Identification and Expression Profiling of the Auxin Response Factors in Dendrobium officinale under Abiotic Stresses
Abstract
:1. Introduction
2. Results
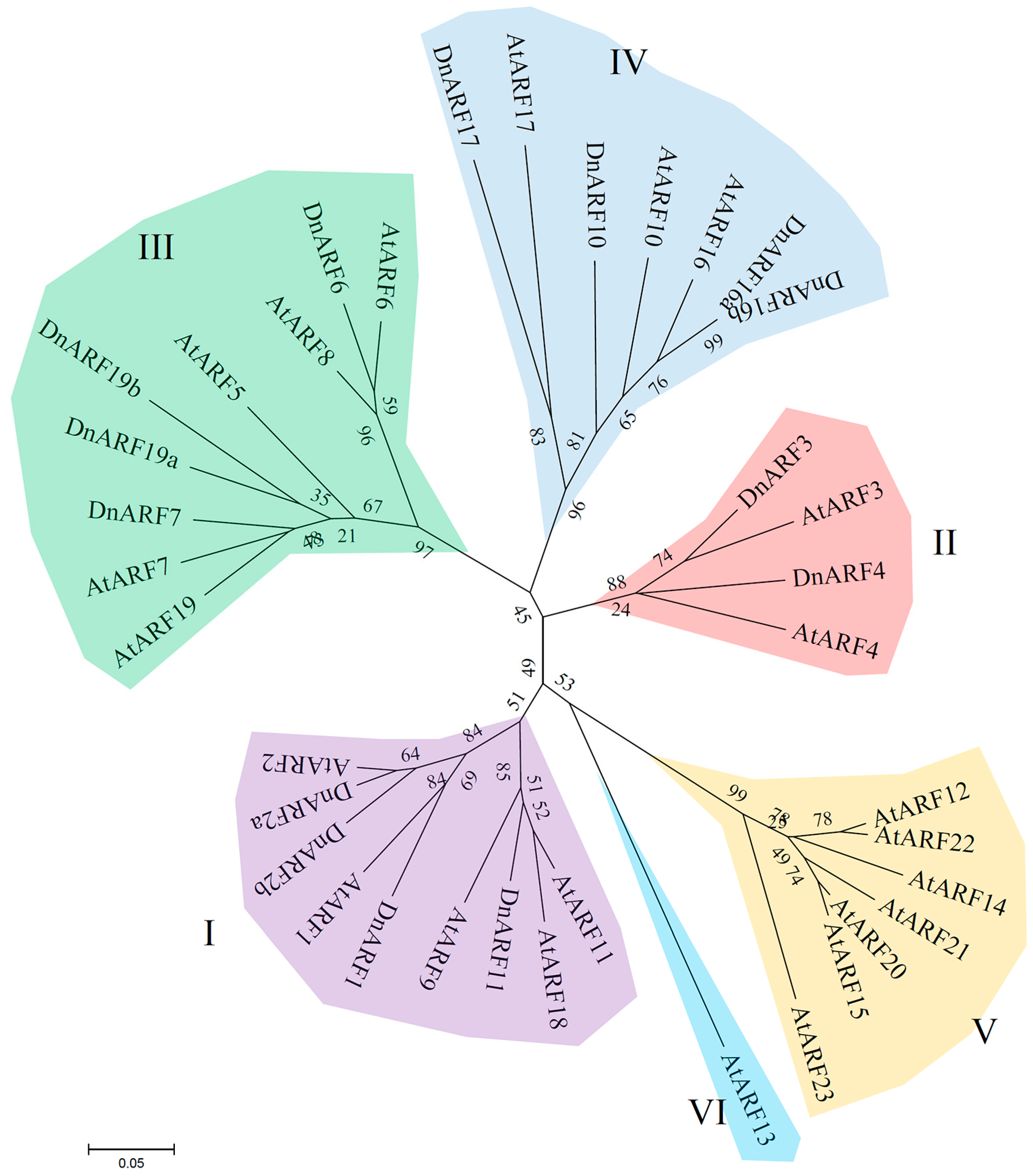
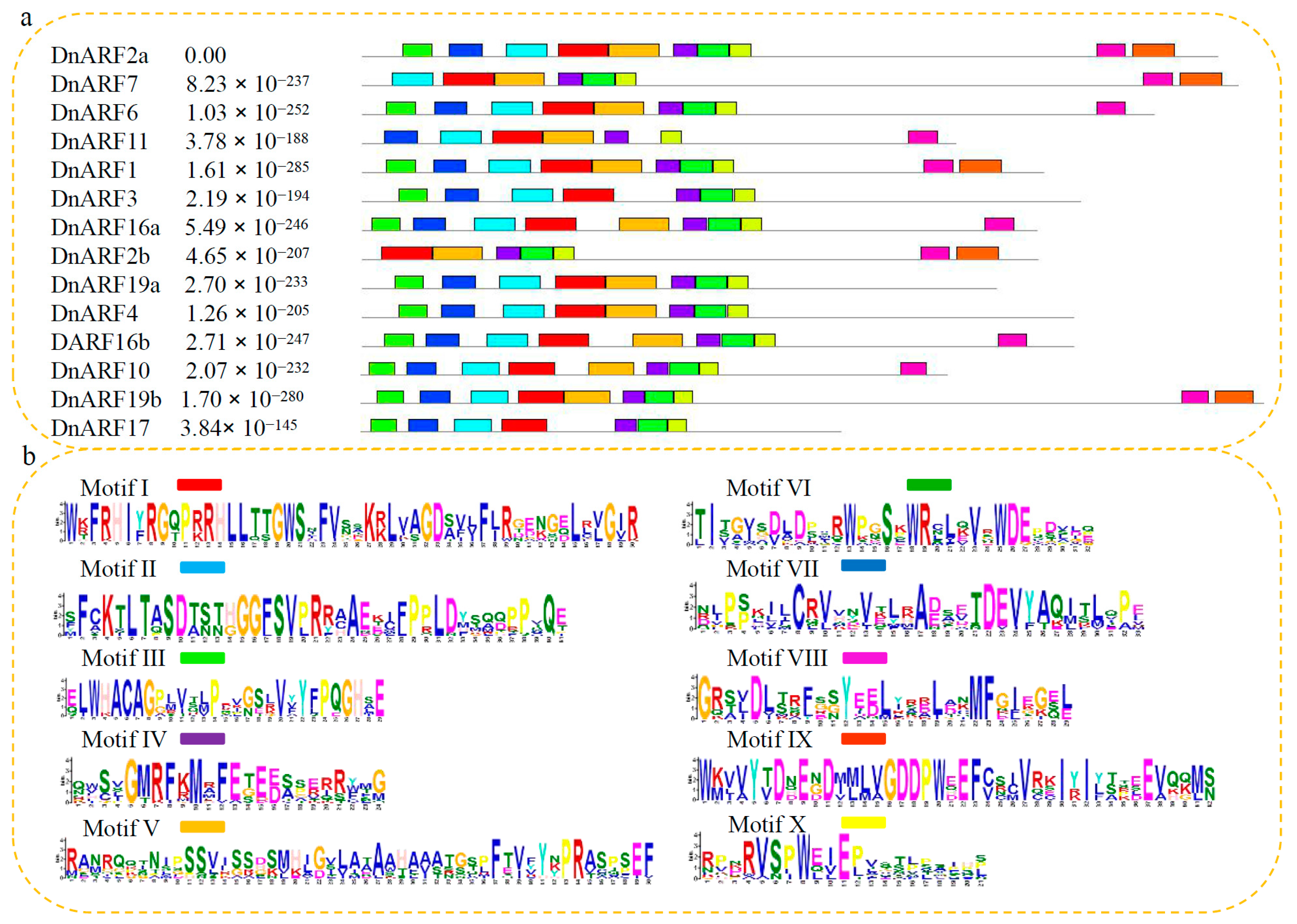
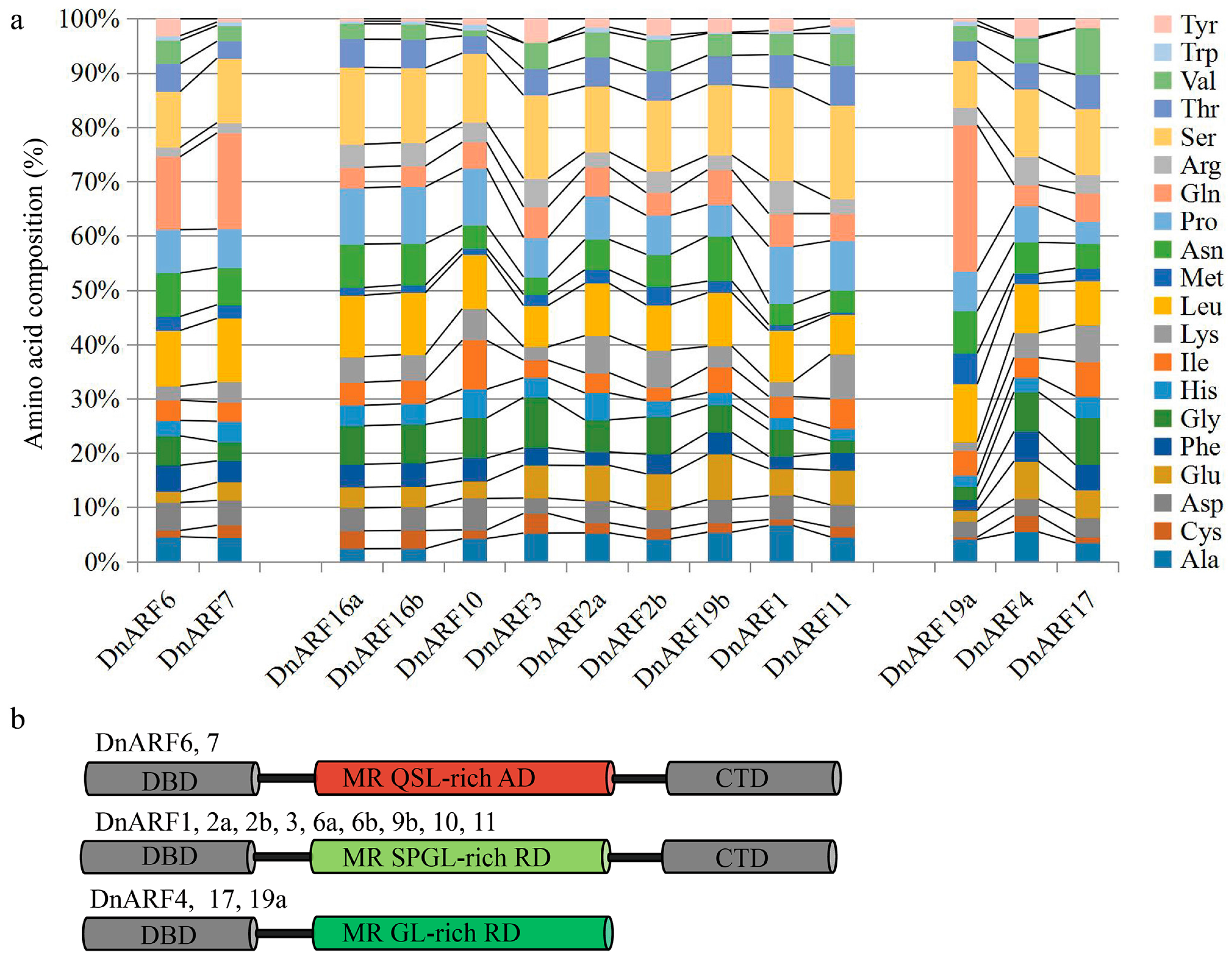
2.1. Isolation of 14 DnARF Genes from the D. officinale Plants
2.2. Isolation of 14 DnARF Genes from the D. officinale Plants
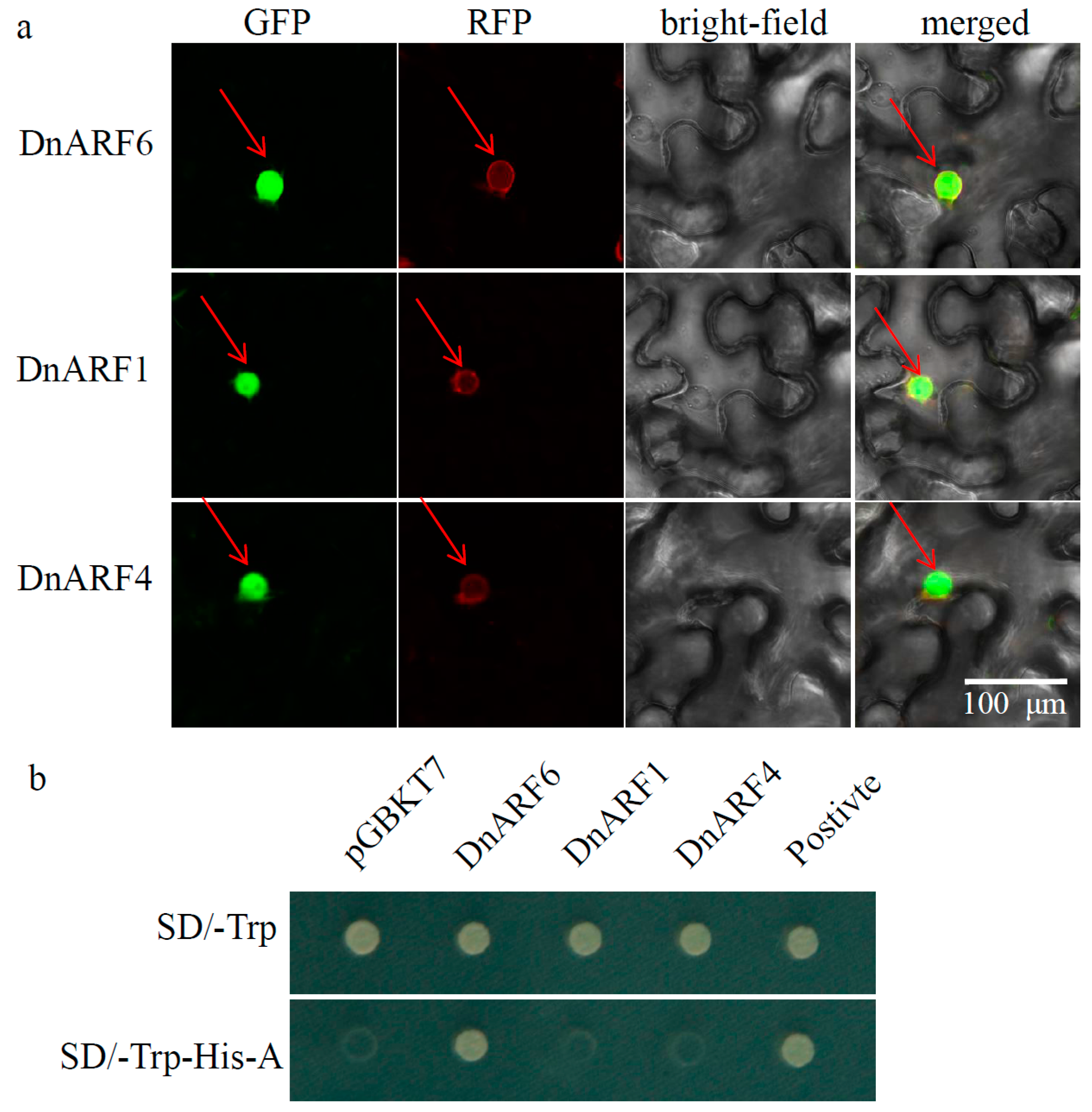
2.3. Subcellular Localization and Transcriptional Activation of Three Selected DnARFs
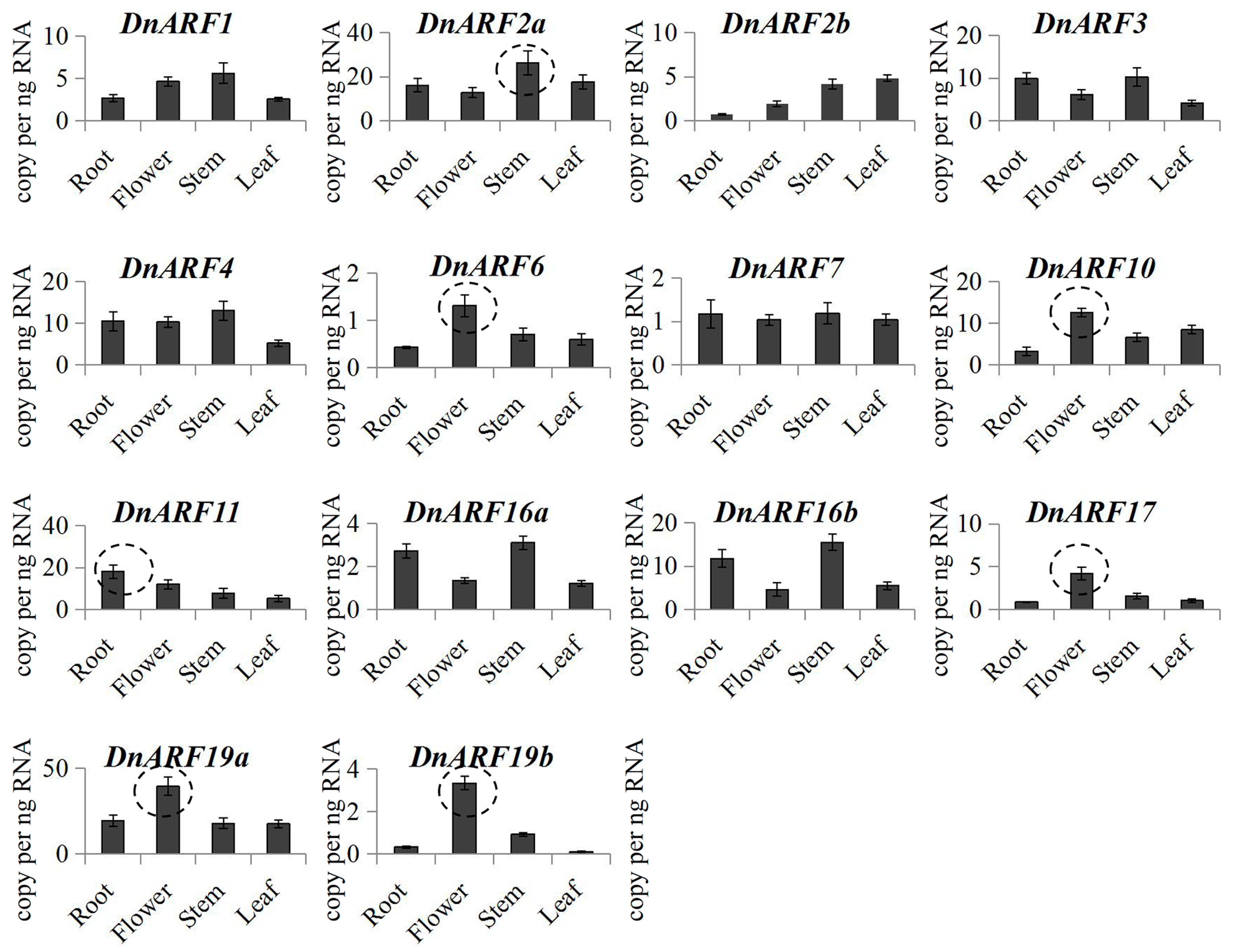
2.4. Expression Patterns for DnARF Genes in Different D. officinale Organs
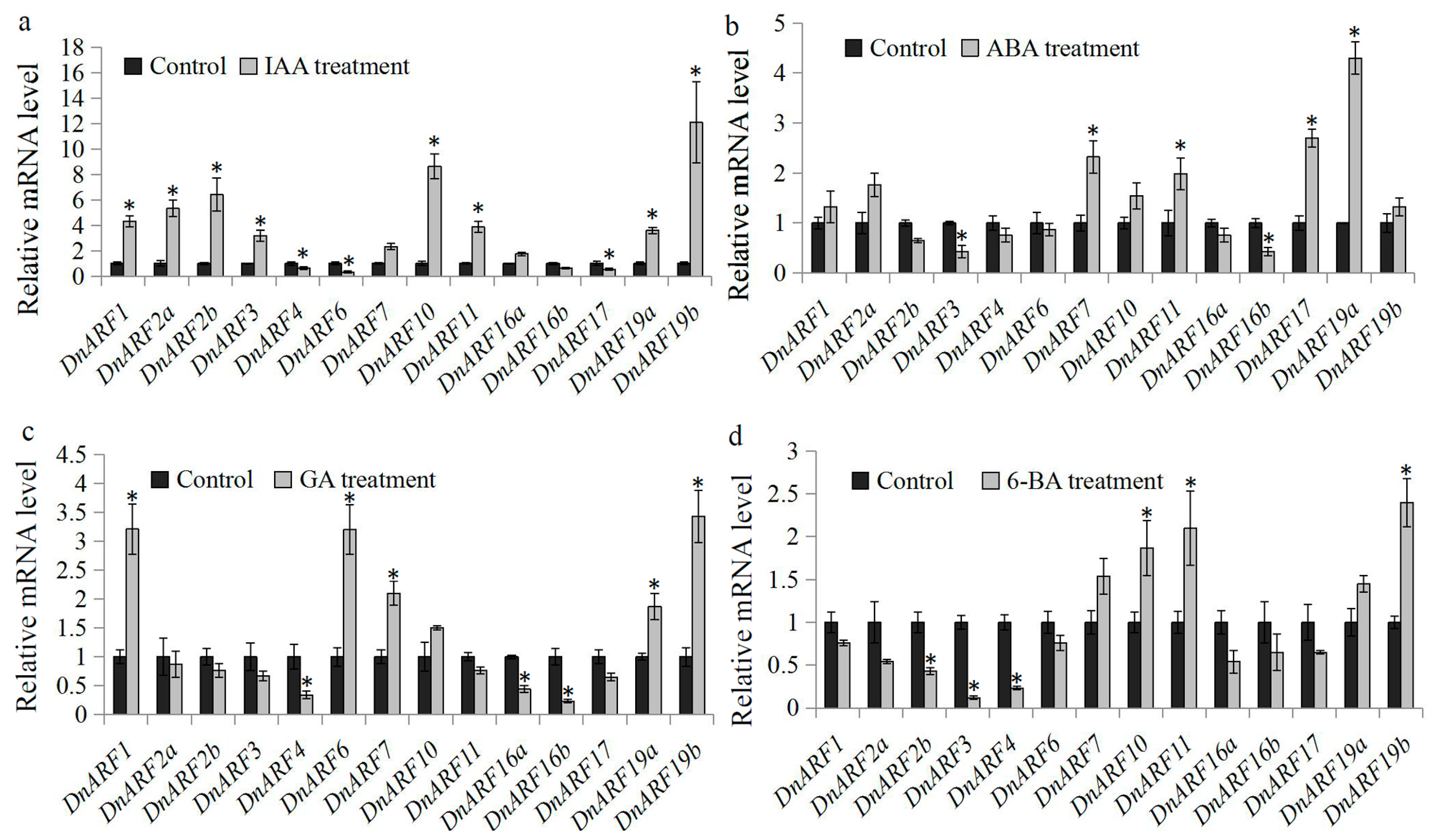
2.5. Expression of DnARF Genes in Response to Various Hormone Treatments
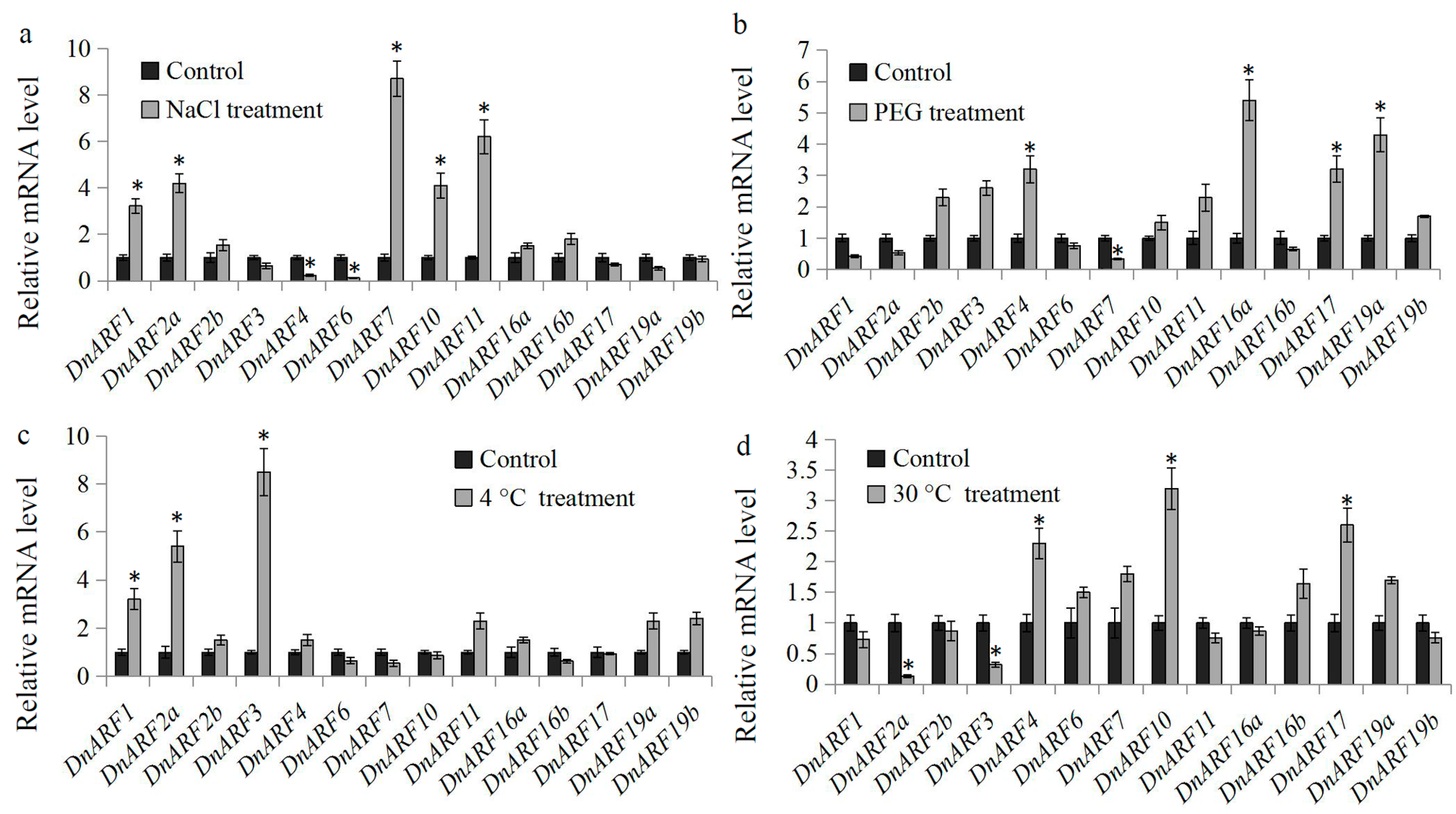
2.6. Expression of DnARF Genes in Response to Various Abiotic Stress Treatments
3. Discussion
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Treatments
4.2. Isolation and Identification of ARF Genes in D. officinale Plants
4.3. Sequence Analysis, Phylogenetic Tree Building, and Prediction of Amino Acid Contents
4.4. RNA Isolation and Quantitative RT-PCR
4.5. Subcellular Localization Analysis
4.6. Analysis of Transcriptional Activation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ARF | Auxin response factor |
| Aux/IAA | Auxin/Indole-3-acetic acid |
| GH3 | Gretchen Hagen3 |
| SAUR | Small Auxin Up RNA |
| AuxREs | auxin response elements |
| DBD | DNA-binding domain |
| AD | activation domain |
| RD | repression domain |
| CTD | C-terminal dimerization domain |
| MS | Murashige and Skoog |
| ABA | abscisic acid |
| 6-BA | 6-benzylaminopurine |
| GA | gibberellic acid |
| PEG | polyethylene glycol |
| HMM | hidden Markov model |
| MEME | Multiple Expectation Maximization for Motif Elicitation |
| GFP | green fluorescent protein |
| ORF | open reading frame |
References
- Ljung, K. Auxin metabolism and homeostasis during plant development. Development 2013, 140, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Krishnakumar, V.; Bidwell, S.; Rosen, B.; Chan, A.; Zhou, S.; Gentzbittel, L.; Childs, K.L.; Yandell, M.; Gundlach, H.; et al. An improved genome release (version mt4.0) for the model legume Medicago truncatula. BMC Genom. 2014, 15, 312. [Google Scholar] [CrossRef]
- Farzinebrahimi, R.; Taha, R.M.; Rashid, K.; Yaacob, J.S. The effect of various media and hormones via suspension culture on secondary metabolic activities of (Cape Jasmine) Gardenia jasminoides ellis. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Tie, S.; Sun, T.; Zhang, L.; Yang, Y.; Qi, J.; Yan, S.; Han, X.; Wang, H.; Shen, C. Genome-wide identification and expression profiling analysis of ZmPIN, ZmPILS, ZmLAX and ZmABCB auxin transporter gene families in maize (Zea mays L.) under various abiotic stresses. PLoS ONE 2015, 10, e0118751. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yue, R.; Yang, Y.; Zhang, L.; Sun, T.; Xu, L.; Tie, S.; Wang, H. Genome-wide identification and expression profiling analysis of the AUX/IAA gene family in Medicago truncatula during the early phase of sinorhizobium meliloti infection. PLoS ONE 2014, 9, e107495. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yue, R.; Sun, T.; Zhang, L.; Xu, L.; Tie, S.; Wang, H.; Yang, Y. Genome-wide identification and expression analysis of auxin response factor gene family in Medicago truncatula. Front. Plant Sci. 2015, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Ulmasov, T.; Hagen, G.; Guilfoyle, T.J. Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. USA 1999, 96, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Lee, I.C.; Kim, J.; Kim, H.J.; Ryu, J.S.; Woo, H.R.; Nam, H.G. Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J. Exp. Bot. 2010, 61, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Sessions, A.; Nemhauser, J.L.; McColl, A.; Roe, J.L.; Feldmann, K.A.; Zambryski, P.C. Ettin patterns the arabidopsis floral meristem and reproductive organs. Development 1997, 124, 4481–4491. [Google Scholar] [PubMed]
- Hardtke, C.S.; Berleth, T. The arabidopsis gene monopteros encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998, 17, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.M.; Stowe-Evans, E.L.; Luesse, D.R.; Muto, H.; Tatematsu, K.; Watahiki, M.K.; Yamamoto, K.; Liscum, E. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 2000, 12, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Vivian-Smith, A.; Johnson, S.D.; Koltunow, A.M. Auxin response factor8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell 2006, 18, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. Auxin response factor 1 and auxin response factor 2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, P.; Ellis, C.M.; Weber, H.; Ploense, S.E.; Barkawi, L.S.; Guilfoyle, T.J.; Hagen, G.; Alonso, J.M.; Cohen, J.D.; Farmer, E.E.; et al. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 2005, 132, 4107–4118. [Google Scholar] [CrossRef] [PubMed]
- Narise, T.; Kobayashi, K.; Baba, S.; Shimojima, M.; Masuda, S.; Fukaki, H.; Ohta, H. Involvement of auxin signaling mediated by IAA14 and ARF7/19 in membrane lipid remodeling during phosphate starvation. Plant Mol. Biol. 2010, 72, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Zouine, M.; Fu, Y.; Chateigner-Boutin, A.L.; Mila, I.; Frasse, P.; Wang, H.; Audran, C.; Roustan, J.P.; Bouzayen, M. Characterization of the tomato ARF gene family uncovers a multi-levels post-transcriptional regulation including alternative splicing. PLoS ONE 2014, 9, e84203. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yue, R.; Sun, T.; Zhang, L.; Yang, Y.; Wang, H. OsARF16, a transcription factor regulating auxin redistribution, is required for iron deficiency response in rice (Oryza sativa L.). Plant Sci. 2015, 231, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yue, R.; Yang, Y.; Zhang, L.; Sun, T.; Tie, S.; Wang, H. OsARF16 is involved in cytokinin-mediated inhibition of phosphate transport and phosphate signaling in rice (Oryza sativa L.). PLoS ONE 2014, 9, e112906. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.V.; Le, D.T.; Nishiyama, R.; Watanabe, Y.; Sulieman, S.; Tran, U.T.; Mochida, K.; Dong, N.V.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; et al. The auxin response factor transcription factor family in soybean: Genome-wide identification and expression analyses during development and water stress. DNA Res. 2013, 20, 511–524. [Google Scholar] [PubMed]
- Xu, Y.X.; Mao, J.; Chen, W.; Qian, T.T.; Liu, S.C.; Hao, W.J.; Li, C.F.; Chen, L. Identification and expression profiling of the auxin response factors (ARFs) in the tea plant (Camellia sinensis (L.) O. Kuntze) under various abiotic stresses. Plant Physiol. Biochem. PPB Soc. Fr. Physiol. Veg. 2016, 98, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zuo, J.; Hou, X.; Yan, Y.; Wei, Y.; Liu, J.; Li, M.; Xu, B.; Jin, Z. The auxin response factor gene family in banana: Genome-wide identification and expression analyses during development, ripening, and abiotic stress. Front. Plant Sci. 2015, 6, 742. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Suo, N.N.; Hu, X.; Wang, S.; Liu, J.J.; Wang, H.Z. Development and characterization of 110 novel EST-SSR markers for Dendrobium officinale (orchidaceae). Am. J. Bot. 2012, 99, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, W.; Zhou, Z.; Liu, J.; Wang, H. Functional characterization of a novel tropinone reductase-like gene in Dendrobium nobile Lindl. J. Plant Physiol. 2013, 170, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Juan, L.I.; Shunxiang, L.I.; Dan, H.; Zhao, X.; Cai, G. Advances in the of resources, constituents and pharmacological effects of Dendrobium officinale. Rev. Sci. Tech. 2011, 29, 74–79. [Google Scholar]
- Wu, J.B.; Zhang, C.L.; Mao, P.P.; Qian, Y.S.; Wang, H.Z. First report of leaf spot caused by nigrospora oryzae on Dendrobium candidum in China. Plant Dis. 2014, 98, 996. [Google Scholar] [CrossRef]
- Shen, C.; Guo, H.; Chen, H.; Shi, Y.; Meng, Y.; Lu, J.; Feng, S.; Wang, H. Identification and analysis of genes associated with the synthesis of bioactive constituents in Dendrobium officinale using RNA-Seq. Sci. Rep. 2017, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Nonogaki, H.; Carrington, J.C. Repression of auxin response factor 10 by MicroRNA160 is critical for seed germination and post-germination stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 2007, 394, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, U.C.; Difazio, S.P.; Brunner, A.M.; Tuskan, G.A. Genome-wide analysis of AUX/IAA and ARF gene families in Populus trichocarpa. BMC Plant Biol. 2007, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; OuYang, W.Z.; Hou, X.J.; Xie, L.L.; Hu, C.G.; Zhang, J.Z. Genome-wide identification, isolation and expression analysis of auxin response factor (ARF) gene family in sweet orange (Citrus sinensis). Front. Plant Sci. 2015, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T.J. AUX/IAA proteins contain a potent transcriptional repression domain. Plant Cell 2004, 16, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, S.; Bai, Y.; Wu, Y.; Zhang, S.; Chen, M.; Guilfoyle, T.J.; Wu, P.; Qi, Y. Functional analysis of the structural domain of ARF proteins in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 3971–3981. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, S165–S183. [Google Scholar] [PubMed]
- Jian, H.; Wang, J.; Wang, T.; Wei, L.; Li, J.; Liu, L. Identification of rapeseed micrornas involved in early stage seed germination under salt and drought stresses. Front. Plant Sci. 2016, 7, 658. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Xu, Y.X.; Ma, J.Q.; Wang, W.W.; Chen, W.; Huang, D.J.; Fang, J.; Li, X.J.; Chen, L. Small RNA and degradome profiling reveals important roles for MicroRNAs and their targets in tea plant response to drought stress. Physiol. Plant. 2016, 158, 435. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Lv, W.; Li, L.; Shi, Q.; Yang, J.; Zhang, M. Reduced expression of bjrce1 gene modulated by nuclear-cytoplasmic incompatibility alters auxin response in cytoplasmic male-sterile Brassica juncea. PloS ONE 2012, 7, e38821. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yue, R.; Yuan, C.; Liu, J.; Zhang, L.; Sun, T.; Yang, Y.; Tie, S.; Shen, C. Auxin signaling is involved in iron deficiency-induced photosynthetic inhibition and shoot growth defect in rice ( Oryza sativa L.). J. Plant Biol. 2015, 58, 391–401. [Google Scholar] [CrossRef]
- Rondinini, S.; Confalonieri, C.; Longhi, P.; Mussini, T. The auxin transporter, OSAUX1, is involved in primary root and root hair elongation and in Cd stress responses in rice (Oryza sativa L.). Plant J. Cell Mol. Biol. 2015, 83, 818. [Google Scholar]
- Feng, S.; Yue, R.; Tao, S.; Yang, Y.; Zhang, L.; Xu, M.; Wang, H.; Shen, C. Genome-wide identification, expression analysis of auxin-responsive GH3 family genes in maize (Zea mays L.) under abiotic stresses. J. Integr. Plant Biol. 2015, 57, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, S.; Shen, C.; Zhang, S.; Chen, Y.; Xu, Y.; Liu, Y.; Wu, Y.; Jiang, D. OsARF12, a transcription activator on auxin response gene, regulates root elongation and affects iron accumulation in rice (Oryza sativa). New Phytol. 2012, 193, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, X.; Liu, H.; Tian, Y.; Lian, J.; Yang, R.; Hao, S.; Wang, X.; Yang, S.; Li, Q.; et al. The genome of Dendrobium officinale illuminates the biology of the important traditional chinese orchid herb. Mol. Plant 2015, 8, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, F.; Cheng, L.; Kong, F.; Peng, Z.; Liu, S.; Yu, X.; Lu, G. Identification, isolation and expression analysis of auxin response factor (ARF) genes in Solanum lycopersicum. Plant Cell Rep. 2011, 30, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yuan, C.; Li, H.; Lin, W.; Yang, Y.; Shen, C.; Zheng, X. Genome-wide identification and characterization of auxin response factor (ARF) family genes related to flower and fruit development in papaya (Carica papaya L.). BMC Genom. 2015, 16, 901. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Tyagi, A.K.; Sharma, A.K. Genome-wide analysis of auxin response factor (ARF) gene family from tomato and analysis of their role in flower and fruit development. Mol. Genet. Genom. 2011, 285, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Pudake, R.N.; Guo, G.; Xing, G.; Hu, Z.; Zhang, Y.; Sun, Q.; Ni, Z. Genome-wide identification and expression profiling of auxin response factor (ARF) gene family in maize. BMC Genom. 2011, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Liu, S.; Guan, X.; Chen, L.; He, Y.; Jie, W.; Gang, L. Genome-wide identification and transcriptional profiling analysis of auxin response-related gene families in cucumber. BMC Res. Notes 2014, 7, 1–13. [Google Scholar]
- Popko, J.; Hansch, R.; Mendel, R.R.; Polle, A.; Teichmann, T. The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol. 2010, 12, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M.; Alabadi, D.; Perez-Gomez, J.; Garcia-Carcel, L.; Phillips, A.L.; Hedden, P.; Blazquez, M.A. Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol. 2006, 142, 553–563. [Google Scholar] [CrossRef] [PubMed]
- De, J.M.; Woltersarts, M.; Garcíamartínez, J.L.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum auxin response factor 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J. Exp. Bot. 2011, 62, 617. [Google Scholar]
- Wang, S.; Bai, Y.; Shen, C.; Wu, Y.; Zhang, S.; Jiang, D.; Guilfoyle, T.J.; Chen, M.; Qi, Y. Auxin-related gene families in abiotic stress response in Sorghum bicolor. Funct. Integr. Genom. 2010, 10, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yang, Y.; Liu, K.; Zhang, L.; Guo, H.; Sun, T.; Wang, H. Involvement of endogenous salicylic acid in iron-deficiency responses in Arabidopsis. J. Exp. Bot. 2016, 67, 4179–4193. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yu, D.; Xue, J.; Lu, J.; Feng, S.; Shen, C.; Wang, H. A transcriptome-wide, organ-specific regulatory map of Dendrobium officinale, an important traditional chinese orchid herb. Sci. Rep. 2016, 6, 18864. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yue, R.; Bai, Y.; Feng, R.; Sun, T.; Wang, X.; Yang, Y.; Tie, S.; Wang, H. Identification and analysis of Medicago truncatula auxin transporter gene families uncover their roles in responses to Sinorhizobium meliloti infection. Plant Cell Physiol. 2015, 56, 1930–1943. [Google Scholar] [CrossRef] [PubMed]

| Gene | Locus ID | ORF (bp) | MR Locations | Deduced Polypeptide | ||
|---|---|---|---|---|---|---|
| Length (aa) | Mol wt (Da) | pI | ||||
| DnARF1 | comp169620_c0_seq10 | 2016 | 358–539 | 672 | 74,848.32 | 5.93 |
| DnARF2a | comp173652_c0_seq13 | 2529 | 387–709 | 843 | 93,698.06 | 6.84 |
| DnARF2b | comp164610_c0_seq15 | 1998 | 203–536 | 666 | 75,067.37 | 8.13 |
| DnARF3 | comp168166_c0_seq7 | 2124 | 380–628 | 708 | 77,816.9 | 6.49 |
| DnARF4 | comp163222_c1_seq4 | 2103 | 373–end | 701 | 77,883.19 | 6.97 |
| DnARF6 | comp171031_c0_seq23 | 2340 | 361–711 | 780 | 87,556.94 | 6.39 |
| DnARF7 | comp173511_c0_seq20 | 2589 | 275–756 | 863 | 97,659.3 | 6.71 |
| DnARF10 | comp160144_c2_seq3 | 1914 | 383–572 | 638 | 71,309.5 | 6.74 |
| DnARF11 | comp170249_c2_seq3 | 1755 | 307–524 | 585 | 65,625.36 | 6.91 |
| DnARF16a | comp165418_c0_seq20 | 1995 | 387–599 | 665 | 73,640.69 | 7.54 |
| DnARF16b | comp160625_c0_seq3 | 2103 | 401–612 | 701 | 77,632.32 | 7.83 |
| DnARF17 | comp134032_c0_seq1 | 1566 | 349–end | 522 | 57,580.99 | 8.40 |
| DnARF19a | comp163679_c1_seq3 | 1875 | 381–end | 625 | 70,303.81 | 8.09 |
| DnARF19b | comp150031_c0_seq2 | 2943 | 360–78 | 981 | 109,549.1 | 5.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yuan, Y.; Fu, D.; Shen, C.; Yang, Y. Identification and Expression Profiling of the Auxin Response Factors in Dendrobium officinale under Abiotic Stresses. Int. J. Mol. Sci. 2017, 18, 927. https://doi.org/10.3390/ijms18050927
Chen Z, Yuan Y, Fu D, Shen C, Yang Y. Identification and Expression Profiling of the Auxin Response Factors in Dendrobium officinale under Abiotic Stresses. International Journal of Molecular Sciences. 2017; 18(5):927. https://doi.org/10.3390/ijms18050927
Chicago/Turabian StyleChen, Zhehao, Ye Yuan, Di Fu, Chenjia Shen, and Yanjun Yang. 2017. "Identification and Expression Profiling of the Auxin Response Factors in Dendrobium officinale under Abiotic Stresses" International Journal of Molecular Sciences 18, no. 5: 927. https://doi.org/10.3390/ijms18050927





