Fasting Enhances the Contrast of Bone Metastatic Lesions in 18F-Fluciclovine-PET: Preclinical Study Using a Rat Model of Mixed Osteolytic/Osteoblastic Bone Metastases
Abstract
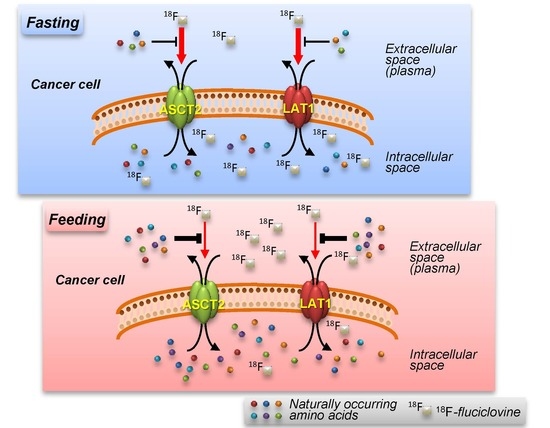
:1. Introduction
2. Results
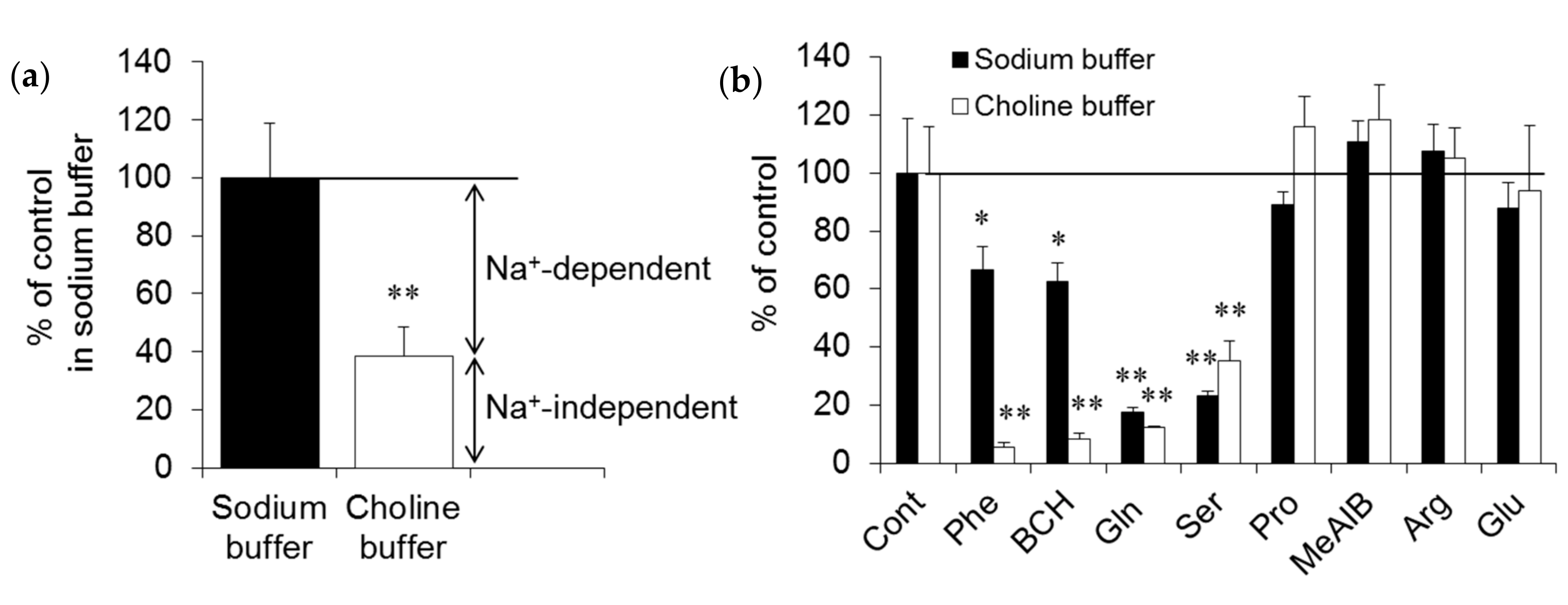
2.1. In Vitro Experiments
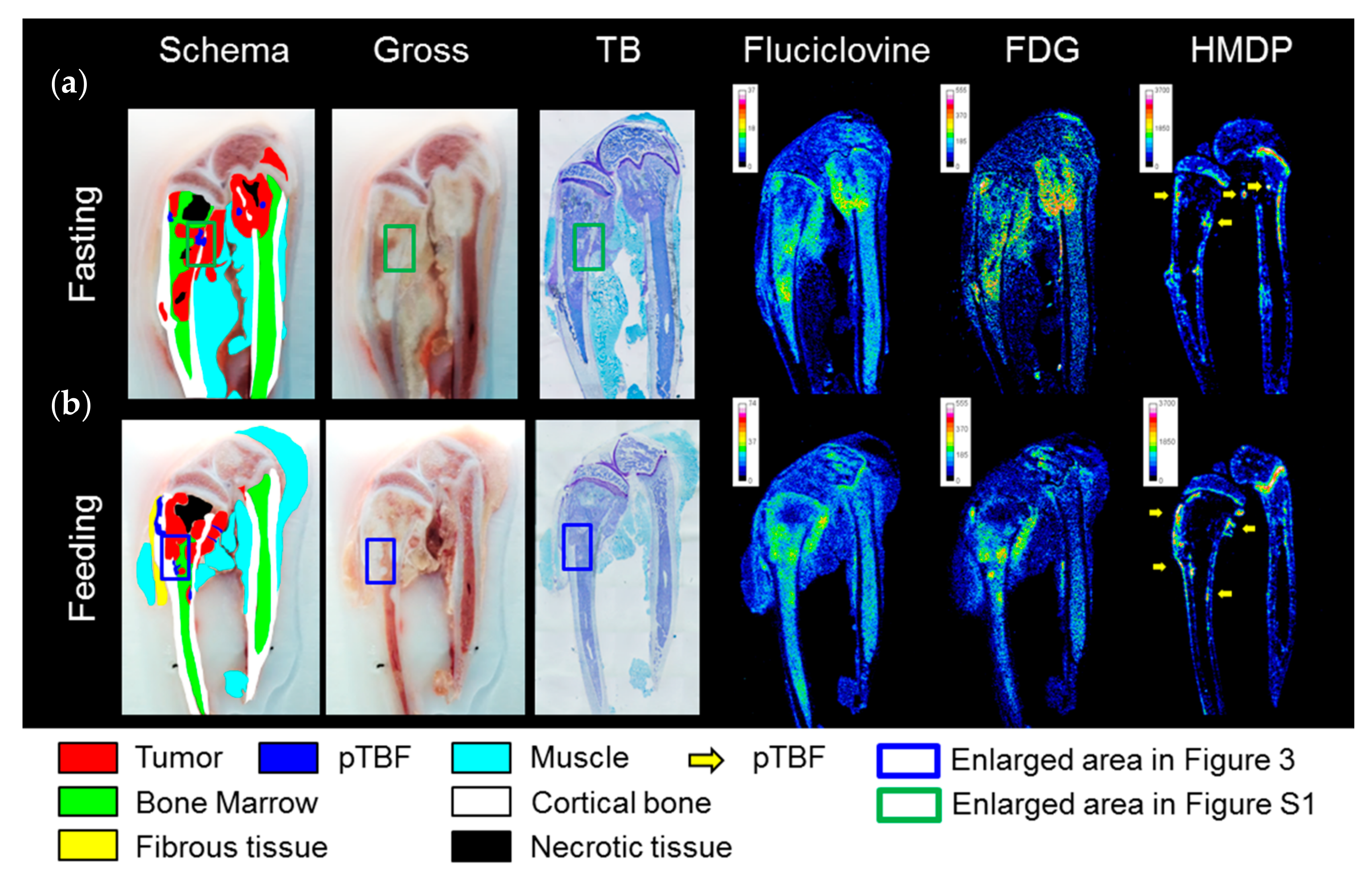
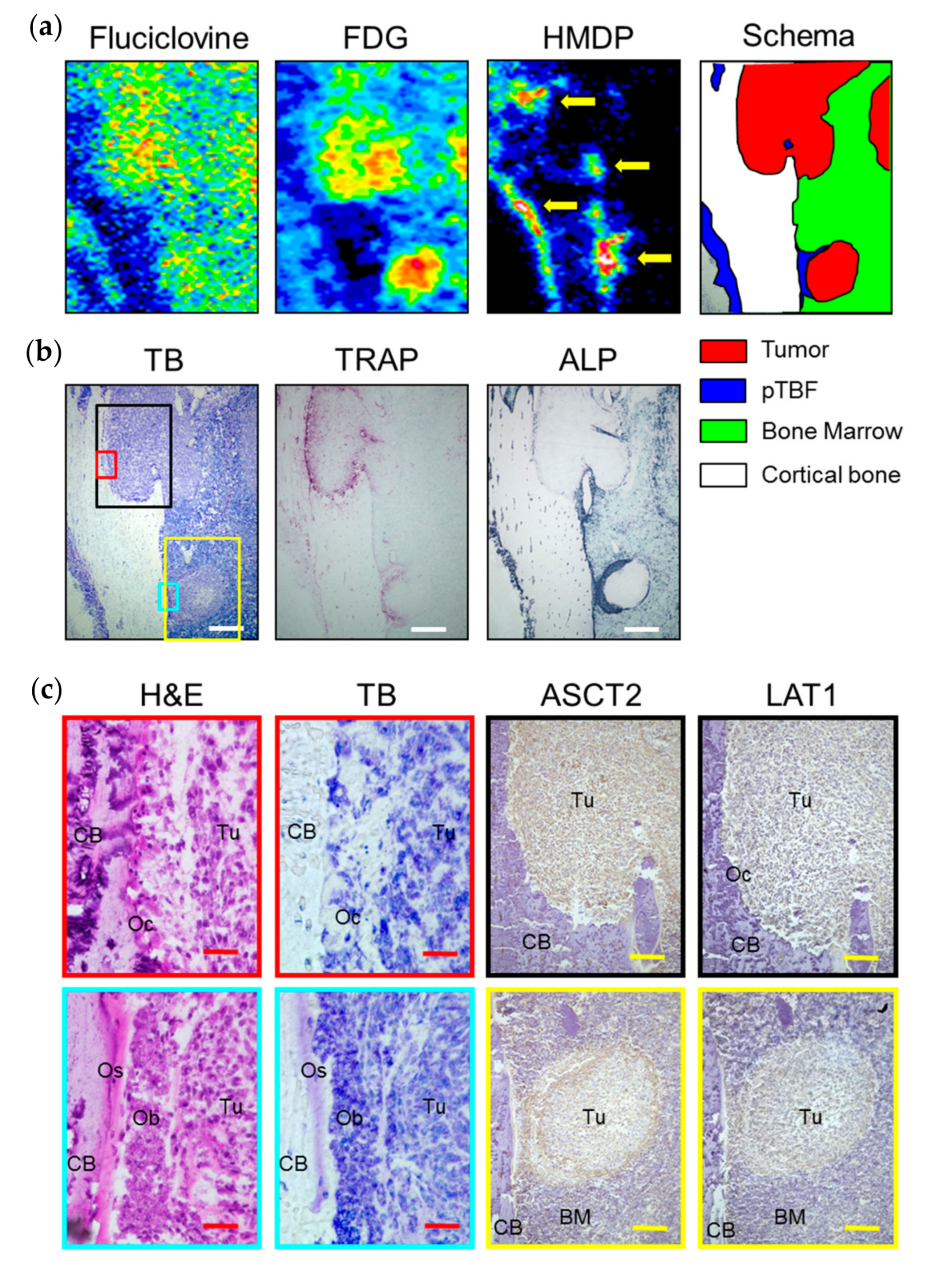
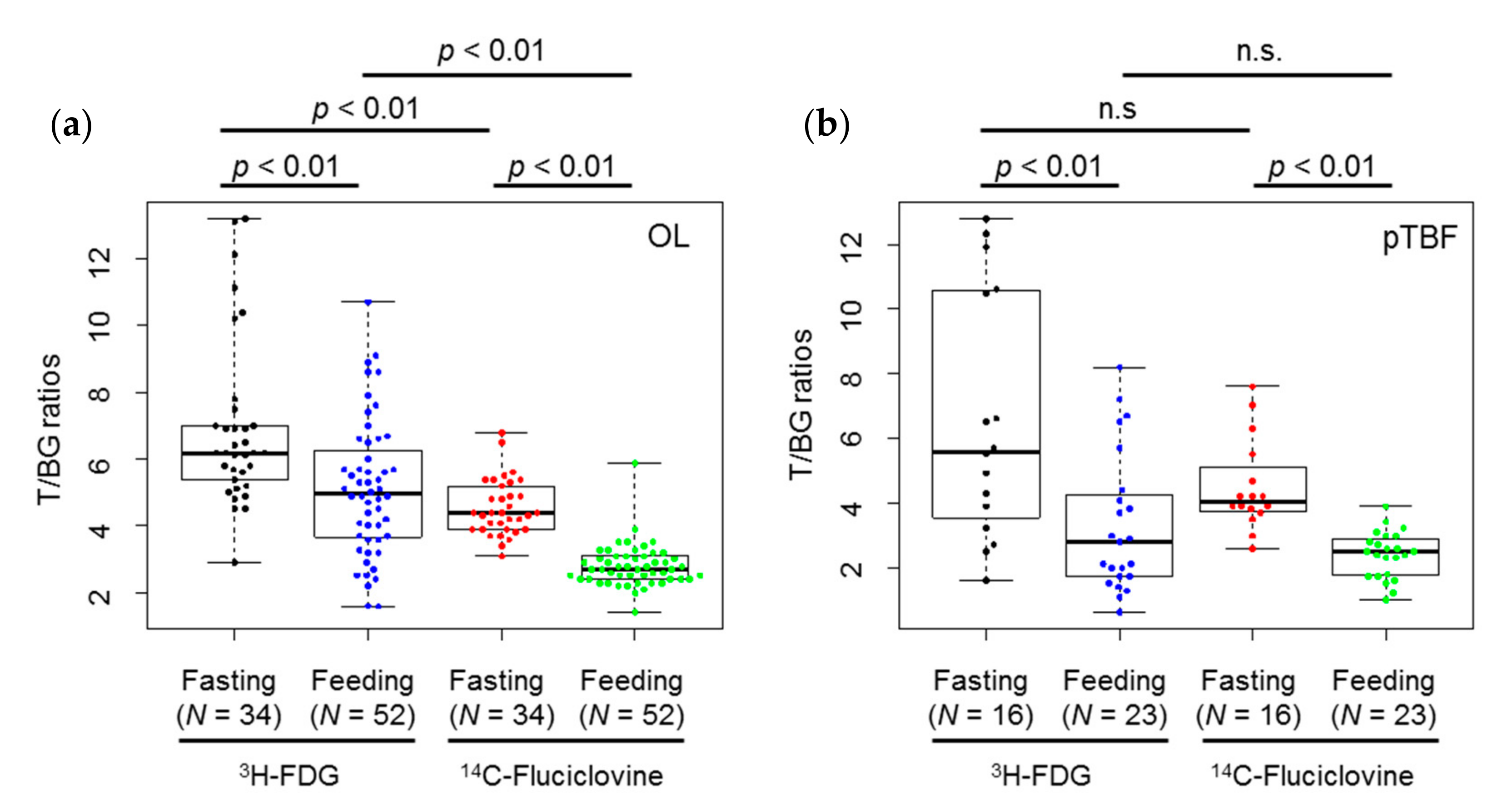
2.2. In Vivo Experiments
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Competitive Inhibition Assay
4.4. Animal Handling
4.5. BCa Bone Metastatic Model
4.6. Triple-Tracer Autoradiography
4.7. Histological Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AA | amino acid |
| AAT | amino acid transporter |
| ALP | alkaline phosphatase |
| ASC | alanine-serine-cysteine |
| ASCT2 | alanine-serine-cysteine transporter 2 |
| BCa | breast cancer |
| BM | bone marrow |
| 11C | carbon-11 |
| 14C | carbon-14 |
| CB | cortical bone |
| 18F | fluorine-18 |
| FDG | 2-deoxy-2-fluoro-d-glucose |
| 3H | tritium-3 |
| H&E | hematoxylin-eosin |
| HMDP | hydroxymethylene diphosphonate |
| LAT1 | L-type amino acid transporter 1 |
| mTORC1 | mammalian target of rapamycin complex 1 |
| 99mTc | technetium-99m |
| Ob | osteoblasts |
| Oc | osteoclasts |
| Os | osteoids |
| PET | positron emission tomography |
| PCa | prostate cancer |
| pTBF | peri-tumor bone formation |
| RCP | radiochemical purity |
| ROI | region-of-interest |
| SCEM | Super Cryoembedding Medium |
| SNAT2 | sodium-dependent neutral amino acid transporter 2 |
| TB | toluidine blue |
| T/BG | targetmean (metastatic leion)-to-backgroundmean (muscle) |
| TRAP | tartrate-resistant acid phosphatase |
References
- Lindholm, P.; Minn, H.; Leskinen-Kallio, S.; Bergman, J.; Ruotsalainen, U.; Joensuu, H. Influence of the blood glucose concentration on FDG uptake in cancer—A PET study. J. Nucl. Med. 1993, 34, 1–6. [Google Scholar] [PubMed]
- Lewis, D.Y.; Soloviev, D.; Brindle, K.M. Imaging tumor metabolism using positron emission tomography. Cancer J. 2015, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jager, P.L. Improving amino acid imaging: Hungry or stuffed? J. Nucl. Med. 2002, 43, 1207–1209. [Google Scholar] [PubMed]
- Lindholm, P.; Leskinen-Kallio, S.; Kirvelä, O.; Någren, K.; Lehikoinen, P.; Pulkki, K.; Peltola, O.; Ruotsalainen, U.; Teräs, M.; Joensuu, H. Head and neck cancer: Effect of food ingestion on uptake of C-11 methionine. Radiology 1994, 190, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.M.; Nanni, C.; Fanti, S.; Oka, S.; Okudaira, H.; Inoue, Y.; Sörensen, J.; Owenius, R.; Choyke, P.; Turkbey, B.; et al. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: Physiologic uptake patterns, incidental findings, and variants that may simulate disease. J. Nucl. Med. 2014, 55, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Goldman, D.A.; Gönen, M.; Pham, H.; Castillo, R.; Lyashchenko, S.K.; Lewis, J.S.; Dang, C. Initial Results of a Prospective Clinical Trial of 18F-Fluciclovine PET/CT in Newly Diagnosed Invasive Ductal and Invasive Lobular Breast Cancers. J. Nucl. Med. 2016, 57, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Hajizamani, S.; Mortaz, E.; Ahmadzadeh, A.; Shahjahani, M.; Shahrabi, S.; Saki, N. Molecular regulation of bone marrow metastasis in prostate and breast cancer. Bone Marrow Res. 2014, 2014, 405920. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Okudaira, H.; Yoshida, Y.; Schuster, D.M.; Goodman, M.M.; Shirakami, Y. Transport mechanisms of trans-1-amino-3-fluoro[1-(14)C]cyclobutanecarboxylic acid in prostate cancer cells. Nucl. Med. Biol. 2012, 39, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Okudaira, H.; Nakanishi, T.; Oka, S.; Kobayashi, M.; Tamagami, H.; Schuster, D.M.; Goodman, M.M.; Shirakami, Y.; Tamai, I.; Kawai, K. Kinetic analyses of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid transport in Xenopus laevis oocytes expressing human ASCT2 and SNAT2. Nucl. Med. Biol. 2013, 40, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Oka, S.; Okudaira, H.; Schuster, D.M.; Goodman, M.M.; Kawai, K.; Shirakami, Y. Comparative evaluation of transport mechanisms of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid and l-[methyl-¹¹C]methionine in human glioma cell lines. Brain Res. 2013, 1535, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Okudaira, H.; Ono, M.; Schuster, D.M.; Goodman, M.M.; Kawai, K.; Shirakami, Y. Differences in transport mechanisms of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid in inflammation, prostate cancer, and glioma cells: Comparison with L-[methyl-11C]methionine and 2-deoxy-2-[18F]fluoro-d-glucose. Mol. Imaging Biol. 2014, 16, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Cho, H.T.; Williams, L.; Zhu, A.; Liang, K.; Huang, K.; Wu, H.; Jiang, C.; Hong, S.; Crowe, R.; et al. Potential Biomarker of L-type Amino Acid Transporter 1 in Breast Cancer Progression. Nucl. Med. Mol. Imaging 2011, 45, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M.; Kovarik, M. Alterations in protein metabolism and amino acid concentrations in rats fed by a high-protein (casein-enriched) diet—Effect of starvation. Food Chem. Toxicol. 2011, 49, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Bergström, M.; Ericson, K.; Hagenfeldt, L.; Mosskin, M.; von Holst, H.; Norén, G.; Eriksson, L.; Ehrin, E.; Johnström, P. PET study of methionine accumulation in glioma and normal brain tissue: Competition with branched chain amino acids. J. Comput. Assist. Tomogr. 1987, 11, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Langen, K.J.; Roosen, N.; Coenen, H.H.; Kuikka, J.T.; Kuwert, T.; Herzog, H.; Stöcklin, G.; Feinendegen, L.E. Brain and brain tumor uptake of l-3-[123I]iodo-α-methyl tyrosine: Competition with natural L-amino acids. J. Nucl. Med. 1991, 32, 1225–1229. [Google Scholar] [PubMed]
- Lahoutte, T.; Caveliers, V.; Franken, P.R.; Bossuyt, A.; Mertens, J.; Everaert, H. Increased tumor uptake of 3-(123)I-Iodo-l-α-methyltyrosine after preloading with amino acids: An in vivo animal imaging study. J. Nucl. Med. 2002, 43, 1201–1206. [Google Scholar] [PubMed]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Naito, S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab. Pharmacokinet. 2005, 20, 452–477. [Google Scholar] [CrossRef] [PubMed]
- Bevington, A.; Brown, J.; Butler, H.; Govindji, S.; M-Khalid, K.; Sheridan, K.; Walls, J. Impaired system A amino acid transport mimics the catabolic effects of acid in L6 cells. Eur. J. Clin. Investig. 2002, 32, 590–602. [Google Scholar] [CrossRef]
- Evans, K.; Nasim, Z.; Brown, J.; Butler, H.; Kauser, S.; Varoqui, H.; Erickson, J.D.; Herbert, T.P.; Bevington, A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J. Am. Soc. Nephrol. 2007, 18, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Hyde, R.; Hajduch, E.; Powell, D.J.; Taylor, P.M.; Hundal, H.S. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005, 19, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1011–E1018. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.M.; Tee, A.R. Leucine and mTORC1: A complex relationship. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1329–E1342. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Bode, B.P. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin. Cancer Biol. 2005, 15, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tiffen, J.; Bailey, C.G.; Lehman, M.L.; Ritchie, W.; Fazli, L. Targeting amino acid transport in metastatic castration-resistant prostate cancer: Effects on cell cycle, cell growth, and tumor development. J. Natl. Cancer Inst. 2013, 105, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Kaira, K.; Ohshima, Y.; Ishioka, N.S.; Shino, M.; Sakakura, K.; Takayasu, Y.; Takahashi, K.; Tominaga, H.; Oriuchi, N.; et al. Prognostic significance of amino-acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br. J. Cancer 2014, 110, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, M.; Kakizaki, S.; Kaira, K.; Tojima, H.; Yamazaki, Y.; Horiguchi, N.; Sato, K.; Oriuchi, N.; Tominaga, H.; Sunose, Y.; et al. Expression of amino acid transporters (LAT1, ASCT2 and xCT) as clinical significance in hepatocellular carcinoma. Hepatol. Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Arakawa, K.; Shimizu, K.; Oriuchi, N.; Nagamori, S.; Kanai, Y.; Oyama, T.; Takeyoshi, I. Relationship between CD147 and expression of amino acid transporters (LAT1 and ASCT2) in patients with pancreatic cancer. Am. J. Transl. Res. 2015, 7, 356–363. [Google Scholar] [PubMed]
- Nikkuni, O.; Kaira, K.; Toyoda, M.; Shino, M.; Sakakura, K.; Takahashi, K.; Tominaga, H.; Oriuchi, N.; Suzuki, M.; Iijima, M.; et al. Expression of Amino Acid Transporters (LAT1 and ASCT2) in Patients with Stage III/IV Laryngeal Squamous Cell Carcinoma. Pathol. Oncol. Res. 2015, 21, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Nakamura, K.; Hirakawa, T.; Imai, H.; Tominaga, H.; Oriuchi, N.; Nagamori, S.; Kanai, Y.; Tsukamoto, N.; Oyama, T.; et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in patients with ovarian tumors. Am. J. Transl. Res. 2015, 7, 1161–1171. [Google Scholar] [PubMed]
- Honjo, H.; Kaira, K.; Miyazaki, T.; Yokobori, T.; Kanai, Y.; Nagamori, S.; Oyama, T.; Asao, T.; Kuwano, H. Clinicopathological significance of LAT1 and ASCT2 in patients with surgically resected esophageal squamous cell carcinoma. J. Surg. Oncol. 2016, 113, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Tsuchihara, K.; Fujii, S.; Sugiyama, M.; Goya, T.; Atomi, Y.; Ueno, T.; Ochiai, A.; Esumi, H. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007, 67, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yoneda, J.; Ohmori, H.; Sasaki, T.; Shimbo, K.; Eto, S.; Kato, Y.; Miyano, H.; Kobayashi, T.; Sasahira, T.; et al. Cancer usurps skeletal muscle as an energy repository. Cancer Res. 2014, 74, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Hattori, R.; Kurosaki, F.; Toyama, M.; Williams, L.A.; Yu, W.; Votaw, J.R.; Yoshida, Y.; Goodman, M.M.; Ito, O. A preliminary study of anti-1-amino-3-18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J. Nucl. Med. 2007, 48, 46–55. [Google Scholar] [PubMed]
- Schuster, D.M.; Votaw, J.R.; Nieh, P.T.; Yu, W.; Nye, J.A.; Master, V.; Bowman, F.D.; Issa, M.M.; Goodman, M.M. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J. Nucl. Med. 2007, 48, 56–63. [Google Scholar] [PubMed]
- Inoue, Y.; Asano, Y.; Satoh, T.; Tabata, K.; Kikuchi, K.; Woodhams, R.; Baba, S.; Hayakawa, K. Phase IIa Clinical Trial of Trans-1-Amino-3-18F-Fluoro-Cyclobutane carboxylic acid in metastatic prostate cancer. Asia Ocean J. Nucl. Med. Biol. 2014, 2, 87–94. [Google Scholar] [PubMed]
- Zepp, M.; Bauerle, T.J.; Elazar, V.; Peterschmitt, J.; Lifshitz-Shovali, R.; Adwan, H.; Armbruster, F.P.; Golomb, G.; Berger, M.R. Treatment of breast cancer lytic skeletal metastasis using a model in nude rats. In Breast Cancer—Current and Alternative Therapeutic Modalities; Gunduz, E., Ed.; InTech: Rijeka, Croatia, 2011; Available online: http://www.intechopen.com/books/breast-cancer-current-and-alternative-therapeutic-modalities/treatment-of-breast-cancer-lytic-skeletal-metastasis-using-a-model-in-nude-rats (accessed on 28 April 2017).
- Kawamoto, T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003, 66, 123–413. [Google Scholar] [CrossRef] [PubMed]
| Lesion | Tracer | Diet | Min. | Max. | Median | 1st Qu. | 3rd Qu. | Mean ± S.D. |
|---|---|---|---|---|---|---|---|---|
| OL | 3H-FDG | Fasting | 2.9 | 13.2 | 6.2 | 5.5 | 7.0 | 6.9 ± 2.5 |
| Feeding | 1.6 | 10.7 | 5.0 | 3.7 | 6.1 | 5.1 ± 2.0 | ||
| 14C-fluciclovine | Fasting | 3.1 | 6.8 | 4.4 | 3.9 | 5.1 | 4.6 ± 0.8 | |
| Feeding | 1.4 | 5.9 | 2.7 | 2.4 | 3.1 | 2.8 ± 0.6 | ||
| pTBF | 3H-FDG | Fasting | 1.6 | 12.8 | 5.6 | 3.7 | 10.5 | 6.6 ± 3.8 |
| Feeding | 0.6 | 8.2 | 2.8 | 1.7 | 4.3 | 3.3 ± 2.2 | ||
| 14C-fluciclovine | Fasting | 2.6 | 7.6 | 4.1 | 3.8 | 4.9 | 4.5 ± 1.4 | |
| Feeding | 1.0 | 3.9 | 2.5 | 1.8 | 2.9 | 2.4 ± 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oka, S.; Kanagawa, M.; Doi, Y.; Schuster, D.M.; Goodman, M.M.; Yoshimura, H. Fasting Enhances the Contrast of Bone Metastatic Lesions in 18F-Fluciclovine-PET: Preclinical Study Using a Rat Model of Mixed Osteolytic/Osteoblastic Bone Metastases. Int. J. Mol. Sci. 2017, 18, 934. https://doi.org/10.3390/ijms18050934
Oka S, Kanagawa M, Doi Y, Schuster DM, Goodman MM, Yoshimura H. Fasting Enhances the Contrast of Bone Metastatic Lesions in 18F-Fluciclovine-PET: Preclinical Study Using a Rat Model of Mixed Osteolytic/Osteoblastic Bone Metastases. International Journal of Molecular Sciences. 2017; 18(5):934. https://doi.org/10.3390/ijms18050934
Chicago/Turabian StyleOka, Shuntaro, Masaru Kanagawa, Yoshihiro Doi, David M. Schuster, Mark M. Goodman, and Hirokatsu Yoshimura. 2017. "Fasting Enhances the Contrast of Bone Metastatic Lesions in 18F-Fluciclovine-PET: Preclinical Study Using a Rat Model of Mixed Osteolytic/Osteoblastic Bone Metastases" International Journal of Molecular Sciences 18, no. 5: 934. https://doi.org/10.3390/ijms18050934