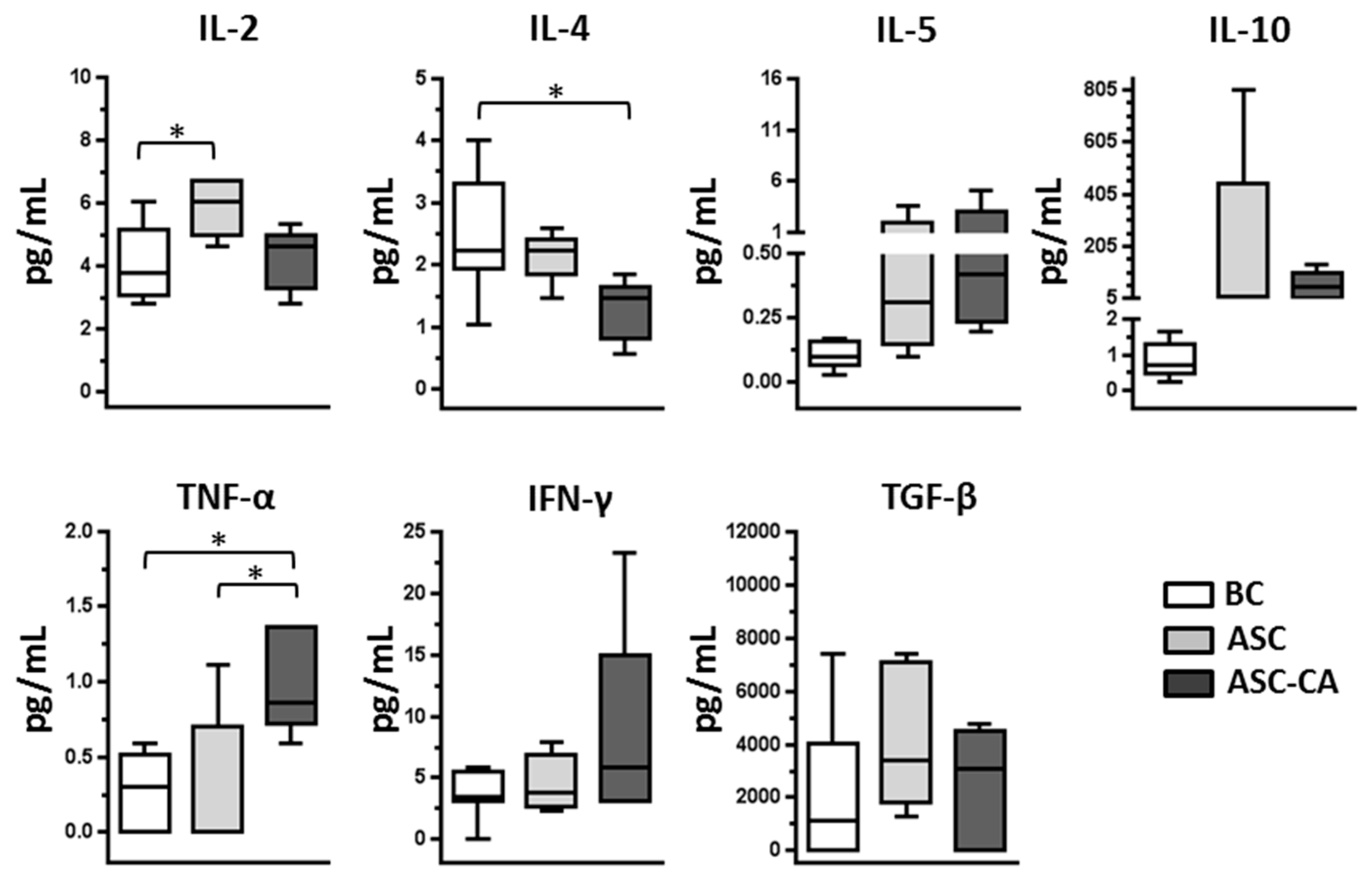
2.3. Cytokines Profile in Blood Plasma and Ascites Supernatant
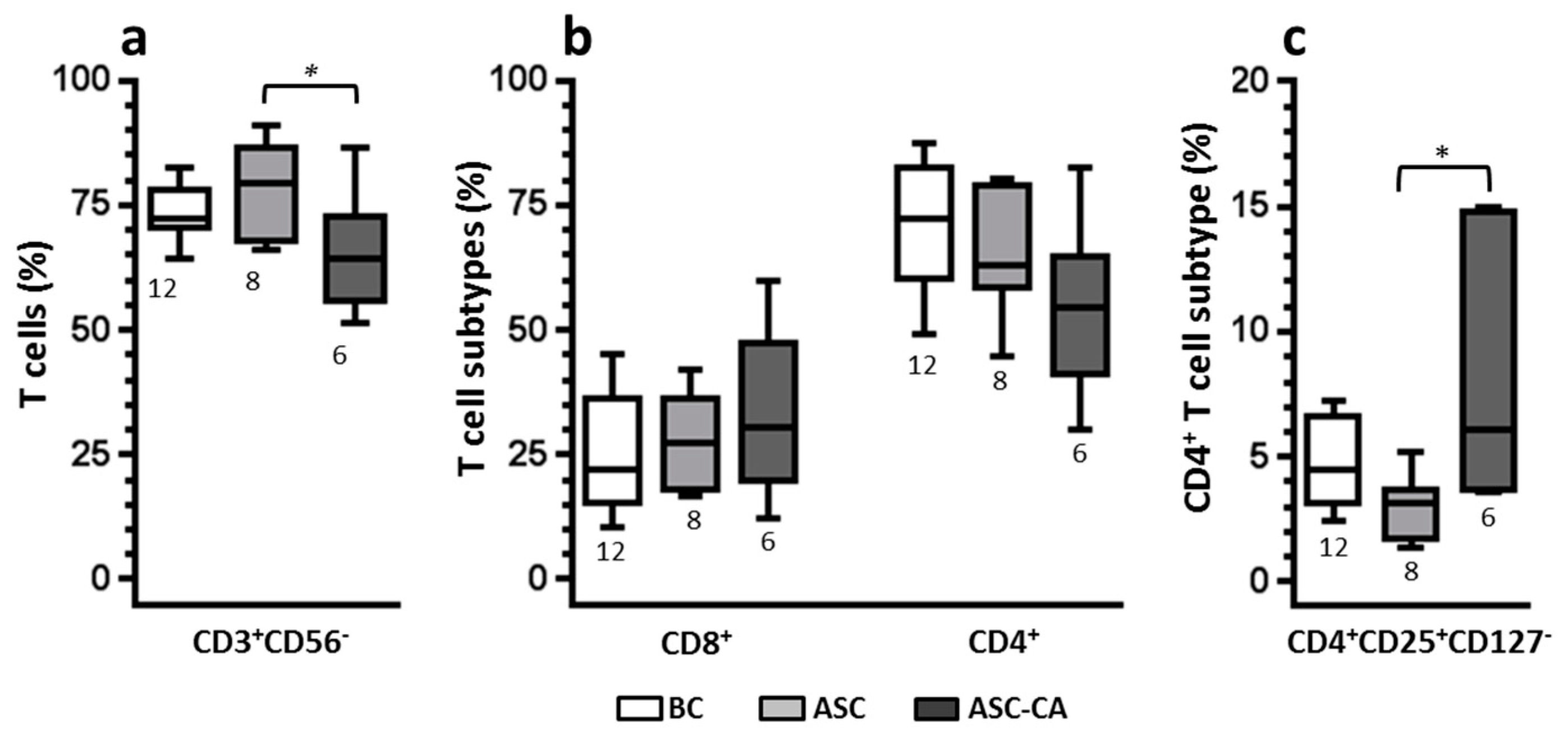
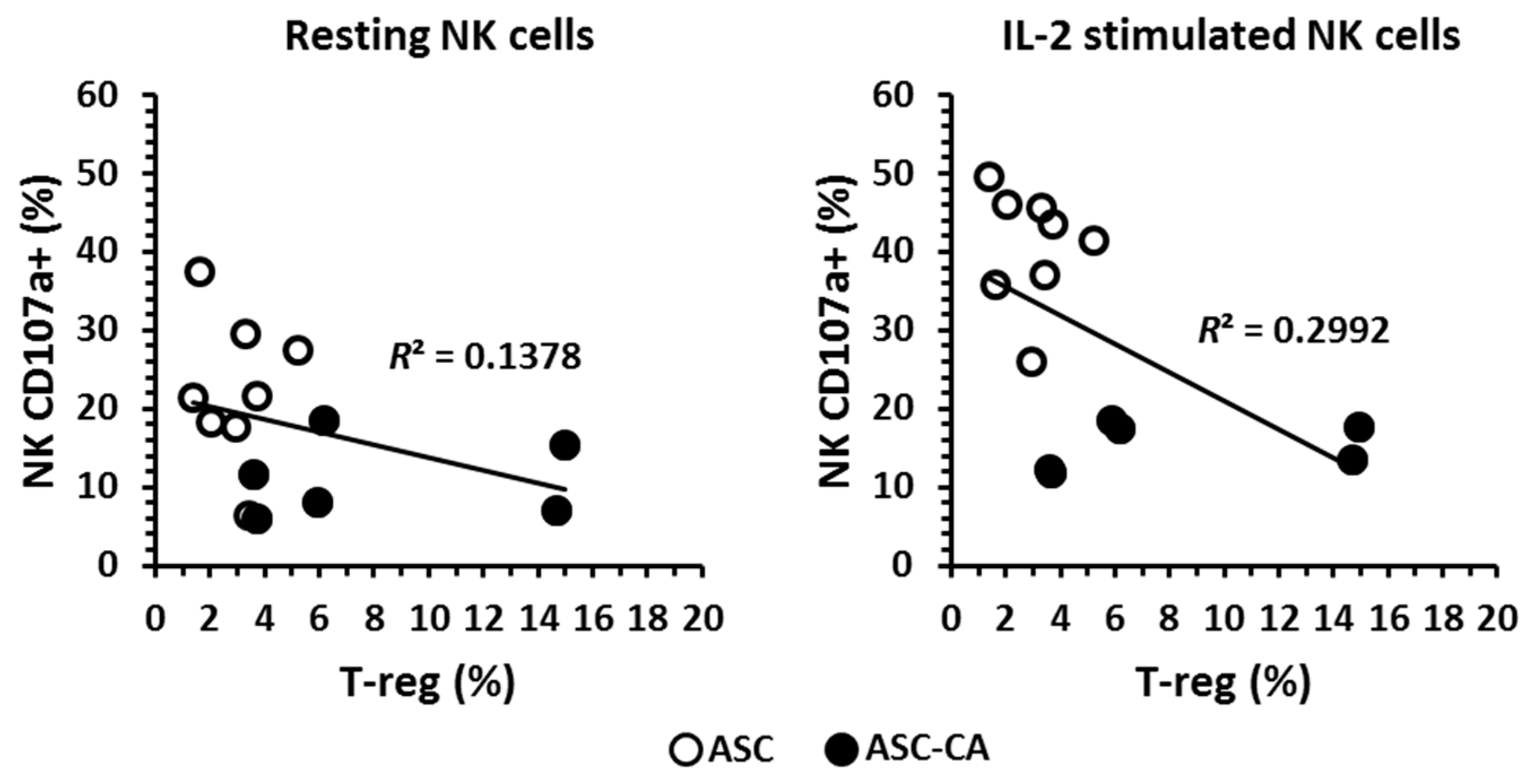
2.4. Phenotype of T Lymphocyte Subpopulations and T-Reg Correlation with NK Cell Function
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Resting and Overnight rhIL-2 Stimulated Effector Cells
4.3. K562 Cell Line Target Cells
4.4. NK Cell Degranulation Assay and Activating Receptor Phenotyping
4.5. Lymphocyte Phenotyping
4.6. Cytokines Profile
4.7. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cellular cytotoxicity |
| APC-Cy7 | Allophycocyanin-cyanine dye |
| ASC | EOC cell-free ascites |
| ASC-CA | Ascites of EOC patients with malignant cells |
| BC | Blood of control women |
| BP | Blood of patients with EOC |
| DNAM-1 | DNAX accessory molecule-1 |
| EOC | Epithelial ovarian cancer |
| EpCAM | Epithelial cell adhesion molecule |
| KIR | Killer Ig-like receptor |
| LILR-B1 | Leukocyte Ig-like receptor B1 |
| vMFI | Variation of mean fluorescence intensity |
| PE | Phycoerythrin dye |
| PE-Cy7 | Phycoerythrin-cyanine dye |
| TAL | Tumor-associated lymphocyte |
| T-reg | Regulatory T-lymphocyte |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.J.; Lan, C.Y.; Huang, Q.D.; Feng, Y.L.; Huang, Y.W.; Liu, J.H. Clinical significance of ascites in epithelial ovarian cancer. Neoplasma 2013, 60, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Naora, H.; Montell, D.J. Ovarian cancer metastasis: Integrating insights from disparate model organisms. Nat. Rev. Cancer 2005, 5, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.L.; Watson, S.A.; Steele, R.J. Malignant ascites. Br. J. Surg. 1996, 83, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.; Luwor, R.B.; Bilandzic, M.; Nazaretian, S.; Stenvers, K.; Pyman, J.; Zhu, H.; Thompson, E.W.; Quinn, M.A.; Findlay, J.K.; et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: Molecular phenotype of chemoresistant ovarian tumors. PLoS ONE 2012, 7, e46858. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Stenvers, K.L. Getting to know ovarian cancer ascites: Opportunities for targeted therapy-based translational research. Front. Oncol. 2013, 3, 256. [Google Scholar] [CrossRef] [PubMed]
- Gavalas, N.G.; Kardimou, A.; Dimopoulos, M.A.; Bamias, A. Immune response in ovarian cancer: How is the immune system involved in prognosis and therapy: Potential for treatment utilization. Clin. Dev. Immunol. 2010, 2010, 791603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, B.; Goode, E.L.; Kimberly, R.K.; Knutson, K.L.; DeRycke, M.S. The immune system in the pathogenesis of ovarian cancer. Crit. Rev. Immunol. 2013, 33, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Coukos, G.; Zou, L.; Xavier, A.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Ménard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-B-dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Teng, M.W.; Swann, J.; Kyparissoudis, K.; Godfrey, D.I.; Hayakawa, Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J. Immunol. 2006, 176, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Landskron, J.; Helland, O.; Torgersen, K.M.; Aandahl, E.M.; Gjertsen, B.T.; Bjorge, L.; Tasken, K. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol. Immunother. 2015, 64, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.Y.; Chu, C.S.; Goletz, T.J.; Schlienger, K.; Yeh, H.; Coukos, G.; Rubin, S.C.; Kaiser, L.R.; June, C.H. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001, 61, 4766–4772. [Google Scholar] [PubMed]
- Liyanage, U.K.; Moore, T.T.; Joo, H.G.; Tanaka, Y.; Herrmann, V.; Doherty, G.; Drebin, J.A.; Strasberg, S.M.; Eberlein, T.J.; Goedegebuure, P.S.; et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002, 169, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.; Kryczek, I.; Cheng, P.; Zou, W.; Curiel, T.J. Regulatory T cells in ovarian cancer: Biology and therapeutic potential. Am. J. Reprod. Immunol. 2005, 54, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Yigit, R.; Massuger, L.F.A.G.; Figdor, C.G.; Torensma, R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol. Oncol. 2010, 117, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, R.; Klein, E.; Pross, H.; Wigzell, H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur. J. Immunol. 1995, 5, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Herberman, R.B.; Nunn, M.E.; Holden, H.T.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer 1975, 16, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Caligiuri, M.A. Isolation and characterization of human natural killer cell subsets. Curr. Protoc. Immunol. 2004. [Google Scholar] [CrossRef]
- Arnon, T.I.; Markel, G.; Mandelboim, O. Tumor and viral recognition by natural killer cells receptors. Semin. Cancer Biol. 2006, 16, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, A.; Lanier, L.L. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 2001, 1, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Moretta, L.; Moretta, A. Unravelling natural killer cell function: Triggering and inhibitory human NK receptors. EMBO J. 2003, 23, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O. Negative signaling by inhibitory receptors: The NK cell paradigm. Immunol. Rev. 2008, 224, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Sivori, S.; Pende, D.; Bottino, C.; Marcenaro, E.; Pessino, A.; Biassoni, R.; Moretta, L.; Moretta, A. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol. 1999, 29, 1656–1666. [Google Scholar] [CrossRef]
- Coudert, J.D.; Zimmer, J.; Tomasello, E.; Cebecauer, M.; Colonna, M.; Vivier, E.; Held, W. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood 2005, 106, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, Y.M.; Meade, J.L.; Holmes, T.D.; McGonagle, D.; Mackie, S.L.; Morgan, A.W.; Cook, G.; Feyler, S.; Richards, S.J.; Davies, F.E. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007, 67, 8444–8449. [Google Scholar] [CrossRef] [PubMed]
- Kaifu, T.; Escaliere, B.; Gastinel, L.N.; Vivier, E.; Baratin, M. B7-H6/NKp30 interaction: A mechanism of alerting NK cells against tumors. Cell. Mol. Life Sci. 2011, 68, 3531–3539. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Lotzova, E.; Savary, C.A.; Freedman, R.; Bowen, J.M. Natural immunity against ovarian tumors. Comp. Immunol. Microbiol. Infect. Dis. 1986, 9, 269–275. [Google Scholar] [CrossRef]
- Lotzova, E.; Savary, C.A.; Freedman, R.S.; Edwards, C.L.; Wharton, J.T. Recombinant IL-2-activated NK cells mediate LAK activity against ovarian cancer. Int. J. Cancer 1988, 42, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Roszkowski, P.I.; Hyc, A.; Malejczyk, J. Natural killer cell activity in patients with ovarian tumors and uterine myomas. Eur. J. Gynaecol. Oncol. 1993, 14, 114–117. [Google Scholar] [PubMed]
- Lutgendorf, S.K.; Sood, A.K.; Anderson, B.; McGinn, S.; Maiseri, H.; Dao, M.; Sorosky, J.I.; de Geest, K.; Ritchie, J.; Lubaroff, D.M. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 2005, 23, 7105–7113. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.P.; Elstrand, M.B.; Holth, A.; Silins, I.; Berner, A.; Trope, C.G.; Davidson, B.; Risberg, B. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am. J. Clin. Pathol. 2006, 125, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kang, M.; Baek, J.H.; Lee, J.I.; Ha, S.Y. Clinical impact of tumor-infiltrating lymphocytes for survival in curatively resected stage IV colon cancer with isolated liver or lung metastasis. Ann. Surg. Oncol. 2013, 20, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Björkström, N.K.; Norell, H.; Bryceson, Y.; van Hall, T.; Baumann, B.C.; Hanson, M.; Schedvins, K.; Kiessling, R.; Ljunggren, H.G.; et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007, 67, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.F.; Petta, C.A.; Derchain, S.F.; Alici, E.; Guimarães, F. Up-regulation of DNAM-1 and NKp30, associated with improvement of NK cells activation after long-term culture of mononuclear cells from patients with ovarian neoplasia. Hum. Immunol. 2014, 75, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, M.; Norell, H.; Bryceson, Y.T.; Poschke, I.; Schedvins, K.; Ljunggren, H.G.; Kiessling, R.; Malmberg, K.J. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J. Immunol. 2009, 183, 4921–4930. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Tabellini, G.; Cantoni, C.; Patrizi, O.; Coltrini, D.; Rampinelli, F.; Matta, J.; Vivier, E.; Moretta, A.; Parolini, S.; et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma imune escape. Oncoimmunology 2015, 4, e1001224. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Mittal, D.; Stagg, J.; Smith, M.J. Targeting cancer-derived adenosine: New therapeutic approaches. Cancer Discov. 2014, 4, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Häusler, S.F.M.; del Barrio, I.M.; Strohschein, J.; Anoop Chandran, P.; Engel, J.B.; Hönig, A.; Ossadnik, M.; Horn, E.; Fischer, B.; Krockenberger, M.; et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol. Immunother. 2011, 60, 1405–1418. [Google Scholar]
- Knutson, K.L.; Karyampudi, L.; Lamichhane, P.; Preston, C. Targeted immune therapy of ovarian cancer. Cancer Metastasis Rev. 2015, 234, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Introna, M.; Mantovani, A. Natural killer cells in human solid tumors. Cancer Metast. Rev. 1983, 2, 337–350. [Google Scholar] [CrossRef]
- Pandey, V.; Oyer, J.L.; Igarashi, R.Y.; Gitto, S.B.; Copik, A.J.; Altomare, D.A. Anti-ovarian tumor response of donor peripheral blood mononuclear cells is due to infiltrating cytotoxic NK cells. Oncotarget 2016, 7, 7318–7328. [Google Scholar] [PubMed]
- Kulbe, H.; Thompson, R.; Wilson, J.L.; Robinson, S.; Hagemann, T.; Fatah, R.; Gould, D.; Ayhan, A.; Balkwill, F. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007, 67, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Y.; Chen, L.; Xu, B.; Wu, C.; Jiang, J. B7-H6 expression correlates with cancer progression and patient´s survival in human ovarian cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9428–9433. [Google Scholar] [PubMed]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Koutsoukou, V.; Terpos, E.; Tsiatas, M.L.; Liakos, C.; Tsitsilonis, O.; Rodolakis, A.; Voulgaris, Z.; Vlahos, G.; Papageorgiou, T.; et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells VEGF and TNFalpha in ascites from advanced ovarian cancer: Association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol. Oncol. 2008, 108, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Putnam, A.L.; Xu-yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; de St. Groth, B.F.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, K.; Kanegane, H.; Kamachi, Y.; Kobayashi, I.; Tsuge, I.; Imaizumi, M.; Sasahara, Y.; Hayakawa, A.; Nozu, K.; Iijima, K.; et al. Identification of FOXP3-negative regulatory T-like (CD4(+)CD25(+)CD127(low)) cells in patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Clin. Immunol. 2011, 141, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Classen, S.; Endl, E.; Kochanek, M.; Weihrauch, M.R.; Debey-Pascher, S.; Knolle, P.A.; Schultze, J.L. Comparative approach to define increased regulatory T cells in different cancer subtypes by combined assessment of CD127 and FOXP3. Clin. Dev. Immunol. 2011, 2011, 734036. [Google Scholar] [CrossRef] [PubMed]
- Boldt, A.; Kentouche, K.; Fricke, S.; Borte, S.; Kahlenberg, F.; Sack, U. Differences in FOXP3 and CD127 expression in Treg-like cells in patients with IPEX syndrome. Clin. Immunol. 2014, 153, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Grossman, W.J.; Verbsky, W.J.; Barchet, W.; Colonna, M.; Atkinson, J.P.; Ley, T.J. Human regulatory T cells can use the perforin pathway to cause autologous target cell death. Immunity 2004, 21, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Von Boehmer, H. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 2005, 6, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.M.; Thornton, A.M.; DiPaolo, R.J.; Shevach, E.M. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 2006, 107, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Friedline, R.H.; Brown, D.S.; Nguyen, H.; Kornfeld, H.; Lee, J.; Zhang, Y.; Appleby, M.; Der, S.D.; Kang, J.; Chambers, C.A. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 2009, 206, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Viel, S.; Marçais, A.; Guimarães, F.S.F.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E.; et al. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 2016, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; Fauriat, C.; Nunes, J.M.; Wood, S.M.; Björkström, N.K.; Long, E.O.; Ljunggren, H.G. Functional analysis of human NK cells by flow cytometry. Methods Mol. Biol. 2010, 612, 335–352. [Google Scholar] [PubMed]
| Patient’s Code | Age (Years) | Stage (FIGO) | Histological Classification | Ascites Cytology Results for EOC Cells |
|---|---|---|---|---|
| 21 | 57 | IIB | High-grade serous adenocarcinoma | Negative |
| 24 | 64 | IIIC | Low-grade serous adenocarcinoma | Positive |
| 30 | 63 | IIIC | High-grade serous adenocarcinoma | Negative |
| 40 | 51 | IIIC | High-grade serous adenocarcinoma | Positive |
| 55 | 56 | IIIC | High-grade serous adenocarcinoma | Positive |
| 61 | 58 | IIIC | High-grade serous adenocarcinoma | Negative |
| 70 | 50 | IIIC | Adenocarcinoma NOS | Negative |
| 73 | 71 | IV | High-grade serous adenocarcinoma | Negative |
| 74 | 43 | IIIC | Adenocarcinoma NOS | Positive |
| 75 | 64 | IIIC | High-grade serous adenocarcinoma | Negative |
| 82 | 45 | IIIC | High-grade serous adenocarcinoma | Positive |
| 86 | 70 | IIIC | Adenocarcinoma NOS | Negative |
| 89 | 79 | IV | High-grade serous adenocarcinoma | Positive |
| 93 | 38 | IIIC | Mucinous adenocarcinoma | Negative |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva, R.F.; Yoshida, A.; Cardozo, D.M.; Jales, R.M.; Paust, S.; Derchain, S.; Guimarães, F. Natural Killer Cells Response to IL-2 Stimulation Is Distinct between Ascites with the Presence or Absence of Malignant Cells in Ovarian Cancer Patients. Int. J. Mol. Sci. 2017, 18, 856. https://doi.org/10.3390/ijms18050856
Da Silva RF, Yoshida A, Cardozo DM, Jales RM, Paust S, Derchain S, Guimarães F. Natural Killer Cells Response to IL-2 Stimulation Is Distinct between Ascites with the Presence or Absence of Malignant Cells in Ovarian Cancer Patients. International Journal of Molecular Sciences. 2017; 18(5):856. https://doi.org/10.3390/ijms18050856
Chicago/Turabian StyleDa Silva, Rodrigo Fernandes, Adriana Yoshida, Daniela Maira Cardozo, Rodrigo Menezes Jales, Silke Paust, Sophie Derchain, and Fernando Guimarães. 2017. "Natural Killer Cells Response to IL-2 Stimulation Is Distinct between Ascites with the Presence or Absence of Malignant Cells in Ovarian Cancer Patients" International Journal of Molecular Sciences 18, no. 5: 856. https://doi.org/10.3390/ijms18050856






